Abstract
Dicoumarol is frequently used as inhibitor of the detoxifying enzyme NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1). In order to test whether dicoumarol may also affect the cellular glutathione (GSH) metabolism, we have exposed cultured primary astrocytes to dicoumarol and investigated potential effects of this compound on the cell viability as well as on the cellular and extracellular contents of GSH and its metabolites. Incubation of astrocytes with dicoumarol in concentrations of up to 100 µM did not acutely compromise cell viability nor was any GSH consumption or GSH oxidation to glutathione disulfide (GSSG) observed. However, unexpectedly dicoumarol inhibited the cellular multidrug resistance protein (Mrp) 1-dependent export of GSH in a time- and concentration-dependent manner with half-maximal effects observed at low micromolar concentrations of dicoumarol. Inhibition of GSH export by dicoumarol was not additive to that observed for the known Mrp1 inhibitor MK571. In addition, dicoumarol inhibited also the Mrp1-mediated export of GSSG during menadione-induced oxidative stress and the export of the GSH–bimane-conjugate (GS–B) that had been generated in the cells after exposure to monochlorobimane. Half-maximal inhibition of the export of Mrp1 substrates was observed at dicoumarol concentrations of around 4 µM (GSH and GSSG) and 30 µM (GS–B). These data demonstrate that dicoumarol strongly affects the GSH metabolism of viable cultured astrocytes by inhibiting Mrp1-mediated export processes and identifies for the first time Mrp1 as additional cellular target of dicoumarol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dicoumarol is a naturally occuring compound that is formed by fungi during spoilage of sweet clover [1,2,3]. It has been initially used as a potent anticoagulant until the mid-1950s as it lowers vitamin K recycling and blood clotting by inhibiting the enzyme vitamin K epoxide reductase [4, 5]. Dicourmarol is also well known for its inhibitory potential on NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1). It efficiently inhibits the NQO1 catalysed two-electron reduction of quinones, thereby avoiding the unwanted one-electron reduction of organic compounds which would cause oxidative stress [4, 6]. Accordingly, dicoumarol has been used to investigate potential cellular functions of NQO1 [7].
The tripeptide glutathione (γ-l-glutamyl-l-cysteinylglycine; GSH) has a pivotal role in the defence of cells against oxidative stress and in the cellular detoxification of harmful endogenous compounds and xenobiotics [8,9,10,11,12]. For example, GSH delivers electrons for the intracellular reduction of peroxides in reactions catalyzed by glutathione peroxidases [13, 14]. During such reactions, GSH is oxidized to GSSG, which is subsequently reduced to GSH by glutathione reductase in a NADPH-dependent reaction [15]. In addition, GSH serves with its reactive thiol-group as substrate for reactions with electrophiles that are catalyzed in cells by GSH-S-transferases [16, 17]. The GSH-conjugates generated by such enzymes can be exported from the cells via multidrug resistance proteins (Mrps). Mrps represent a large family of ATP-dependent export pumps that belong to the ABC-transporters and mediate the ATP-dependent transport of organic anions from mammalian cells [18, 19]. Among the family of Mrps, Mrp1 is prominently expressed in many cell types and has been shown to mediate the transport of a large variety of different intracellular substrates, including GSH conjugates, GSSG and also GSH [20, 21].
In brain, astrocytes are bidirectional communication partners with neurons [22]. Astrocytes cover with their endfeed the brain capillaries almost completely and have therefore direct access to blood-derived energy substrates and amino acids [23, 24]. Astrocytes fulfill also very important functions in the protection of the brain against oxidative stress and toxins [9,10,11, 25, 26]. Along this line, astrocytes supply neighboring neurons with precursors for GSH synthesis, which involves Mrp1-mediated export of GSH and subsequent extracellular cleavage of the tripeptide [11, 12].
Astrocytes express several Mrp-family members both in vitro [27,28,29] and in vivo [29,30,31]. Among those, Mrp1 appears to be especially important in the antioxidative and detoxifying pathways of astrocytes, as Mrp1 is predominantly responsible for the cellular export of GSH [32, 33], which is involved in the supply of GSH precursors from astrocytes to neurons [11, 12, 34]. In addition, the rapid export of GSSG that accumulates during oxidative stress in astrocytes is exclusively mediated by Mrp1 [33, 35] and is considered as mechanism that helps to prevent a rapid oxidation of the intracellular thiol reduction potential during oxidative stress [33, 36]. Finally, the conjugate of GSH with bimane (GS–B), that is formed after exposure of astrocytes to monochlorobimane (MCB) [37] as well as other GSH conjugates [38, 39] are also exported by Mrp1. All those astrocytic Mrp1-mediated export processes are efficiently inhibited by the compound MK571 [32, 33, 35, 37].
Dicoumarol has frequently been applied to cells to investigate NQO1-mediated detoxification processes [40,41,42]. Concerning the GSH metabolism only few studies have investigated dicoumarol and reported that dicoumarol has no or at best little effects on GSH contents in cell lines of peripheral origin and in hepatocytes [43, 44], while to our knowledge effects of dicoumarol on the GSH metabolism of brain cells have not been reported. As dicoumarol has been shown to diminish cellular resistance of neural cells towards compounds such as l-dopa [42, 45, 46], we investigated whether dicoumarol may—in addition to inhibiting NQO1 activity—also affect the metabolism of GSH in cultured brain cells. Here, we show for the first time that dicoumarol potently inhibits the Mrp1-mediated export of GSH, GSSG and GS–B from viable astrocytes at least as efficiently as the frequently used Mrp1-inhibitor MK571. Thus, Mrp1 has to be considered as additional cellular target of a dicoumarol treatment.
Materials and Methods
Materials
Dicoumarol and menadione were purchased from Sigma-Aldrich (Steinheim, Germany) and MK571 from Biomol (Hamburg, Germany). Dulbecco’s modified Eagle’s medium was from Gibco (Darmstadt, Germany), fetal calf serum was obtained from Biochrom (Berlin, Germany) and penicillin/streptomycin solution from Invitrogen-Gibco (Darmstadt, Germany). Bovine serum albumin (BSA), NADPH, NADH and sulfosalicylic acid were obtained from Applichem (Darmstadt, Germany) and the enzyme glutathione reductase was purchased from Roche Diagnostics (Mannheim, Germany). All other chemicals of the highest purity available were obtained from Merck (Darmstadt, Germany), Sigma-Aldrich (Steinheim, Germany), Fluka (Buchs, Switzerland), Roth (Karlsruhe, Germany) or Riedel-de Haën (Seelze, Germany). Sterile cell culture plates, unsterile 96-well microtiter plates and unsterile black 96-well microtiter plates were from Sarstedt (Nümbrecht, Germany).
