Abstract
Activation of glial cells has been observed in neurodegenerative diseases including Alzheimer’s disease (AD). Aggregation of amyloid β (Aβ) is profusely observed as characteristic pathology in AD brain. In our previous study using microglial cell line BV-2, tissue-type transglutaminase (TG2) was found to be involved in phagocytosis (Kawabe et al., in Neuroimmunomodulation 22(4):243–249, 2015; Kawabe et al., Neurochem Res 2017). In the present study, we examined whether TG2 and milk fat globule EGF factor 8 protein (MFG-E8), an adaptor protein promotes macrophage to engulf apoptotic cells, were involved in Aβ endocytosis. When the neuronal/glial mixed culture was stimulated freshly prepared Aβ1−42 for 3 days, the incorporation of Aβ was observed by immunofluorescence staining technique in Iba-1-positive microglia. Cystamine, a broad competitive inhibitor of TGs, suppressed it. When aggregated Aβ was added to the mixed culture, the immunoreactivity of MFG-E8 surrounding Aβ was observed, and then followed by microglial endocytosis. Using western blotting technique, MFG-E8 was detected in cell lysate of astrocyte culture, and was also detected in the medium. When microglia culture was incubated with astrocyte conditioned medium, MFG-E8 levels in microglia tended to increase. It is likely that microglia might utilize MFG-E8 released from astrocytes as well as that expressed in themselves in order to endocytose Aβ aggregation. Furthermore, we confirmed that MFG-E8 could bind with TG2 in microglia culture by immunoprecipitate technique. These results suggest that microglia might uptake Aβ as a complex of aggregated Aβ/MFG-E8/TG2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In pathological mechanism of various neurodegenerative diseases, it is well known that the activation of glial cells play important roles for both exacerbation and recovery [1–3]. Therefore, the elucidation of the mechanism of glial activation is essential for overcome such diseases. Astrocytes play various important roles in CNS, such as maintenance of blood brain barrier, scavenging some neurotransmitters, control of ionic balance in brain parenchyma. These functions of astrocytes serve the maintenance of brain homeostasis [4–6]. Functional changes of astrocytes are involved in neurodegenerative processes in various CNS diseases including Alzheimer’s diseases (AD).
Microglia categorized into macrophage is considered to be a major cell for immunity in CNS. Activated microglia release nitric oxide (NO) and proinflammatory cytokines to damage neurons, and also produce neurotrophins to protect neurons. In addition, they engulf invading microorganisms and scavenge cell debris and damaged cells [1, 2]. Microglial phagocytosis contributes to maintaining of homeostasis of CNS and to developing neural network physiologically. However, it is reported that NO production and phagocytosis of hyper-activated microglia cause neuronal damage in neurodegenerative diseases [3].
Endocytosis is generally divided into two categories, pinocytosis and phagocytosis, with size of particle up-taken; pinocytosis is defined as uptake of particles smaller than 1–2 µm and phagocytosis is as uptake over 2 µm [7–9]. In case of scavenging apoptotic cells, phagocytotic cells need to contact with “eat me” signal of target apoptotic cells by their receptors [10, 11]. Phosphatidylserine (PS) is known to be a major “eat me” signal, most of which normally exist in inner leaflet of cell membrane; however, when cells are undergone apoptosis, PS is exposed on surface of outer leaflet.
An adaptor protein, milk fat globule EGF factor 8 protein (MFG-E8) consists of four domains, E1 and E2 homologous regions to epidermal growth factor (EGF) and C1 and C2 homologous regions to coagulation factor 8, and proline/threonine rich domain is additionally inserted between them resulting to be called a long-form of MFG-E8 [12]. C2 region of MFG-E8 binds to PS and RGD motif in E2 region of MFG-E8 binds a vitronectin receptor (VR) of macrophage formed by integrin αVβ3/5 dimer, thereby, macrophage can recognize and uptake the apoptotic cells [13, 14]. It is reported that microglia also release and utilize MFG-E8 to recognize neurons when stimulated by lipopolysaccharide (LPS) or amyloid β (Aβ) [3, 15, 16]. However, it has not been reported whether MFG-E8 directly participate in Aβ uptake by microglia other than the incorporation of the apoptotic cells.
