Abstract
Oleuropein, a well-known olive polyphenol, has been shown to mediate neuroprotection in Alzheimer’s disease and cerebral ischemia. We investigated the effects of oleuropein on pentylenetetrazole (PTZ)-induced seizures in male NMRI mice, with diazepam as the standard drug. We also examined the possible involvement of opioidergic/nitrergic pathways in the probable effects of oleuropein. Intraperitoneal (i.p.) administration of different doses of oleuropein (10, 20 and 30 mg/kg) significantly increased the seizure threshold 60 min prior to induction of seizure, in a dose-dependent manner. Administration of naltrexone (10 mg/kg, i.p.), an opioid receptor antagonist, completely reversed the anticonvulsant effects of oleuropein (10 mg/kg). On the other hand, the anticonvulsant effect of oleuropein (10 mg/kg) was blocked by a non-effective dose of nonspecific inhibitor of nitric oxide synthase (NOS), L-NAME (1 and 10 mg/kg, i.p) and a selective inhibitor of neuronal NOS, 7-nitroindazole (30 mg/kg, i.p.). However, the nitric oxide precursor, l-arginine (30 and 60 mg/kg, i.p.) potentiated the anticonvulsant activity of oleuropein (10 mg/kg). A selective inducible NOS inhibitor, aminoguanidine (100 mg/kg, i.p.) did not change the anticonvulsant activity of oleuropein. It seems that the opioidergic system and constitutive neuronal NOS may be involved in the anticonvulsant properties of oleuropein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oleuropein is the major bioactive component of Olea europaea, which is widely known as the olive tree, and is present in high amounts in unprocessed olive fruit and leaves. Oleuropein have been shown to possess multiple neuroprotective effects such as brain hypoxia–reoxygenation [1, 2], audiogenic seizures related to a high-fat diet [3], hippocampal neuronal damage [4], cerebral ischemia [5, 6], brain damage after hypoxia–reoxygenation in diabetic rats [7], and aging [8] in experimental models. Despite these reported neuroprotective effects of oleuropein, the effectiveness of oleuropein in pentylenetetrazole (PTZ)-induced seizures remains obscure.
It has been shown that tolerance to the antinociceptive effect of morphine was inhibited by oleuropein [9]. It has also been found that oleuropein could prevent withdrawal signs in rats dependent on morphine [10]. It is well known that opioids have both anticonvulsant and proconvulsant effects on seizure threshold [11, 12]. On the other hand, our previous studies have shown that nitric oxide (NO) is linked to opioidergic modulation of seizure threshold [13, 14]. Furthermore, oleuropein has been reported to have both the ability to scavenge NO and increase the expression of nitric oxide synthase (NOS) enzymes in mouse peritoneal macrophages [15,16,17,18].Therefore, the aim of this study was to determine whether oleuropein may alter seizure threshold through opioidergic and nitrergic pathways on PTZ-induced seizures in mice (Scheme 1).
Materials and methods
Animals
In this study, male NMRI mice weighing 23–30 g were used for acute administration [19]. The animals were housed in a temperature-controlled room (24 ± 1 °C) in standard polycarbonate cages with four or five mice in each cage on a 12-h light/dark cycle with free access to food and water. All behavioral experiments were performed between 9:00 am and 2:00 pm. All experiments and manipulations were carried out in accordance with the Institutional Animal Care and Use Committee (Department of Pharmacology, School of Medicine, Tehran University of Medical Sciences) guidelines. The animals were randomly assigned to different groups, plus each mouse was used only once and each treatment group consisted of 8–12 animals. Additionally, every effort was made to reduce animal suffering and to use only the number of animals necessary to produce reliable scientific data.