Astrocyte Cultures
Astrocyte-rich primary cultures were prepared from the brains of newborn Wistar rats as previously described in detail [47]. 300,000 viable cells were seeded in 1 mL culture medium containing 90% Dulbecco’s modified Eagle’s medium (with 25 mM glucose), 10% fetal calf serum, 18 U/mL penicillin G, 18 µg/mL streptomycin sulfate, 44.6 mM NaHCO3 and 1 mM pyruvate in wells of 24-well plates. The cultures were incubated at 37 °C in the humidified atmosphere of a Sanyo incubator (Osaka, Japan) with 10% CO2. The culture medium was renewed every 7 days and 24 h prior to the experiments. The experiments of the present study were performed on confluent cultures of an age between 15 and 21 days.
Experimental Incubation of the Cells
To investigate GSH export from cultured astrocytes, the cellular and extracellular contents of total glutathione (GSx = amount of GSH plus twice the amount of GSSG) and GSSG were determined after the indicated incubations. The cultures were washed twice with 1 mL prewarmed (37 °C) incubation buffer (IB; 20 mM HEPES, 5 mM d-glucose, 145 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 0.8 mM Na2HPO4, pH 7.4) and incubated at 37 °C with 200 µL IB containing 100 µM of the γ-glutamyltranspeptidase inhibitor acivicin [48] in the absence or the presence of the compounds indicated in the figures and the tables. After the given incubation periods, the incubation media were collected and the cells were washed with 1 mL ice-cold phosphate-buffered saline (PBS; 10 mM potassium phosphate buffer, pH 7.4, containing 150 mM NaCl) before the extracellular and cellular GSx and GSSG contents were determined.
To investigate the potential reversibility of the inhibition of the GSH export by dicoumarol from astrocytes, the cells were pre-incubated for 2 h at 37 °C in 200 µL IB containing 100 µM acivicin in the absence or the presence of 30 µM dicoumarol. After two washing steps each with 1 mL prewarmed (37 °C) IB, the cells were further incubated for up to 4 h in 200 µL IB containing 100 µM acivicin in the absence or the presence of 30 µM dicoumarol. The amounts of extracellular GSx were determined for main incubation periods of 1 h, 2 h or 4 h, while the cellular GSx content and the extracellular lactate dehydrogenase (LDH) activity were determined after the 4 h main incubation.
During oxidative stress, cultured astrocytes oxidize GSH to GSSG which is subsequently exported via Mrp1 [33, 35]. In the current study, menadione was applied to induce cellular oxidation of GSH to GSSG. Cultured astrocytes were washed twice with 1 mL prewarmed (37 °C) IB and then incubated with 200 µL of IB containing 100 µM acivicin for 60 min at 37 °C without or with 100 µM menadione in the absence or the presence of the given concentrations of dicoumarol and/or MK571. Subsequently, the incubation medium was harvested for quantification of extracellular LDH acitivty and the contents of extracellular GSx and GSSG, while the cells were washed with 1 mL of ice-cold PBS before the cellular contents of GSx and GSSG were determined.
Upon incubation with monochlorobimane (MCB), astrocytes have been reported to form the GSH–bimane conjugate (GS–B), which is exported via Mrp1 [37]. To investigate a potential inhibition by dicoumarol of the GS–B export, cultured astrocytes were washed twice with 1 mL prewarmed IB and incubated in 500 µL IB containing 100 µM acivicin and 10 µM MCB without or with other substances as indicated in the figures. After the incubation, the incubation media were collected and analyzed for LDH activity and the contents of extracellular GS–B and GSx, while the cells were washed twice with 1 mL PBS and lysed to determine cellular GS–B and GSx contents.
Determination of Cell Viability and Cellular Protein Content
A potential loss in cell viability was monitored by the quantification of the release of the cytosolic enzyme LDH as described previously in detail [47, 49]. In addition, the membrane integrity of cells was investigated by staining with the fluorescent dyes propidium iodide (PI) and Hoechst 33342 as described previously [47]. PI-positive staining is only observed, if a given treatment causes permeabilization of the cell membrane, while all cell nuclei present are stained with the membrane permeable Hoechst 33342. The protein content of the cultures was measured by the Lowry method [50] using BSA as standard protein.
Determination of the Contents of GSx, GSSG and GS–B
The extracellular and cellular contents of GSx and GSSG were determined by a microtiter plate-based modification of the colorimetric Tietze method as described previously [47]. For the quantification of extracellular GSx or GSSG contents, 10 µL media samples were mixed with 10 µL 1% (w/v) sulfosalicylic acid. The cells were lysed with 200 µL 1% (w/v) sulfosalicylic acid and 10 µL of these lysates were used for the quantification of the cellular GSx and GSSG contents.
The amounts of cellular and extracellular GS–B were determined as described previously [37]. The treated cells were lysed in 500 µL lysis buffer (1% (w/v) Triton X-100 in 20 mM potassium phosphate buffer, pH 6.5). 200 µL of cell lysates or of the harvested incubation media were transfered into wells of a black 96-well microtiter plate and the GS–B fluorescence was recorded at 520 nm after excitation at 390 nm. GS–B was quantified by comparison of the fluorescence of samples with those of GS–B standards in incubation buffer or lysis buffer, which had been prepared as previously described in detail [37].
Visualization of Cellular GS–B by Fluorescence Microscopy
Astrocytes were incubated with 10 µM MCB in IB for 5 or 30 min before the cellular GS–B fluorescence was monitored with a Nicon Eclipse TS-100 fluorescence microscope using a preheated (37 °C) microscope stage and an excitation wavelength of 330–380 nm. The fluorescence emission was recorded with a long pass filter at wavelengths above 420 nm.
Presentation of Data
The quantitative data shown represent means ± standard deviation (SD) of values that were obtained in at least three experiments performed on independently prepared astrocyte cultures. Microscopic images are derived from a representative experiment that was reproduced twice on independently prepared cultures with almost identical results. The analysis of significance between multiple groups of data was performed by ANOVA followed by the Bonferroni post hoc test, while the t test was used for statistical comparison of two sets of data. p > 0.05 was considered as not significant.
Results
Test for Potential Adverse Effects of Dicoumarol on the Viability of Cultured Astrocytes
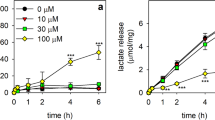
To investigate potential adverse consequences of a treatment of astrocytes with dicoumarol, cultured astrocytes were exposed to this compound in concentrations of up to 30 µM for up to 6 h or to 100 µM dicoumarol for 4 h. None of these incubation conditions caused any significant increase in the detectable activity of extracellular LDH compared to cells that had been incubated in the absence of dicoumarol but under otherwise identical conditions (Fig. 1a, b). This absence of any obvious toxicity of a dicoumarol treatment was confirmed by PI staining which revealed hardly any detectable PI-positive cells after exposure of the cultures to 30 µM or 100 µM dicoumarol for 6 h (Fig. 2d, f) compared to control cultures that had been incubated without dicoumarol (Fig. 2a, b). In contrast, many cells in astrocyte cultures that had been exposed to the toxic AgNO3 [51] were PI-positive (Fig. 2g, h).