Transglutaminase (TG), a crosslinking enzyme consisting eight isozymes identified, catalyzes Ca2+-dependently to form ε-(γ-glutamyl)lysine isopeptide bond crosslinking between glutamine and lysine residues in proteins [17]. Tissue-type TG (TG2) ubiquitously expressed in various cells is involved in cell adhesion and construct of cytoskeleton [18, 19]. In addition, extracellular TG2 binds to integrin and fibronectin in a Ca2+-independent manner [20, 21]. These functions contribute to extracellular matrix formation, tissue structures stabilization and epithelial barrier.
Also in phagocytosis, TG2 plays important roles. It was reported in peritoneal macrophage that TG2 can bind both of integrin β3 and MFG-E8, and TG2 protein is involved in recognition of apoptotic cells; suggesting that TG2 mediates the binding between VR and MFG-E8 in TG enzyme activity-independent manner [22, 23]. Also in brain macrophage, microglia, TG2 might be involved in binding of MFG-E8 to VR. We previously demonstrated in mouse microglial cell line BV-2 activated by LPS or amphotericin B that TG2 would contribute to microglial phagocytosis because a TG inhibitor blocked phagocytotic activity [24, 25].
In the present study, we assessed involvement of MFG-E8 and TG2 in Aβ uptake by microglia and the cellular derivation of MFG-E8 protein in CNS, using three kinds of cell cultures; neuronal/glial mixed culture, microglia culture and astrocyte culture.
Experimental Procedures
Materials
Deoxyribonuclease I (DNase I), trypsin, fetal bovine serum (FBS) and anti-Aβ antibody were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Dulbecco’s modified Eagle medium (DMEM) and horse serum were from Gibco BRL (Grand Island, NY, USA). Cystamine dihydrochloride was from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Anti-TG2 antibody was from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Anti-MFG-E8 antibody was from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Horseradish peroxidase (HRP) conjugated goat anti-mouse IgG (H + L) and HRP conjugated goat anti-rabbit IgG (H + L) antibodies (secondary antibodies) were from Bio-Rad Laboratories Inc. (Hercules, CA, USA). Aβ1−42 was from Peptide Institute, Inc. (Osaka, Japan).
Preparation of Cell Culture
This study was carried out in compliance with the Guideline for Animal Experimentation at Osaka Prefecture University, with an effort to minimize the number of animals used and their suffering.
Neuronal/Glial Mixed Culture
Neuronal/glial mixed culture was prepared from hippocampus of 20-day-old embryos, which were taken out from pregnant Wistar rats deeply anesthetized. Hippocampi were cleared of meninges, cut into about 1-mm3 blocks, and treated with 0.25% trypsin in Ca2+, Mg2+-free phosphate-buffered saline (PBS) containing 5.5 mM glucose for 20 min at 37 °C with gentle shaking. An equal volume of horse serum supplemented with 0.1 mg/ml of DNase I was added to the medium to inactivate the trypsin. Then, the tissues were centrifuged at 350×g for 5 min. The tissue sediments were triturated through a yellow-chip-mounted pipette with DMEM containing 10% fetal bovine serum (FBS), 100 mg/l streptomycin and 5 × 104 units/l penicillin. After filtering cell suspensions through a lens-cleaning paper (Fujifilm Co., Tokyo, Japan), the cells were plated on poly-l-lysine-coated 24-well slide glass (4 mm diameter each, Matsunami Glass Ind. Ltd., Osaka, Japan) at a density of 8.0 × 103 cells/well. Cultures were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37 °C for 1 day, and medium was changed with DMEM containing 5% FBS. After 2 days (at 3 days in vitro), the cells were used for the experiments. The neuronal/glial mixed culture consisted with 10% neuron, 85% astrocytes, and 5% microglia as the result of immunostaining using antibodies against a neuronal marker, MAP2 (Sigma), an astrocyte marker, glial fibrillary acidic protein (GFAP) (Biosensis Pty Ltd., Thebarton, Australia), and a microglial marker, Iba-1 (Wako).