Chemicals
Oleuropein, PTZ, L-NAME [L-NG-nitro-l-arginine methyl ester hydrochloride], l-arginine, 7-nitroindazole (7-NI), aminoguanidine (AG) and naltrexone (NTX) were purchased from Sigma (St. Louis, MO, USA). All drugs except 7-NI were dissolved in sterile isotonic saline solution to a concentration at which the required doses were administered intraperitoneally (i.p.) in a volume of 10 ml/kg. PTZ was administered intravenously. 7-NI was suspended in 1% solution of Tween 80. The solutions were prepared immediately before each experiment. Appropriate vehicle controls were prepared for each experiment.
Seizure paradigms
A 30-gauge butterfly needle was inserted into the tail vein while the mice were restrained in a mouse restrainer. The needle was then secured to the tail with a narrow piece of adhesive tape, with the mouse moving freely. PTZ (0.5%) was infused at a constant rate of 1 ml/min [12, 13] using an infusion pump (Harvard, USA). Infusion was halted when forelimb clonus followed by full clonus of the body was observed. The minimal dose of PTZ (mg/kg mouse weight) needed to induce clonic seizures was used as an index of seizure threshold [13, 20].
Experiments
In experiment 1, to determine the optimum time needed to develop the effects of oleuropein acid on PTZ-induced seizure threshold, animals received an acute i.p. injection of saline or oleuropein (20 mg/kg) at 30, 60 and 90 min prior to determination of PTZ-induced clonic seizure threshold. This dose was chosen based on published data [9, 21].
In experiment 2, mice in separate groups received a single injection of different doses of oleuropein [0 (saline), 1, 5, 10, 20 and 30 mg/kg, i.p.] at 60 min before evaluation of the PTZ-induced seizure threshold. In this step, the potent anticonvulsant dose of oleuropein was assessed for further experiments.
In experiment 3, mice in separate groups received a single injection of an opioid receptor antagonist, NTX [0 (saline), 10 mg/kg, i.p.] alone and at 30 min before oleuropein (10 mg/kg, i.p.). The PTZ-induced seizure threshold was assessed 60 min after the injection of oleuropein.
In experiments 4 and 5, animals in separate groups received an injection of nonspecific NO synthase [NOS] inhibitor, L-NAME [0 (saline), 1 and 10 mg/kg, i.p.] as well as the precursor of NO, l-arginine [0 (saline), 30 and 60 mg/kg, i.p.] alone and at 15 min before the effective dose of oleuropein (10 mg/kg, i.p.). In addition, 7-NI as a selective neuronal NOS inhibitor at a dose of 30 mg/kg, and AG as a selective inducible NOS inhibitor at a dose of 100 mg/kg were administered 15 min before the effective dose of oleuropein (10 mg/kg, i.p.) or saline in experiment 6. PTZ-induced seizure threshold was assessed 60 min after the injection of oleuropein acid.
Diazepam (0.05 mg/kg, i.p.) was injected 30 min before seizure threshold measurement, as a positive control. The doses of L-NAME, l-arginine, NTX [13, 22], 7-NI and AG [23, 35] were chosen based on previously published studies.
Measurement of NO metabolites
NO end product (nitrite) was measured using the Griess reaction [23, 24]. Mice were killed by cervical dislocation, and the blood was drawn from the carotid arteries and the jugular veins at cervical dislocation. Blood was centrifuged to separate plasma from red blood cells, and the supernatants were stored at −20 °C for 1–3 h until assay.
The samples were mixed with the same volume of Griess reagent to form a purple azo dye in a reaction pathway. The Griess reagent (1.25% HCl containing 5 g/l sulfanilamide with 0.25 g/l N-naphtyl ethylenediamine) was added at a rate of 0.1 ml/min. The absorbance of the dye product was measured at 540 nm with regard to a standard nitrite curve generated using NaNO2 by ELISA reader. The results were expressed as nM/ml.
Statistical analysis
Data are presented as mean ± SEM of PTZ minimal dose (mg/kg) and were analyzed using the SPSS statistical software package (ver. 15). One- or two-way analyses of variance (ANOVA) followed by post hoc Tukey’s tests was used to analyze the data where appropriate. Tests of homogeneity of variance were used to ensure normal distribution of the data. A P value of <0.05 was defined as statistically significant.