Time- and concentration-dependent effects of dicoumarol on the viability and the GSx content of cultured astrocytes. The cells were incubated for up to 6 h (a, c, e, g) or for 4 h (b, d, f, h) with the indicated concentrations of dicoumarol. The extracellular LDH activity (a, b) as an indicator for a potential loss of cell vitality, the extracellular GSx content (c, d), the cellular GSx content (e, f) and the sum of the extracellular plus the cellular GSx contents (g, h) were determined. The initial cellular specific GSx content of the cultures was 37.3 ± 1.8 nmol/mg (a, c, e, g) and 40.4 ± 9.3 nmol/mg (b, d, f, g) and the cultures contained 120 ± 29 (a, c, e, g) and 141 ± 12 µg protein per well (b, d, f, g). The data shown represent means ± SD of values that had been obtained in experiments performed on three independently prepared astrocyte cultures. Statistical analysis of the significance of differences of data compared to those of the control incubation (absence of dicoumarol) was performed by ANOVA followed by the Bonferroni post hoc test. The levels of significance are indicated by *p < 0.05, **p < 0.01 and ***p < 0.001
Test for potential effects of dicoumarol on the membrane integrity of cultured primary astrocytes. The cells were incubated in the absence (a, b) or the presence of 30 µM (c, d) or 100 µM (e, f) dicoumarol for 6 h. As positive control for cell toxicity, cells were alternatively exposed to 100 µM silver nitrate (g, h). Cells with permeabilised cell membranes are indicated by a positive fluorescence signal for propidium iodide (b, d, f, h). The presence of all cell nuclei is indicated by staining of the cultures with the membrane permeable fluorescent dye Hoechst 33342 (a, c, e, g). The images shown are derived from a representative experiment performed on a 20 days-old culture. The results were reproduced twice on independently prepared cultures with similar results. The scale bar in b indicates 100 µm and applies to all panels
Effects of Dicoumarol on the Cellular and Extracellular GSx Contents of Astrocytes
During incubation of cultured astrocytes in the absence of dicoumarol, an almost linear increase in extracellular GSx content was observed (Fig. 1c) that was accompanied by a corresponding loss in the cellular GSx content (Fig. 1e). Accordingly, the sum of cellular plus extracellular GSx was not altered during the incubation under such control conditions (Fig. 1g). If dicoumarol was present in concentrations of 3 µM, 10 µM and 30 µM during the incubation, the extracellular GSx accumulation was significantly lowered by around 45%, 70% and 90%, respectively (Fig. 1c), while the cellular loss in GSx was reduced (Fig. 1e) and the sum of cellular plus extracellular GSx remained almost constant (Fig. 1g). A more detailed analysis of the concentration dependent effects of dicoumarol on the cellular and extracellular GSx contents after 4 h of incubation revealed that half-maximal inhibitory effects on the extracellular GSx accumulation and on the loss in cellular GSx were observed at dicoumarol concentrations of around 4 µM (Fig. 1d, f; Table 1), while 10 µM dicoumarol had to be present to obtain a maximal inhibitory effect (Fig. 1d, f).
To analyse whether exposure of astrocyte cultures to dicoumarol may cause any alteration in the ratio of GSH to GSSG, the cells were incubated for 4 h without or with 30 µM dicoumarol and the GSx and GSSG contents were determined for cell lysates and media samples. Compared to control cells that had been incubated in the absence of dicoumarol, only the specific extracellular GSx content was significantly lowered in dicoumarol-treated cells by around 65%, which was accompanied by a minor but significant increase in the cellular GSx content. In contrast, the cellular and extracellular contents of GSSG were not altered compared to control cells and remained very low in both cells and media of dicoumarol-treated cultures (Table 2).
These data demonstrate that exposure of cultured astrocytes to dicoumarol does not alter the high GSH to GSSG ratio and strongly suggest that dicoumarol inhibits the basal release of GSH from viable astrocytes.
Test for Potential Additive Inhibitory Effects of Dicoumarol and MK571 on the GSH Export from Cultured Astrocytes
GSH export from cultured astrocytes is predominantly mediated by Mrp1, which can be inhibited by MK571 [32, 33]. The inhibition of extracellular GSx accumulation by MK571 during a 4 h incubation was almost identical to that observed for 30 µM dicoumarol and a coincubation with both dicoumarol and MK571 did not further lower the extracellular GSx accumulation compared to a treatment with one of the inhibitors alone (Table 2). In addition, none of the substances caused a substantial increase in cellular or extracellular GSSG levels within 15 min (data not shown) or within 4 h (Table 2) nor was the cell viability compromised, as indicated by the absence of any significant increase in extracellular LDH activity compared to control cells (Table 2). These data suggest that dicoumarol inhibits the Mrp1-mediated basal release of GSH from viable astrocytes.
Test for Potential Persistence of the Inhibition of GSH Export After Removal of Dicoumarol
To investigate whether the presence of dicoumarol is required to maintain the observed inhibition of astrocytic GSH export, cultured astrocytes were pre-incubated in the absence or the presence of 30 µM dicoumarol for 2 h, washed and subsequently incubated for further 4 h in the absence or the presence of 30 µM dicoumarol. After the 2 h pre-incubation, the extracellular GSx values were higher for cultures that had been pre-incubated without dicoumarol compared to dicoumarol-treated cells (Fig. 3a). Exposure to dicoumarol of cells that had been pre-incubated without dicoumarol for the main incubation lowered significantly the extracellular GSx accumulation compared to that of astrocytes that had been incubated without dicoumarol during pre- and main incubation (Fig. 3a). In contrast, the extracellular GSx accumulation was found significantly increased for cultures that had been pre-incubated with dicoumarol for 2 h and further incubated in the absence of dicoumarol (Fig. 3a, b). Accordingly, elevated GSx contents were determined in cells after incubations with dicoumarol (Fig. 3c). Calculation of the rate of extracellular GSx accumulation for the main incubation revealed a high value of around 8.5 nmol GSx/(mg × h) in cultures that had not been exposed to dicoumarol during the pre- and main incubation (Fig. 3b). This rate was significantly lowered by around 60%, if dicoumarol had been present during the main incubation (Fig. 3b). In contrast, a low extracellular accumulation rate of around 2 nmol GSx/(mg × h) was observed for astrocytes that had been incubated with dicoumarol during pre- and main incubation, while compared to this value a doubling of the GSx accumulation rate was observed after removal of dicoumarol for the main incubation (Fig. 3b), demonstrating that the inhibition by dicoumarol of extracellular GSx accumulation is not irreversible but at least partially reversible. None of the conditions applied compromised the cell viability as no substantial LDH release was detectable for the experimental conditions used (Fig. 3d).