Astrocytes Culture
Astrocytes were prepared as described previously from 20-day-old cortex of Wistar rat embryos [26] by the similar manner as described above for the mixed culture. The cells were plated on polyethyleneimine-coated 100-mm diameter plastic dishes (Iwaki, Asahi Glass Co., Tokyo, Japan) at a density of 0.8–1.3 × 105 cells/cm2 . Cultures were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37 °C with changing medium every 3 days. After 1 week, astrocytes were replated to remove neurons. On days 12–14, they were replated onto adequate plates or dishes using an ordinary trypsin-treatment technique at a density of 4 × 104 cells/well for 96-well plate (Sumitomo Bakelite Co., Tokyo, Japan) or 8 × 105 cells/35 mm dish (Thermo Fisher Scientific Inc., Waltham, MA, USA), and stabilized for 1 day, then we used for the experiments.
More than 90% of the cells were immunoreactively positive to GFAP using the antibody (Biosensis) and FITC-conjugated anti-mouse IgG antibody. Less than 10% of the cells were positive to Iba-1 using the antibody (Wako) and rhodamine-conjugated anti-rabbit IgG antibody.
Microglial Culture
Primary cultures of rat microglia were prepared as described previously [27]. Briefly, the whole brains of neonatal Wistar rats were homogenized with DMEM using a Pasteur pipette, and treated with 0.25% trypsin in Ca2+, Mg2+-free PBS containing 5.5 mM glucose for 15 min at 37 °C with gentle shaking. An equal volume of horse serum supplemented with 0.1 mg/ml of DNase I was added to the medium to inactivate the trypsin, and the tissues were centrifuged at 350×g for 5 min. The tissue sediments were triturated through a yellow-tip-mounted pipette with DMEM containing 10% FBS, 100 mg/l streptomycin and 5 × 104 units/l penicillin. The cells were plated on polyethyleneimine-coated plastic bottles, and cultured at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The medium was changed every week. On day 14, microglia were harvested from mostly-glial mixed cultures with shaking bottles for 1 h at 100 rpm, and replated onto 35 mm dish (for suspension culture; Sumitomo Bakelite Co., Tokyo, Japan) at a density of 8 × 105 cells/dish. After 30 min, the medium was changed to remove non-adherent cells, and the remaining microglia were allowed to stabilized for 1 day in DMEM containing 10% FBS before the experiments. In our culture, more than 95% of the cells were positive to Iba-1.
Preparation of Aβ Solution
Aβ1−42 was dissolved in sufficient volume of 1,1,1,3,3,3-hexafluoro-2-propanol and air-dried overnight. Then, a condensate was resolved in dimethyl sulfoxide (DMSO) at a concentration of 1 mM as a stock solution and kept at −80 °C before using. This stock solution was used for “freshly prepared Aβ” after adequate dilution. In some experiments, “aggregated Aβ” was used. The medium was added with Aβ stock solution at the concentration of 10 µM and preincubated for 7 days at 37 °C, then used for aggregated Aβ stimulation, usually at 100 nM of Aβ monomer concentration.
Astrocyte Conditioned Medium (ACM)
Cultured astrocytes were replated on 35 mm dish (Thermo Fisher Scientific Inc.) and stabilized for 24 h, and changed to fresh medium. Then after 24 h again, the medium was collected and centrifuged at 3000×g and the supernatant was used for ACM.