Results
Effect of time in oleuropein treatment on seizure threshold
Figure 1 shows the time course of the effect of oleuropein on seizure threshold. As shown, i.p. administration of oleuropein (20 mg/kg) did not affect the PTZ-induced seizure threshold at 30 and 90 min, whereas it significantly reduced the seizure threshold at 60 min after administration [F (3, 23) = 11.911, P < 0.001, P < 0.05, respectively].
Effect of oleuropein (20 mg/kg, i.p) on PTZ-induced clonic seizure threshold in mice. Oleuropein was administered at 30, 60 and 90 min before PTZ and its effects were compared with those of the control group administered PTZ at the same time. Data are expressed as mean ± SEM of seizure threshold in at least 8 mice. ***P < 0.001 compared with corresponding saline group
Effect of different doses of oleuropein on seizure threshold
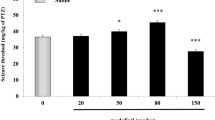
Figure 2 illustrates the effect of acute i.p. administration of oleuropein (1, 5, 10, 20 and 30 mg/kg) on seizure threshold. Seizure threshold was determined 60 min after administration of different doses of oleuropein. The low dose of oleuropein (1 mg/kg) did not alter seizure threshold. However, ANOVA analysis and subsequent post hoc analysis showed a significant increase in seizure threshold at ≥5 mg/kg [F (5, 47) = 13.851; P < 0.001; Fig. 2].
The effect of different doses of oleuropein (1, 5, 10, 20 and 30 mg/kg, i.p.) administration on the PTZ-induced seizure threshold in mice. Oleuropein was administered 60 min prior to PTZ. Data are expressed as mean ± SEM of seizure threshold in at least 8 mice. *P < 0.05, ***P < 0.001 compared with saline-treated group
Effect of NTX on effective doses of oleuropein
Figure 3 illustrates the effect of NTX (10 mg/kg, i.p.) alone or in combination with oleuropein (10 mg/kg, i.p.) on the clonic seizure threshold induced by PTZ. Comparison of the effect of oleuropein with the effect of saline show no significant difference in seizure threshold [F (1, 15) = 2.635; P > 0.05, compared with the saline-treated group; Fig. 3].
Effect of naltrexone on the anticonvulsant effect of oleuropein. Naltrexone (10 mg/kg, i.p.) was administered 15 min before oleuropein (10 mg/kg, i.p.). Data are expressed as mean ± SEM of seizure threshold in at least 8 mice. ≠≠ P < 0.01 compared with oleuropein-treated group; ***P < 0.001 compared with saline-treated group
Acute administration of NTX (10 mg/kg, i.p.) 15 min before oleuropein (10 mg/kg, i.p.) significantly altered the anticonvulsant effect of oleuropein [F (1, 17) = 11.234; P < 0.01, compared with the corresponding oleuropein-treated group; Fig. 3].
Effect of L-NAME on the anticonvulsant effect of oleuropein
Figure 4 illustrates the effect of different doses of L-NAME (1 and 10 mg/kg, i.p.) alone or in combination with oleuropein (10 mg/kg, i.p.) on the clonic seizure threshold induced by PTZ. Comparison of the effect of L-NAME with the effect of saline showed no significant difference in seizure threshold [F (2, 23) = 1.125; P > 0.05, compared with the saline-treated group; Fig. 4].
Effect of L-NAME on the anticonvulsant effect of oleuropein. L-NAME (1 and 10 mg/kg, i.p.) administered 15 min before oleuropein (10 mg/kg, i.p.). Data are expressed as mean ± SEM of seizure threshold in at least 8 mice. ≠≠ P < 0.01; ≠≠≠ P < 0.001 compared with oleuropein-treated group; ***P < 0.001 compared with saline-treated group
Acute administration of different doses of L-NAME (1 and 10 mg/kg, i.p.) 15 min before oleuropein (10 mg/kg, i.p.) significantly altered the anticonvulsant effect of oleuropein [F (5, 47) = 10.041; P < 0.001, compared with the corresponding oleuropein acid-treated group; Fig. 4].