Test for the reversibility of the dicoumarol-mediated inhibition of extracellular GSx accumulation in cultured astrocytes. The cells were pre-incubated for 2 h in the absence (−; 0 µM) or in the presence (+) of 30 µM dicoumarol. After a washing step, the cells were further incubated for up to 4 h in the absence (−; 0 µM) or the presence (+) of 30 µM dicoumarol. After the pre-incubation and at the indicated time points of the main incubation media samples were collected for determination of the extracellular GSx content (a), while the specific cellular GSx content (c) and the extracellular LDH activity (d) as an indicator for a loss in cell viability were determined after the 4 h main incubation. The rates of extracellular GSx accumulation (b) were calculated for the almost linear increases in extracellular GSx contents between 3 and 6 h after start of the pre-incubation. The values given in panel a represent the sum of the extracellular GSx content determined after the pre-incubation plus the extracellular GSx amount that had been accumulated during the given main incubation. The initial cellular GSx content was 43.4 ± 1.3 nmol/mg protein and the cultures contained 130 ± 10 µg protein per well. The data represent means ± SD of values that have been obtained in experiments on three independently prepared astrocyte cultures. Statistically significant differences between the data obtained for incubations with or without dicoumarol (t test) are indicated by #p < 0.05, ##p < 0.01 and ###p < 0.001
Effects of Dicoumarol on the Menadione-Induced GSSG Export from Astrocytes
The data described so far strongly suggest that dicoumarol inhibits Mrp1-mediated GSH export from cultured astrocytes. As Mrp1 also mediates export of GSSG from astrocytes under oxidative stress conditions [33, 35], it was tested whether dicoumarol may also inhibit Mrp1-mediated GSSG export from astrocytes. During basal incubation conditions, cultured astrocytes do only contain minute amounts of GSSG and also the extracellular GSSG content was very low even after 4 h of incubation (Table 2). In order to study GSSG export from astrocytes, conditions had to be established which cause intracellular oxidation of GSH to GSSG. This was achieved for the current study by application of the electron cycler menadione, which induces oxidative stress in cells [52, 53].
Exposure of cultured astrocytes to 100 µM menadione caused a rapid initial loss in cellular GSx content within 5 min that was followed by a slower and almost time proportional further decrease in cellular GSx content (Fig. 4a). This slower decrease was matched by extracellular GSx accumulation (Fig. 4b). Analysis of the contribution of GSSG to the GSx values determined for cells and media revealed that after menadione application GSSG accounted almost exlusively for the GSx contents determined (Fig. 4a–d). The cellular viability was not compromised during the incubation of astrocytes with 100 µM menadione for up to 60 min (Fig. 4f), but the total amount of cellular plus extracellular GSx was lowered by up to 50% in menadione-treated cells during the 60 min incubation (Fig. 4e). This loss is likely to be caused either by a direct conjugation of menadione with GSH as previously described [54] and/or by indirect formation of GSH-protein mixed disulfides in cells that contain high concentrations of GSSG [8, 55].
Time-dependent inhibition of the menadione-induced GSSG export by dicoumarol and/or MK571. Cultured astrocytes were incubated for up to 60 min in the absence or the presence of 100 µM menadione without or with 30 µM dicoumarol and/or 50 µM MK571. The cellular (a) and extracellular (b) GSx contents, the cellular (c) and extracellular (d) GSSG contents, the sum of cellular plus extracellular GSx (e) contents and the extracellular LDH activity (f) as indicator for a potential loss of cell vitality were determined. The initial cellular GSx content of the cultures was 43.9 ± 2.4 nmol/mg, the initial cellular GSSG content was 2.0 ± 0.5 nmol GSx/mg and the cultures contained 101 ± 13 µg protein/well. The data represent means ± SD of values that had been obtained in experiments on three independently prepared astrocyte cultures. Statistical analysis for the significance of differences of data compared to those of the control incubation (with menadione in the absence of inhibitors) was performed by ANOVA followed by the Bonferroni post hoc test. The level of significance is indicated by *p < 0.05, **p < 0.01 and ***p < 0.001. Men menadione, DC dicoumarol, MK MK571
The menadione-induced stress paradigm was used to test for a potential effect of dicoumarol on the Mrp1-mediated GSSG export from astrocytes. In menadione-treated astrocytes, both dicoumarol and MK571 alone or in combination strongly inhibited the cellular loss in GSx and GSSG (Fig. 4a, c) and the extracellular accumulation of GSx and GSSG (Fig. 4b, d). In contrast, presence of the inhibitors did not substantially affect the total amounts of GSx in cells plus media (Fig. 4e) nor was the cell viability compromised by any of the treatments (Fig. 4f).
A more detailed analysis of the concentration-dependent effects of dicoumarol on the cellular and extracellular GSSG contents of astrocytes after menadione-treatment for 30 min revealed, that half-maximal inhibition of the extracellular GSSG accumulation (Fig. 5c) was observed at dicoumarol concentrations of around 4 µM (Table 1), while around 30 µM dicoumarol had to be present to obtain maximal inhibition of GSSG export from menadione-treated astrocytes (Fig. 5).
Concentration-dependent inhibition of the menadione-induced GSSG export by dicoumarol. Cultured astrocytes were incubated for 30 min in the presence of 100 µM menadione without or with dicoumarol in the indicated concentrations. The extracellular (a) and cellular (b) GSx contents, the extracellular (c) and the cellular (d) GSSG contents, the sum of extracellular plus cellular GSx contents (e) and the extracellular LDH activity (f) as indicator for a potential loss of cell vitality were determined. The initial cellular GSx content of the cultures was 39.2 ± 5.6 nmol/mg, the initial cellular GSSG content was 1.8 ± 0.7 nmol GSx/mg and the cultures contained 119 ± 29 µg protein/well. The data represent means ± SD of values that had been obtained in experiments on three independently prepared astrocyte cultures. Statistical analysis for the significance of differences of data compared to those obtained for the control (with menadione in the absence of dicoumarol) was performed by ANOVA followed by the Bonferroni post hoc test and the level of significance is indicated by **p < 0.01 and ***p < 0.001
Effects of Dicoumarol on the Export of the GSH-Bimane Conjugate from Astrocytes
Mrp1 has been reported to mediate the export of the GS–B conjugate that is formed in astrocytes after application of MCB [37]. GS–B formation in MCB-treated astrocytes and its subsequent export was confirmed by fluorescence microscopy. After a 5 min incubation of astrocytes with 10 µM MCB, strong cellular GS–B fluorescence was observed (Fig. 6b) compared to control cells that had been incubated in the absence of MCB (Fig. 6a), but this cellular fluorescence intensity was almost completely diminished after further 25 min of incubation (Fig. 6f). In contrast, the strong cellular GS–B fluorescence signal detected after 5 min of incubation in MCB-treated astrocytes that had been co-incubated with dicourmarol and/or MK571 (Fig. 6c–e) was maintained during a subsequent 25 min incubation (Fig. 6g–i).