Immunostaining
Neuronal/glial mixed culture was stimulated with freshly prepared Aβ for 3 days. The cells were fixed by 4% paraformaldehyde for 8 min at 37 °C, and permeabilized with 100% methanol for 5 min at −20 °C. After blocking with 5% bovine serum albumin (BSA) in PBS for 30 min, the cells were incubated with antibodies against Iba-1 (1:2000), Aβ (1:2000), and MFG-E8 (1:100) in blocking buffer at 4 °C overnight followed by an incubation with FITC-conjugated anti-mouse IgG antibody (1:10,000) or rhodamine-conjugated anti-rabbit IgG antibody (1:2000) at room temperature for 1 h. Then, nuclear stain was performed using hoechst33342 for 10 min, and the cells were mounted with SlowFade® Diamond (Molecular Probes). To visualize fluorescent immunostaining, Olympus BX61VS microscope was used.
Western Blotting
Astrocyte culture and microglia culture were stimulated with aggregated Aβ (preincubated at 37 °C for 7 days) for 24 h. The cells were homogenized in 20 mM Tris–HCl buffer (pH 7.5) containing 1 mM EDTA and protease inhibitor cocktail (Sigma P8340). To detect proteins in the medium, the medium was concentrated. Culture medium from astrocytes was centrifuged at 3000×g, and then the supernatant was concentrated 20-fold using Nanosep® centrifugal device (3 kDa cut-off; Pall, NY, USA).
Each sample including cell homogenate and concentrated medium was added at a volume ratio of 4:1 to 50 mM Tris–HCl buffer (pH 6.8) containing 50% glycerol, 10% sodium dodecyl sulfate, 0.05% bromophenol blue and 25% 2-mercaptoethanol, followed by mixing and boiling at 100 °C for 5 min. Each aliquot in a certain amount of protein or concentrated medium was loaded on a 10% polyacrylamide gel for electrophoresis at a constant voltage of 120 V for 2 h at room temperature and subsequent blotting to a polyvinylidene fluoride membrane previously treated with 100% methanol. After blocking by 5% skimmed milk dissolved in 20 mM Tris–HCl buffer (pH 7.5) containing 137 mM NaCl and 0.05% Tween 20, the membrane was reacted with antibodies against TG2 (1:2000), MFG-E8 (1:500), and β-actin (1:100,000) followed by a reaction with secondary antibodies. Proteins reactive with those antibodies were detected with the aid of ECL detection reagents (Millipore) using lumino-image-analyzer (LAS-4000, Fujifilm). Laser densitometric analysis was performed to standardize the results of western blotting. Protein concentrations were determined by the method of Bradford using coomassie brilliant blue (CBB) color solution (Nacalai Tesque, Kyoto, Japan), according to the manufacturer’s protocol, with BSA as the standard.
Immunoprecipitation to Detect Binding Between MFG-E8 and TG2
Microglia culture was stimulated with aggregated Aβ (preincubated at 37 °C for 7 days) for 24 h. The cells were homogenized in 20 mM Tris–HCl buffer (pH 7.5) containing 1 mM EDTA and protease inhibitor cocktail. Anti-MFG-E8 antibody (5 µg) was mixed with SureBeads™ Protein A magnetic beads (Bio-Rad), incubated for 30 min at room temperature, and washed three times with PBS containing 0.1% Tween-20 (PBS-T). The cell homogenate was added to the beads which bind anti-MFG-E8 antibody, and incubated on a rotator for 3 h at room temperature. After washing with PBS-T, elution buffer [20 mM glycine (pH 2.0)] was added and incubated for 5 min, followed by addition of neutralized buffer [1 M phosphate buffer (pH 7.4)]. Each sample was added at a volume ratio of 1:1 to 66 mM Tris–HCl buffer (pH 6.8) containing 26% glycerol, 2% sodium dodecyl sulfate, 0.01% bromophenol blue, followed by mixing and boiling at 100 °C for 10 min. Each aliquot was examined by western blotting using anti-TG2 antibody as described above.
Data Analysis
For statistical analysis of the data, Student’s t test was used. Differences between treatments were considered statistically significant when p < 0.05.