Effect of l-arginine on the anticonvulsant effect of oleuropein
Figure 5 shows the effect of different doses of l-arginine (30 and 60 mg/kg, i.p.) alone or in combination with oleuropein (10 mg/kg, i.p.) on the clonic seizure threshold induced by PTZ. Comparison of the effect of l-arginine with the effect of saline showed no significant difference in seizure threshold [F (2, 23) = 2.635; P > 0.05, compared with the saline-treated group; Fig. 5].
Acute administration of different doses of l-arginine (30 and 60 mg/kg, i.p.) 15 min before oleuropein (10 mg/kg, i.p.) did not change the anticonvulsant effect of oleuropein [F (5, 47) = 11.839; P < 0.001, compared with the saline-treated group; Fig. 5].
Effect of 7-NI and AG on the anticonvulsant effect of oleuropein
Figure 6 shows the effect of 7-NI (selective neuronal NOS inhibitor) and AG (selective inducible NOS inhibitor) alone or in combination with oleuropein (10 mg/kg, i.p.) on the clonic seizure threshold induced by PTZ. Comparison of the effect of 7-NI (30 mg/kg, i.p.) and AG (100 mg/kg, i.p.) with the effect of saline showed no significant difference in seizure threshold (P > 0.05 compared with the oleuropein-treated group). Furthermore, administration of AG before oleuropein did not affect the anticonvulsant property of oleuropein; however, the anticonvulsant effect of oleuropein was completely prevented by an injection of 7-NI 15 min before oleuropein [F (5, 47) = 9.501; P < 0.001].
Effect of 7-nitroindazole (7-NI) and aminoguanidine (AG) on the anticonvulsant effect of oleuropein. 7-NI (30 mg/kg, i.p.) and AG (100 mg/kg, i.p.) were administered 15 min before oleuropein (10 mg/kg, i.p.). Data are expressed as mean ± SEM of seizure threshold in at least 8 mice. ### P < 0.001 compared with oleuropein-treated group; **P < 0.01, ***P < 0.001 compared with saline-treated group
NO metabolite (NOx) levels in plasma
Table 1 shows a comparison between plasma NOx levels in the different experimental groups. Plasma NOx levels were significantly elevated in the oleuropein (10 mg/kg, i.p.)-, and l-arginine (60 mg/kg, i.p.)-treated groups, and also in the mice that were administered l-arginine (60 mg/kg, i.p.) 15 min before oleuropein (10 mg/kg, i.p.) compared to the saline-treated group (44.21 ± 2.70, 43.02 ± 3.26, 45.11 ± 2.96, and 37.75 ± 2.27 nmol/ml, respectively). However, nitrite levels were not significantly different in the mice that received L-NAME (10 mg/kg, ip) 15 min before oleuropein (10 mg/kg, i.p.) compared with the saline-treated group (37.33 ± 2.27, and 37.75 ± 2.27 nm/ml, respectively).
Discussion
In the current study, we showed for the first time that oleuropein could modulate the anticonvulsant effect in a PTZ-induced seizure model in mice. We demonstrated that NTX (opioid receptor antagonist) blocked the effect of oleuropein. In addition, the anticonvulsant effect of oleuropein was completely reversed by acute pretreatment with L-NAME (a nonselective NOS inhibitor) and 7-NI (a selective inhibitor of neuronal NOS). However, l-arginine (a precursor of NOS) potentiated the anticonvulsant effect of oleuropein on seizure threshold. The anticonvulsant activity of oleuropein did not change after treatment with AG as a selective inhibitor of inducible NOS. Based on these data, we speculate that the opioidergic system and neuronal nitrergic pathway could be involved in the anticonvulsant properties of oleuropein.