Time-dependent inhibition of GS–B export by dicoumarol and/or MK571. Astrocyte cultures were incubated with 10 µM MCB for 0 min (a) or for 5 min (b–e), 30 min (f–i) or the indicated time periods (j–o) in the absence (a, b, f, j–o) or the presence of 30 µM dicoumarol (c, g, j–o), 50 µM MK571 (d, h, j–o) or 30 µM dicoumarol plus 50 µM MK571 (e, i, j–o), before fluorescence microscopic pictures were taken (a–i) or the cellular and extracellular amounts of GSx and GS–B were determined (j–o). The size bar in panel a represents 50 µm and applies to the panels a–i, which show fluorescence images from a representative experiment, while the data shown in panels j–o are means ± SD of values that had been obtained in experiments on three independently prepared astrocyte cultures. The initial cellular GSx content of the cultures was 44.6 ± 7.4 nmol/mg and the cultures contained 145 ± 13 µg protein/well. Statistical analysis for the significance of differences of the quantitative data compared to those of control incubations (absence of inhibitors) was performed by ANOVA followed by the Bonferroni post hoc test. The level of significance is indicated by *p < 0.05, **p < 0.01 and ***p < 0.001
To confirm the fluorescence microscopical results, the cellular and extracellular contents of GSx and GS–B were quantified for cultured astrocytes that had been exposed to MCB and/or the Mrp1 inhibitors. Application of MCB to astrocytes caused a rapid disappearance of cellular GSx (Fig. 6j) that was accompanied by a rapid but transient increase in cellular GS–B fluorescence (Fig. 6m). During the incubation with MCB, extracellular GSx accumulation was not observed (Fig. 6k) but substantial amounts of extracellular GS–B were determined (Fig. 6n). None of the conditions applied lead to any obvious loss in cell viability as no increase in extracellular LDH was observed (data not shown). The loss in the sum of cellular plus extracellular GSx (around 30 nmol/mg) during the incubation with MCB (Fig. 6l) was accompanied by a matching increase in the respective GS–B values (Fig. 6o), leading to an almost constant sum of cellular plus extracellular amounts of GSx plus GS–B, which was close to the initital cellular GSx content of untreated cells (44.6 ± 7.4 nmol/mg).
Dicoumarol and MK571 alone or in combination did not affect the initial loss in the cellular GSx content after application of MCB to astrocytes (Fig. 6j), but efficiently slowed down the loss in cellular GS–B fluorescence (Fig. 6m) and the extracellular accumulation of GS–B (Fig. 6n). The potential of 30 µM dicoumarol or 50 µM MK571 to prevent extracellular GS–B accumulation was lower than that observed for the combination of both compounds (Fig. 6n), while the sum of cellular and extracellular amounts of GSx (Fig. 6l) or GS–B (Fig. 6o) was found almost identical for all conditions applied (Fig. 6j).
To explore the concentration-dependent inhibitory effect of dicoumarol on the export of GS–B from MCB-treated astrocytes, the cells were incubated for 30 min with dicoumarol in concentrations of up to 100 µM. After this incubation, extracellular GSx was not observed (Fig. 7a) and the GSx content of the MCB-treated cultures was not significantly different in cultures that had been incubated without or with up to 100 µM dicoumarol (Fig. 7c,e). In contrast, the strong accumulation of extracellular GS–B in MCB-treated astrocytes in the absence of dicoumarol (around 20 nmol/mg) was inhibited by dicoumarol in a concentration-dependent manner (Fig. 7b) while the cellular GS–B content was found to be increased (Fig. 7d). None of the conditions applied compromised the cell viability as demonstrated by the absence of any increase in the extracellular LDH activity (data not shown). Half-maximal inhibition of the extracellular accumulation of GS–B was observed for a dicoumarol concentration of around 30 µM (Table 1), while around 100 µM dicoumarol was required to almost completely inhibit the extracellular accumulation of GS–B and the cellular loss of GS–B in MCB-treated astrocytes (Fig. 7b, d).
Concentration-dependent inhibition by dicoumarol of the GS–B export from astrocytes. The cells were incubated for 30 min with 10 µM MCB in the absence or the presence of the indicated concentrations of dicoumarol before the extracellular and cellular amounts of GSx (a, c) and GS–B (b, d) were determined and the sum of cellular plus extracellular GSx (e) or GS–B (f) contents was calculated. The initial cellular GSx content of the cultures was 43.4 ± 3.4 nmol/mg and the cultures contained 125 ± 18 µg protein/well. The data represent means ± SD of values that had been obtained in experiments on three independently prepared astrocyte cultures. Statistical analysis for significance of differences of the data compared to those of control incubations (absence of dicoumarol) was performed by ANOVA followed by the Bonferroni post hoc test. The level of significance is indicated by **p < 0.01 and ***p < 0.001
Discussion
Dicoumarol is a well-known inhibitor of the protective enzyme NQO1 and has frequently been used to investigate a potential involvement of NQO1 in the protection of cells against various compounds and oxidants [42, 45, 46, 56]. Here we report for the first time that dicoumarol also inhibits efficiently already in low micromolar concentrations the Mrp1-mediated cellular export of GSH, GSSG and GSH conjugates.
Exposure of cultured astrocytes to dicoumarol in concentrations of up to 100 µM did not cause any obvious alteration of cell morphology nor any increase in extracellular LDH activity or increased PI staining, confirming the low toxic potential of this compound previously reported for cultured neural cells [42, 57]. Concerning the cellular GSH metabolism, dicoumarol does not directly interact with GSH as no disappearance of GSH was observed in the presence of dicoumarol in cell-free experiments (data not shown) and during cell incubations, nor does dicoumarol cause oxidative stress in cells, which would lead to an increase in the low ratio of cellular GSSG to GSH. This contrasts the situations observed for treatments of astrocytes with compounds inducing oxidative stress [35, 58] or conjugating to GSH [12, 59, 60]. Dicoumarol did also not stimulate the export of GSH as shown for various structurally very diverse compounds including formaldehyde [61], arsenicals [62, 63] or antiretroviral protease inhibitors [64, 65]. In contrast, dicoumarol caused an unexpected time- and concentration-dependent inhibition of the Mrp1-mediated export of GSH, GSSG and GS–B from astrocytes.