Results
Involvement of TG in Aβ Uptake by Microglia in Neuronal/Glial Mixed Culture
In the previous study, we demonstrated that TG2 might be involved in endocytosis activity using fluorescent beads and dead cells in microglia [24, 25]. In the present study, we evaluated uptake of Aβ by microglia in neuronal/glial mixed culture. To assess the involvement of TGs, we examined the effect of cystamine, a broad competitive inhibitor of TGs, on Aβ uptake by microglia. Neuronal/glial mixed culture was stimulated by freshly prepared Aβ (100 nM) for 3 days, and immunostaining against Aβ and Iba-1 was performed. We quantified the cells with and without Aβ staining around nuclei of Iba-1-positive microglia, and the percentages of Aβ uptake microglia were evaluated. Under these conditions, more than 80% of microglia incorporated Aβ. We could observe some Aβ staining at the position apart from Iba-1 staining; however, most of Aβ staining was associated with Iba-1 staining. In the presence of 10 nM cystamine, percentage of Aβ staining around nuclei of Iba-1-positive microglia decreased remarkably to about 30% (Fig. 1).
Aβ uptake by microglia and the inhibition by cystamine in neuronal/glial mixed culture. Neuronal/glial mixed culture was incubated with freshly prepared Aβ (100 nM) with or without 10 nM cystamine for 3 days. The Aβ uptake by microglia was detected by immunofluorescent staining. Microglial marker Iba-1 (red) and Aβ (green) were immunostained using each specific antibody, and nuclei were stained with hoechst33342 (blue). Representative photographs are shown in a. The graph shows the ratio of Aβ uptake microglia/total microglia cell numbers in b. Data are mean ± SD of three samples. *p < 0.05, significantly different from Aβ 100 nM. Scale bar 60 µm. (Color figure online)
Aβ Uptake by Microglia via MFG-E8 in Neuronal/Glial Mixed Culture
To assess the involvement of MFG-E8 in Aβ uptake by microglia, neuronal/glial mixed culture was stimulated with freshly prepared Aβ (100 nM) for 3 days and immunostaining was performed against Iba-1, Aβ and MFG-E8 (Fig. 2a). Similar to Fig. 1a (upper), most of Iba-1-positive microglia was co-stained with Aβ in double-staining against Aβ and Iba-1 (Fig. 2A–a, c). Immunoreactivity for MFG-E8 was also colocalized with it for Aβ in double-staining against Aβ and MFG-E8 (Fig. 2A–b, d).
Aβ uptake by microglia in neuronal/glial mixed culture. a Freshly prepared Aβ (100 nM) was added to neuronal/glial mixed culture and incubated for 3 days. The Aβ uptake by microglia was detected by immunofluorescent staining. Aβ (green) and either microglial marker Iba-1 (red) (a, c) or MFG-E8 (red) (b, d) were immunostained with each specific antibody, and nuclei were stained with hoechst33342 (blue). Representative photographs are shown in low magnification (a, b) and high magnification of red-lined box in a, b (c, d). b Aggregated Aβ (pre-incubated at 37 °C for 7 days) was added to neuronal/glial mixed culture and incubated for 1 (e, g) or 2 days (f, h). The localization of Aβ and MFG-E8 was detected by immunofluorescent staining. MFG-E8 (red) and Aβ (green) were immunostained with each specific antibody, and nuclei were stained with hoechst33342 (blue). Representative photos are shown in low magnification (e, f) and high magnification of red-lined box in e, f (g, h). Scale bars 100 µm (a, b, e, f), and 10 µm (c, d, g, h). (Color figure online)
Next, Aβ uptake was assessed when aggregated Aβ, which was pre-incubated for 7 days at 37 °C, was added to neuronal/glial mixed culture (Fig. 2b). After 1 day of Aβ addition, aggregated Aβ were observed as green dots on the slide glass and many of them were surrounded by MFG-E8, red particles (Fig. 2B–e, g). After 2 days of Aβ addition, most of Aβ had been incorporated into cells, much less green dots on the slide glass, and colocalized with MFG-E8, yellow dots in some cells (Fig. 2B–f, h).