Oleuropein, a natural polyphenolic compound belonging to the secoiridoids group, possesses diverse pharmacological and healing properties. A growing body of evidence supports the protective characteristics of oleuropein in the central nervous system against audiogenic seizures related to a high-fat diet [3], Alzheimer’s disease [25, 26], hippocampal neuronal damage [4], and cerebral ischemia [5, 6, 27]. In our study, oleuropein at doses of 10, 20 and 30 mg/kg showed anticonvulsant activities. The mechanisms suggested for this reaction of oleuropein were reduction of amyloid β-protein (Aβ) degradation [3, 25], calcium antagonistic activity [28], suppression of the inflammatory response [29, 30], and clearing various endogenous and exogenous free radicals and oxidants [18, 31].
It has been reported that oleuropein could potentiate the antinociceptive property of morphine in a subeffective dose and suppress low-dose morphine hyperalgesia in rats through Ca2+ channel blocking activity [10]. In addition, oleuropein prevents the development of morphine antinociceptive tolerance through inhibition of morphine-induced L-type calcium channel overexpression [9]. In the current study, NTX suppressed the anticonvulsant activity of oleuropein. These data suggest the modulation of opioidergic receptors in the effects of oleuropein in PTZ-induced seizure in mice.
NO is a known modulator of seizure susceptibility with diverse anti- and proconvulsant effects in different models of seizure [13]. Furthermore, previous reports have shown that three different isoforms of NOS are found to be modulators of both anti- and proconvulsant effects in different seizure models. For example, Sardo et al. reported that the anti-epileptic effect of levetiracetam was mediated via neuronal NOS [34]. Although in our recent study, the selective inducible NOS inhibitor reversed the anticonvulsant effect of vasopressin on seizure threshold [35]. In another study, both the pro- and anticonvulsive effect of D-penicillamine was inhibited by 7-NI, a selective neuronal inhibitor of NOS [23]. Our evidence suggests that the anticonvulsant activities of oleuropein may be mediated through an NO-dependent mechanism on the basis that L-NAME and 7-NI blocked the aforementioned phase of oleuropein. As shown in previous studies, oleuropein enhances NO production by mouse macrophages [17, 26], and causes an increase in NOS expression in these cells [15, 32]. Furthermore, the anti-hypertensive effects of oleuropein might be mediated by improving the release of NO [33].
In conclusion, the current findings underline for the first time that oleuropein shows anticonvulsant activity in PTZ-induced seizures. This phenomenon could be blocked by opioid receptor antagonists and neuronal NOS inhibitors, suggesting the involvement of opioidergic/nitrergic pathways in the convulsive impacts of oleuropein in the PTZ model of clonic seizures in mice.