Unstressed astrocytes export GSH efficiently with a Km-value of around 25 mM [66] in a process that is predominantly mediated by Mrp1 [32, 33]. As shown by comparison with the frequently used Mrp1 inhibitor MK571 [32, 33], also dicoumarol lowered extracellular GSH accumulation in a concentration-dependent and at least partially reversible manner. However, in contrast to MK571, which shows a biphasic modulation of astrocytic GSH export with a stimulation of GSH export at low micromolar concentrations of around 3 µM and an inhibition of GSH export at a high MK571 concentration of 50 µM [32, 33], no stimulation of GSH export was observed for astrocytes that had been exposed to low dicoumarol concentrations. For dicoumarol a classical concentration-dependent inhibition of astrocytic GSH export was observed with a half-maximal inhibition determined for 4 µM dicoumarol. Thus, dicoumarol appears to be a more potent inhibitor of astrocytic Mrp1-mediated GSH export than MK571 as it inhibits this export already efficiently at very low micromolar concentrations, while such low concentrations of MK571 stimulate GSH export from astrocytes [32, 33].
GSSG export in oxidatively stressed astrocytes is exclusively mediated by Mrp1 [33, 35]. As under unstressed conditions hardly any GSSG is detectable in cultured astrocytes [67], the cells have to be exposed to conditions which strongly induce cellular GSH oxidation to GSSG before GSSG export can be studied. For the current study, oxidative stress was induced in astrocytes by the application of the naphthoquinone menadione, which is known to cause severe oxidative stress in cultured astrocytes [52] and other cells [53]. As the cell membrane integrity was not impaired by the menadione treatment and as MK571 almost completely inhibited the export of GSSG from menadione-treated cells, it can be concluded that the extracellular GSSG determined under such conditions had been exported from viable cells via Mrp1, consistent with literature data on the GSSG export from astrocytes during oxidative stress [33, 35, 68]. The observed menadione-induced GSSG export from astrocytes was strongly inhibited in the presence of dicoumarol in a time- and concentration-dependent manner with half-maximal inhibition observed for around 4 µM dicoumarol. Thus, also concerning inhibition of GSSG export, dicoumarol appears to be even more potent than MK571, which has to be applied in a concentration of 50 µM to efficiently inhibit GSSG export form astrocytes during oxidative stress [33, 35, 67]. The rapid accumulation of GSSG in menadione-treated astrocytes was not affected by the presence of dicoumarol, nor was the rapid formation of reactive oxygen species that we observed for such conditions prevented by dicoumarol (data not shown). These data suggest that the enzyme NQO1, which is efficiently inhibited by dicoumarol [4, 6], is not involved in the generation of reactive oxygen species and oxidative stress in menadione-treated astrocytes.
In addition to GSH and GSSG, Mrp1 is also mediating the export of the GSH conjugate GS–B from cultured astrocytes [37]. To test for potential inhibition by dicoumarol of Mrp1-mediated GS–B export, astrocytes were incubated with 10 µM MCB to generate substantial amounts of cellular GS–B. The rapid loss of cellular GS–B during the incubation of viable astrocytes in the absence of an Mrp1 inhibitor was prevented by MK571, as previously described [37], but also by dicoumarol in a concentration-dependent manner with half-maximal inhibition found at a concentration of 30 µM.
Thus, as previously reported for MK571 [32, 33, 35] also dicoumarol was found to inhibit the export of the three Mrp1 substrates GSH, GSSG and GS–B from cultured astrocytes. The substantial differences in the concentrations of dicoumarol required to inhibit the export of GSH, GSSG and GS–B by 50% are consistent with the different transport efficiencies of Mrp1 for its three substrates, as demonstrated by the reported Km-values of Mrp1 (25 mM for GSH [66], 93 µM for GSSG [39] and 35 nM for GS–B [69]). Thus, assuming that dicoumarol binds to Mrp1 at the substrate binding site with a given affinity, efficient inhibition of a high affinity Mrp1 substrate such as GS–B will require the presence of higher concentrations of dicoumarol compared to an inhibition of the export of a substrate with a higher Km value such as GSSG or GSH.
Dicoumarol has frequently been applied in cell culture studies to investigate protective functions of NQO1 against toxins and oxidants and an accelerated damage in presence of dicoumarol has been interpreted as evidence for a protective function of NQO1 in the investigated stress paradigm [41, 45, 70, 71]. However, as our new data demonstrate that already low micromolar concentrations of dicoumarol efficiently inhibit Mrp1-mediated export processes, a potential contribution of an inhibition of Mrp1 by dicoumarol in the effects reported has to be considered. For example, the inhibition of the Mrp1-mediated export of cellular GSSG under oxidative stress conditions will lead to a drastic oxidation in the cellular thiol-reduction potential, which will in turn render the cells more vulnerable towards oxidative stress. Furthermore, since dicoumarol not only inhibits the Mrp1-mediated export of GSH and GSSG, but also of the GSH conjugate GS–B, the export of other GSH conjugates and/or potentially toxic compounds that are substrates of Mrp1, such as 4-hydroxynonenal [72], are likely to be also inhibited by dicoumarol, thereby accelerating cytotoxicity.
In conclusion, our study demonstrates for the first time that Mrps have to be considered as cellular target of dicoumarol and that dicoumarol inhibits the Mrp1-mediated export of GSH, GSSG and GS–B at least in cultured astrocytes. This inhibitory potential of dicoumarol should be considered for future experiments and for the interpretation of literature data of studies investigating the protective role of NQO1 in cellular defence processes. In addition, as dicoumarol inhibits Mrp1-mediated export processes already in low micromolar concentrations, it appears to be a good and cheap alternative to the frequently used Mrp1 inhibitor MK571 for experiments studying Mrp1-mediated export mechanisms, at least for such studies that are not influenced by the strong inhibitory potential of dicoumarol towards NQO1.