Expression of MFG-E8 in Astrocyte Culture
It was reported that MFG-E8 had long- and short-forms depending on the structure with and without proline/threonine rich domain, respectively [28]. We examined the expression of MFG-E8 in astrocyte culture. By double-immunostaining, MFG-E8 was observed in GFAP-positive astrocytes (data not shown). The cells were stimulated with 100 nM aggregated Aβ for 24 h, and MFG-E8 expression in the cells and MFG-E8 in the medium were evaluated by western blotting (Fig. 3). Both of long- and short-forms of MFG-E8 were detected in whole cell lysate of astrocyte culture, although the band of long-form was much more prominent than that of short-form. Aβ stimulation did not significantly change the expressions of both forms (Fig. 3a).
Expression of MFG-E8 in astrocyte culture and detection of MFG-E8 protein in the medium. Astrocyte culture was stimulated with 100 nM aggregated Aβ for 24 h and the expression of MFG-E8 was detected by western blotting using whole cell lysate (a) and medium (b). Typical bands of western blotting for MFG-E8 are shown in the photograph. The graph shows MFG-E8/β-actin ratio of the density for cellular expression, and density of MFG-E8 bands for medium which was equivalently 20-fold concentrated. Data are mean ± SD of three samples
In the medium analysis, both of long- and short-forms of MFG-E8 were also detected; even though the bands observed more broadly than that of cell lysate, probably due to the concentrated sample. Aβ stimulation did not significantly change the protein levels (Fig. 3b).
Expressions of MFG-E8 and TG2 in Microglia Culture
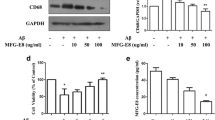
Also in microglia culture, we confirmed the expression of MFG-E8. By double-immunostaining, MFG-E8 was observed in Iba-1-positive microglia (data not shown). The cells were stimulated with aggregated Aβ (100 nM) and MFG-E8 amount in the cells was evaluated by western blotting. In whole cell lysate of microglia culture, only long-form of MFG-E8 was detected, and Aβ stimulation did not significantly change the expression (Fig. 4a). When the cells were incubated with ACM for 24 h, cellular amount of long-form MFG-E8 tended to increase (Fig. 4b). In the medium analysis, long-form of MFG-E8 was also detected. Aβ stimulation did not significantly change the protein levels (data not shown).
MFG-E8 and TG2 expressions in microglia culture. Microglia culture was incubated with 100 nM aggregated Aβ (a) or ACM (b) for 24 h and the expression of MFG-E8 was detected by western blotting using whole cell lysate. Typical bands of western blotting for MFG-E8 are shown in the photograph. The graph shows MFG-E8/β-actin ratio of the density of detection bands. Data are mean ± SD of three samples. c Microglia culture was incubated with 100 nM aggregated Aβ for 24 h and the expression of TG2 was detected by western blotting using whole cell lysate. Typical bands of western blotting for TG2 are shown in the photograph. The graph shows TG2/β-actin ratio of the density of detection bands. Data are mean ± SD of three samples. *p < 0.05, significantly different from control (CTL)
We also examined the expression of TG2 in whole cell lysate of microglial culture. The expression of TG2 significantly increased by the stimulation with aggregated Aβ (100 nM) for 24 h (Fig. 4c).
Binding of MFG-E8 with TG2 in Microglia Culture
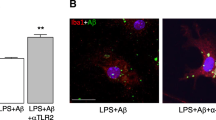
To assess whether MFG-E8 directly interacts to TG2 in microglia, we performed immunoprecipitation using cell homogenates of microglia culture with anti-MFG-E8 antibody followed by detection with anti-TG2 antibody by western blotting. The binding between MFG-E8 and TG2 was confirmed regardless of the Aβ stimulation (100 nM) for 24 h (Fig. 5).