References
González-Correa JA, Muñoz-Marín J, Arrebola MM, Guerrero A, Narbona F, López-Villodres JA, De La Cruz JP (2007) Dietary virgin olive oil reduces oxidative stress and cellular damage in rat brain slices subjected to hypoxia–reoxygenation. Lipids 42(10):921–929
González-Correa JA, Navas MD, Lopez-Villodres JA, Trujillo M, Espartero JL, De La Cruz JP (2008) Neuroprotective effect of hydroxytyrosol and hydroxytyrosol acetate in rat brain slices subjected to hypoxia–reoxygenation. Neurosci Lett 446(2):143–146
Kuem N, Song SJ, Yu R, Yun JW, Park T (2014) Oleuropein attenuates visceral adiposity in high-fat diet-induced obese mice through the modulation of WNT10b- and galanin-mediated signalings. Mol Nutr Food Res 58(11):2166–2176
Dekanski D, Selaković V, Piperski V, Radulović Ž, Korenić A, Radenović L (2011) Protective effect of olive leaf extract on hippocampal injury induced by transient global cerebral ischemia and reperfusion in Mongolian gerbils. Phytomedicine 18(13):1137–1143
Bu Y, Rho S, Kim J, Kim MY, Lee DH, Kim SY, Choi H, Kim H (2007) Neuroprotective effect of tyrosol on transient focal cerebral ischemia in rats. Neurosci Lett 414(3):218–221
Mohagheghi F, Bigdeli MR, Rasoulian B, Zeinanloo AA, Khoshbaten A (2010) Dietary virgin olive oil reduces blood brain barrier permeability, brain edema, and brain injury in rats subjected to ischemia-reperfusion. Sci World J 10:1180–1191
De La Cruz JP, Del Río S, Arrebola MM, López-Villodres JA, Jebrouni N, González-Correa JA (2010) Effect of virgin olive oil plus acetylsalicylic acid on brain slices damage after hypoxia-reoxygenation in rats with type 1-like diabetes mellitus. Neurosci Lett 471(2):89–93
Pitozzi V, Jacomelli M, Zaid M, Luceri C, Bigagli E, Lodovici M, Ghelardini C, Vivoli E, Norcini M, Gianfriddo M, Esposto S (2010) Effects of dietary extra-virgin olive oil on behaviour and brain biochemical parameters in ageing rats. Br J Nutr 103(11):1674–1683
Zare L, Esmaeili-Mahani S, Abbasnejad M, Rasoulian B, Sheibani V, Sahraei H, Kaeidi A (2012) Oleuropein, chief constituent of olive leaf extract, prevents the development of morphine antinociceptive tolerance through inhibition of morphine-induced L-type calcium channel overexpression. Phytother Res 26(11):1731–1737
Esmaeili Mahani S, Zare L (2013) Olive (Olea europaea L.) leaf extract and its main component (oleuropein) mitigate the development of morphine physical dependence in rats. Physiol Pharmacol 16(4):360–370
Lauretti GR, Ahmad I, Pleuvry BJ (1994) The activity of opioid analgesics in seizure models utilizing N-methyl-dl-aspartic acid, kainic acid, bicuculline and pentylenetetrazole. Neuropharmacology 33(2):155–160
Foote F, Gale K (1984) Proconvulsant effect of morphine on seizures induced by pentylenetetrazol in the rat. Eur J Pharmacol 105(1):179–184
Homayoun H, Khavandgar S, Namiranian K, Gaskari SA, Dehpour AR (2002) The role of nitric oxide in anticonvulsant and proconvulsant effects of morphine in mice. Epilepsy Res 48(1):33–41
Khavandgar S, Homayoun H, Dehpour AR (2003) Mediation of nitric oxide in inhibitory effect of morphine against electroshock-induced convulsions in mice. Pharmacol Biochem Behav 74(4):795–801
de la Puerta R, Domínguez ME, RuÍz-GutÍerrez V, Flavill JA, Hoult JR (2001) Effects of virgin olive oil phenolics on scavenging of reactive nitrogen species and upon nitrergic neurotransmission. Life Sci 69(10):1213–1222
Visioli F, Galli C (1994) Oleuropein protects low density lipoprotein from oxidation. Life Sci 55(24):1965–1971
Visioli F, Bellosta S, Galli C (1998) Oleuropein, the bitter principle of olives, enhances nitric oxide production by mouse macrophages. Life Sci 62(6):541–546
Visioli F, Poli A, Gall C (2002) Antioxidant and other biological activities of phenols from olives and olive oil. Med Res Rev 22(1):65–75