References
Hroboňová K, Machyňáková A, Čižmárik J (2018) Determination of dicoumarol in Melilotus officinalis L. by using molecularly imprinted polymer solid-phase extraction coupled with high performance liquid chromatography. J Chromatogr 1539:93–102
Last JA (2002) The missing link: the story of Karl Paul Link. Toxicol Sci 66:4–6
Poulton JE, McRee DE, Conn EE (1980) Intracellular localization of two enzymes involved in coumarin biosynthesis in Melilotus alba. Plant Physiol 65:171–175
Timson D (2017) Dicoumarol: a drug which hits at least two very different targets in vitamin K metabolism. Curr Drug Targets 18:500–510
Stafford DW (2005) The vitamin K cycle. J Thromb Haemost 3:1873–1878
Aras D, Cinar O, Cakar Z, Ozkavukcu S, Can A (2016) Can dicoumarol be used as a gonad-safe anticancer agent: an in vitro and in vivo experimental study. Mol Hum Reprod 22:57–67
Hong J, Zhang P, Yoon IN, Kim H (2016) Inhibition of NAD(P)H:quinone oxidoreductase 1 by dicumarol reduces tight junction in human colonic epithelial cells. J Life Sci 26:531–536
Deponte M (2017) The incomplete glutathione puzzle: just guessing at numbers and figures? Antioxid Redox Signal 27:1130–1161
Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649–671
Dringen R, Brandmann M, Hohnholt MC, Blumrich E-M (2015) Glutathione-dependent detoxification processes in astrocytes. Neurochem Res 40:2570–2582
Hirrlinger J, Dringen R (2010) The cytosolic redox state of astrocytes: maintenance, regulation and functional implications for metabolite trafficking. Brain Res Rev 63:177–188
Schmidt M, Dringen R (2012) Glutathione (GSH) synthesis and metabolism. In: Choi I-Y, Gruetter R (eds) Neural metabolism in vivo. Springer, New York, pp 1029–1050
Flohé L (2016) The impact of thiol peroxidases on redox regulation. Free Radic Res 50:126–142
Jiao Y, Wang Y, Guo S, Wang G (2017) Glutathione peroxidases as oncotargets. Oncotarget 8:80093
Couto N, Wood J, Barber J (2016) The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Biol Med 95:27–42
Mazzetti AP, Fiorile MC, Primavera A, Bello ML (2015) Glutathione transferases and neurodegenerative diseases. Neurochem Int 82:10–18
Tew KD, Townsend DM (2012) Glutathione-S-transferases as determinants of cell survival and death. Antioxid Redox Signal 17:1728–1737
Deeley RG, Westlake C, Cole SP (2006) Transmembrane transport of endo-and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 86:849–899
Keppler D (2011) Multidrug resistance proteins (MRPs, ABCCs): importance for pathophysiology and drug therapy. In: Fromm MF, Kim RB (eds) Drug transporters. Springer, New York, pp 299–323
Cole S (2014) Targeting multidrug resistance protein 1 (MRP1, ABCC1): past, present, and future. Annu Rev Pharmacol Toxicol 54:95–117
Cole SP (2014) Multidrug resistance protein 1 (MRP1, ABCC1): A ‘multitasking’ ABC transporter. J Biol Chem 289:30880–30888
Allen NJ, Barres BA (2009) Neuroscience: glia more than just brain glue. Nature 457:675–677
Argente-Arizon P, Guerra-Cantera S, Garcia-Segura LM, Argente J, Chowen JA (2017) Glial cells and energy balance. J Mol Endocrinol 58:R59–R71
Parpura V, Heneka MT, Montana V, Oliet SHR, Schousboe A, Haydon PG, Stout RF, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A (2012) Glial cells in (patho)physiology. J Neurochem 121:4–27
Schmidt MM, Dringen R (2012) Glutathione (GSH) synthesis and metabolism. In: Choi I-Y, Gruetter R (eds) Neural metabolism in vivo. Springer, Boston, pp 1029–1050
McBean GJ (2017) Cysteine, glutathione, and thiol redox balance in astrocytes. Antioxidants 6:62
Hirrlinger J, König J, Dringen R (2002) Expression of mRNAs of multidrug resistance proteins (Mrps) in cultured rat astrocytes, oligodendrocytes, microglial cells and neurones. J Neurochem 82:716–719
Ballerini P, Di Iorio P, Ciccarelli R, Nargi E, D’alimonte I, Traversa U, Rathbone MP, Caciagli F (2002) Glial cells express multiple ATP binding cassette proteins which are involved in ATP release. Neuroreport 13:1789–1792
Dallas S, Miller DS, Bendayan R (2006) Multidrug resistance-associated proteins: expression and function in the central nervous system. Pharmacol Rev 58:140–161
Hirrlinger J, Moeller H, Kirchhoff F, Dringen R (2005) Expression of multidrug resistance proteins (Mrps) in astrocytes of the mouse brain: a single cell RT-PCR study. Neurochem Res 30:1237–1244
Nies A, Jedlitschky G, König J, Herold-Mende C, Steiner H, Schmitt H-P, Keppler D (2004) Expression and immunolocalization of the multidrug resistance proteins, MRP1–MRP6 (ABCC1–ABCC6), in human brain. Neuroscience 129:349–360
Hirrlinger J, Schulz JB, Dringen R (2002) Glutathione release from cultured brain cells: multidrug resistance protein 1 mediates the release of GSH from rat astroglial cells. J Neurosci Res 69:318–326
Minich T, Riemer J, Schulz JB, Wielinga P, Wijnholds J, Dringen R (2006) The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J Neurochem 97:373–384
Dringen R, Pfeiffer B, Hamprecht B (1999) Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci 19:562–569
Hirrlinger J, König J, Keppler D, Lindenau J, Schulz JB, Dringen R (2001) The multidrug resistance protein MRP1 mediates the release of glutathione disulfide from rat astrocytes during oxidative stress. J Neurochem 76:627–636
Akerboom TP, Sies H (1989) Transport of glutathione, glutathione disulfide, and glutathione conjugates across the hepatocyte plasma membrane. Methods Enzymol 173:523–534
Waak J, Dringen R (2006) Formation and rapid export of the monochlorobimane–glutathione conjugate in cultured rat astrocytes. Neurochem Res 31:1409–1416
Jedlitschky G, Keppler D (2002) Transport of leukotriene C4 and structurally related conjugates. Vitam Horm 64:153–184
Leier I, Jedlitschky G, Buchholz U, Center M, Susan P, Deeley RG, Keppler D (1996) ATP-dependent glutathione disulphide transport mediated by the MRP gene-encoded conjugate export pump. Biochem J 314:433–437
Beyer RE, Segura-Aguilar J, Di Bernardo S, Cavazzoni M, Fato R, Fiorentini D, Galli MC, Setti M, Landi L, Lenaz G (1996) The role of DT-diaphorase in the maintenance of the reduced antioxidant form of coenzyme Q in membrane systems. Proc Natl Acad Sci USA 93:2528–2532
Cullen JJ, Hinkhouse MM, Grady M, Gaut AW, Liu J, Zhang YP, Weydert CJ, Domann FE, Oberley LW (2003) Dicumarol inhibition of NADPH:quinone oxidoreductase induces growth inhibition of pancreatic cancer via a superoxide-mediated mechanism. Cancer Res 63:5513–5520
Huenchuguala S, Muñoz P, Graumann R, Paris I, Segura-Aguilar J (2016) DT-diaphorase protects astrocytes from aminochrome-induced toxicity. Neurotoxicology 55:10–12
Karczewski JM, Peters JG, Noordhoek J (1999) Quinone toxicity in DT-diaphorase-efficient and-deficient colon carcinoma cell lines. Biochem Pharmacol 57:27–37
Ruiz-Larrea MB, Garrido MJ, Lacort M (1993) Estradiol-induced effects on glutathione metabolism in rat hepatocytes. J Biochem 113:563–567
Drukarch B, Jongenelen CA, van Muiswinkel FL (2001) NAD(P)H:quinone oxidoreductase (NQO1) protects astroglial cells against L-Dopa toxicity. In: Dansette PM, Snyder R, Delaforge M, Gibson GG, Greim H, Jollow DJ, Monks TJ, Sipes IG (eds) Biological reactive intermediates VI. Springer, Boston, pp 237–240
Kapinya KJ, Harms U, Harms C, Blei K, Katchanov J, Dirnagl U, Hörtnagl H (2003) Role of NAD(P)H:quinone oxidoreductase in the progression of neuronal cell death in vitro and following cerebral ischaemia in vivo. J Neurochem 84:1028–1039
Tulpule K, Hohnholt M, Hirrlinger J, Dringen R (2014) Primary cultures of astrocytes and neurons as model systems to study the metabolism and metabolite export from brain cells. In: Hirrlinger J, Waagepetersen HS (eds) Neuromethods: brain energy metabolism. Springer, New York, pp 45–72
Dringen R, Kranich O, Hamprecht B (1997) The γ-glutamyl transpeptidase inhibitor acivicin preserves glutathione released by astroglial cells in culture. Neurochem Res 22:727–733
Dringen R, Kussmaul L, Hamprecht B (1998) Detoxification of exogenous hydrogen peroxide and organic hydroperoxides by cultured astroglial cells assessed by microtiter plate assay. Brain Res Protoc 2:223–228
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Luther EM, Koehler Y, Diendorf J, Epple M, Dringen R (2011) Accumulation of silver nanoparticles by cultured primary brain astrocytes. Nanotechnology 22:375101
Abe K, Saito H (1996) Menadione toxicity in cultured rat cortical astrocytes. Jpn J Pharmacol 72:299–306
Klotz L-O, Hou X, Jacob C (2014) 1, 4-Naphthoquinones: from oxidative damage to cellular and inter-cellular signaling. Molecules 19:14902–14918
Ross D, Thor H, Orrenius S, Moldeus P (1985) Interaction of menadione (2-methyl-1, 4-naphthoquinone) with glutathione. Chem-Biol Interact 55:177–184
Vogel R, Wiesinger H, Hamprecht B, Dringen R (1999) The regeneration of reduced glutathione in rat forebrain mitochondria identifies metabolic pathways providing the NADPH required. Neurosci Lett 275:97–100
Dinkova-Kostova AT, Talalay P (2010) NAD (P) H: quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 501:116–123
Li J, Lin JC, Wang H, Peterson JW, Furie BC, Furie B, Booth SL, Volpe JJ, Rosenberg PA (2003) Novel role of vitamin k in preventing oxidative injury to developing oligodendrocytes and neurons. J Neurosci 23:5816–5826
Dringen R, Hamprecht B (1997) Involvement of glutathione peroxidase and catalase in the disposal of exogenous hydrogen peroxide by cultured astroglial cells. Brain Res 759:67–75
Ehrke E, Arend C, Dringen R (2015) 3-Bromopyruvate inhibits glycolysis, depletes cellular glutathione, and compromises the viability of cultured primary rat astrocytes. J Neurosci Res 93:1138–1146
Schmidt MM, Dringen R (2010) Fumaric acid diesters deprive cultured primary astrocytes rapidly of glutathione. Neurochem Int 57:460–467
Tulpule K, Dringen R (2011) Formaldehyde stimulates Mrp1-mediated glutathione deprivation of cultured astrocytes. J Neurochem 116:626–635
Meyer N, Koehler Y, Tulpule K, Dringen R (2013) Arsenate accumulation and arsenate-induced glutathione export in astrocyte-rich primary cultures. Neurochem Int 62:1012–1019
Tadepalle N, Koehler Y, Brandmann M, Meyer N, Dringen R (2014) Arsenite stimulates glutathione export and glycolytic flux in viable primary rat brain astrocytes. Neurochem Int 76:1–11
Arend C, Brandmann M, Dringen R (2013) The antiretroviral protease inhibitor ritonavir accelerates glutathione export from cultured primary astrocytes. Neurochem Res 38:732–741
Brandmann M, Tulpule K, Schmidt MM, Dringen R (2012) The antiretroviral protease inhibitors indinavir and nelfinavir stimulate Mrp1-mediated GSH export from cultured brain astrocytes. J Neurochem 120:78–92
Tulpule K, Schmidt MM, Boecker K, Goldbaum O, Richter-Landsberg C, Dringen R (2012) Formaldehyde induces rapid glutathione export from viable oligodendroglial OLN-93 cells. Neurochem Int 61:1302–1313
Hirrlinger J, Dringen R (2005) Multidrug resistance protein 1-mediated export of glutathione and glutathione disulfide from brain astrocytes. Methods Enzymol 400:395–409
Hirrlinger J, Schulz JB, Dringen R (2002) Effects of dopamine on the glutathione metabolism of cultured astroglial cells: implications for Parkinson’s disease. J Neurochem 82:458–467
Homma M, Suzuki H, Kusuhara H, Naito M, Tsuruo T, Sugiyama Y (1999) High-affinity efflux transport system for glutathione conjugates on the luminal membrane of a mouse brain capillary endothelial cell line (MBEC4). J Pharmacol Exp Ther 288:198–203
Kishi T, Takahashi T, Mizobuchi S, Mori K, Okamoto T (2002) Effect of dicumarol, a NAD (P) H: quinone acceptor oxidoreductase 1 (DT-diaphorase) inhibitor on ubiquinone redox cycling in cultured rat hepatocytes. Free Radic Res 36:413–419
Nemeikait A, Šarlauskas J, Anusevičius Ž, Nivinskas H, Narimantas Č (2003) Cytotoxicity of RH1 and related aziridinylbenzoquinones: involvement of activation by NAD (P) H: quinone oxidoreductase (NQO1) and oxidative stress. Arch Biochem Biophys 416:110–118
Renes J, de Vries EE, Hooiveld GJ, Krikken I, Jansen PL, Müller M (2000) Multidrug resistance protein MRP1 protects against the toxicity of the major lipid peroxidation product 4-hydroxynonenal. Biochem J 350:555–561
Acknowledgements
Christian Arend and Ralf Dringen would like to acknowledge the substantial financial support of the Tönjes-Vagt-Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Raabe, J., Arend, C., Steinmeier, J. et al. Dicoumarol Inhibits Multidrug Resistance Protein 1-Mediated Export Processes in Cultured Primary Rat Astrocytes. Neurochem Res 44, 333–346 (2019). https://doi.org/10.1007/s11064-018-2680-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2680-y