Honar H, Riazi K, Homayoun H, Demehri S, Dehghani M, Vafaie K, Ebrahimkhani MR, Rashidi N, Gaskari SA, Dehpour AR (2004) Lithium inhibits the modulatory effects of morphine on susceptibility to pentylenetetrazole-induced clonic seizure in mice: involvement of a nitric oxide pathway. Brain Res 1029(1):48–55
Majewska MD, Bell JA, London ED (1990) Regulation of the NMDA receptor by redox phenomena: inhibitory role of ascorbate. Brain Res 537(1):328–332
Park S, Choi Y, Um SJ, Yoon SK, Park T (2011) Oleuropein attenuates hepatic steatosis induced by high-fat diet in mice. J Hepatol 54(5):984–993
Shafaroodi H, Baradaran N, Moezi L, Dehpour S, Kabiri T, Dehpour AR (2011) Morphine sensitization in the pentylenetetrazole-induced clonic seizure threshold in mice: role of nitric oxide and μ receptors. Epilepsy Behav 20(4):602–606
Rahimi N, Sadeghzadeh M, Javadi-Paydar M, Heidary MR, Jazaeri F, Dehpour AR (2014) Effects of D-penicillamine on pentylenetetrazole-induced seizures in mice: involvement of nitric oxide/NMDA pathways. Epilepsy Behav 39:42–47
Tsikas D (2007) Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. J Chromatogr 851(1):51–70
Del Vecchio RA, Gold LH, Novick SJ, Wong G, Hyde LA (2004) Increased seizure threshold and severity in young transgenic CRND8 mice. Neurosci Lett 367(2):164–167
Luccarini I, Grossi C, Rigacci S, Coppi E, Pugliese AM, Pantano D, Casamenti F (2015) Oleuropein aglycone protects against pyroglutamylated-3 amyloid-β toxicity: biochemical, epigenetic and functional correlates. Neurobiol Aging 36(2):648–663
Mohagheghi F, Bigdeli MR, Rasoulian B, Hashemi P, Pour MR (2011) The neuroprotective effect of olive leaf extract is related to improved blood–brain barrier permeability and brain edema in rat with experimental focal cerebral ischemia. Phytomedicine 18(2):170–175
Rauwald HW, Brehm O, Odenthal KP (1994) Screening of nine vasoactive medicinal plants for their possible calcium antagonistic activity. Strategy of selection and isolation for the active principles of Olea europaea and Peucedanum ostruthium. Phytother Res 8(3):135–140
Wang L, Geng C, Jiang L, Gong D, Liu D, Yoshimura H, Zhong L (2008) The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. Euro J Nutr 47(5):235–243
Cullinen K (2006) Olive oil in the treatment of hypercholesterolemia. Med Health RI 89(3):113
Owen RW, Giacosa A, Hull WE, Haubner R, Würtele G, Spiegelhalder B, Bartsch H (2000) Olive-oil consumption and health: the possible role of antioxidants. Lancet Oncol 1(2):107–112
Ueda S, Tokuda H, Iwashima A, Nishino H (1994) Chemoprevention of skin and lung cancer by Gardenia iridoid. In: Proceedings International Congress on Natural Products Research
Nekooeian AA, Khalili A, Khosravi MB (2014) Effects of oleuropein in rats with simultaneous type 2 diabetes and renal hypertension: a study of antihypertensive mechanisms. J Asian Nat Prod Res 16(9):953–962
Sardo P, D’Agostino S, Rizzo V, Carletti F, Lonobile G, Ferraro G (2009) In the rat maximal dentate activation model of partial complex epilepsy, the anticonvulsant activity of levetiracetam is modulated by nitric oxide-active drugs. J Neural Transm 116:831–839
Javadian N, Rahimi N, Javadi-Paydar M, Doustimotlagh A, Dehpour AR (2016) The modulatory effect of nitric oxide in pro- and anti-convulsive effects of vasopressin in PTZ-induced seizures threshold in mice. Epilepsy Res 126:134–140
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Rahimi, N., Delfan, B., Motamed-Gorji, N. et al. Effects of oleuropein on pentylenetetrazol-induced seizures in mice: involvement of opioidergic and nitrergic systems. J Nat Med 71, 389–396 (2017). https://doi.org/10.1007/s11418-017-1071-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-017-1071-z