Binding of MFG-E8 with TG2 in microglia culture. Microglia culture was incubated with 100 nM aggregated Aβ for 24 h. To assess the binding of TG2 and MFG-E8 in microglia, the cell lysate was immuprecipitated (IP) using anti-MFG-E8 antibody and the precipitate was examined by western blotting (WB) using anti-TG2 antibody
Discussion
In the previous study, we reported in activated microglia that TG2 might be involved in endocytosis of beads and dead cells, because of inhibition of the endocytosis by cystamine, a TG inhibitor [24, 25]. In the present study, microglial Aβ uptake in neuronal/glial mixed culture was also inhibited by cystamine. In peritoneal macrophage from TG2-knocked out mice show impaired engulfment of apoptotic cells; suggesting the involvement of TG2 protein [22, 23]. We also observed that aggregated Aβ stimulation increased TG2 expression in microglia culture. These results suggest that TG2 might be involved in Aβ uptake by microglia.
In the present study in neuronal/glial mixed culture, Aβ uptake by microglia was detected in both conditions stimulated by freshly prepared Aβ for 3 days and by aggregated Aβ for 1 or 2 days. It was reported that uptake of soluble Aβ monomer would be observed in resting microglia and the soluble Aβ uptake would not activate microglia [29], on the other hand, uptake of aggregated Aβ was reported to activate microglia [30]. In the present study, microglial morphology incorporating Aβ looked to be activated (data not shown); suggesting that microglia might incorporate Aβ as the aggregated Aβ which is formed in the medium.
Furthermore, in the present study, when neuronal/glial mixed culture was stimulated with freshly prepared Aβ for 3 days, MFG-E8 was detected around the nuclei of Iba-1-positive microglia incorporating Aβ. When aggregated Aβ was added to neuronal/glial mixed culture, Aβ on the slide glass was surrounded with MFG-E8 on day 1, and then followed by uptake into cells on day 2. These results suggest that aggregated Aβ bound with MFG-E8 would be incorporated by microglia.
MFG-E8 has been considered to be an adaptor protein for phagocytosis of apoptotic cells. It is reported that MFG-E8 binds to PS and that RGD motif of MFG-E8 binds to a VR of macrophage, thereby, macrophage can recognize and uptake the cells [14]. In co-culture of neurons and microglia, it is reported that NO derived from LPS-stimulated microglia causes exposure of PS on neurons and that microglia recognize neuronal PS to uptake the neurons [15]. It has been reported that MFG-E8 was used for phagocytosis of apoptotic cells as described above; however, in our present results, MFG-E8 might be used also for Aβ uptake as well as apoptotic cell phagocytosis.
To assess the cellular derivation of MFG-E8, we examined the expression of MFG-E8 in astrocyte culture and in microglia culture. In peripheral tissues, MFG-E8 protein was mainly released from macrophages and used for phagocytosis [13]. It was reported that microglia derived from MFG-E8 knockout mice could endocytose neurons using MFG-E8 containing in the conditioned medium of astrocytes derived from wild-type mice [16]. However, the details of the expression pattern in MFG-E8 protein was unclear in the brain. The present study demonstrated that astrocytes expressed both of long- and short-forms of MFG-E8 and released them, and that microglia expressed only long-form of MFG-E8.
Stimulation by aggregated Aβ for 24 h did not significantly affect the expressions of MFG-E8 in astrocytes and microglia, and the protein levels of MFG-E8 in the medium were neither affected. On the other hand, when microglia culture was incubated with ACM for 24 h, MFG-E8 protein in microglial cell lysate tended to increase. It is likely that microglia might utilize MFG-E8 released from astrocytes as well as that expressed in themselves, although the possibility is not excluded that some factors in ACM increase MFG-E8 expression in microglia culture.
Furthermore, the binding between TG2 and MFG-E8 was confirmed in microglial homogenates by immunoprecipitation. It is possible that TG2/MFG-E8 complex might be involved in the recognition and phagocytosis of aggregated Aβ by microglia. The binding of MFG-E8 with TG2 in cells is the first report, even though TG2 was reported to bind each of MFG-E8 and VR using recombinant proteins [31].
In conclusion, TG2 and MFG-E8 might be involved in microglial Aβ uptake. Astrocytes expressed MFG-E8 and released it into the medium. It is likely that microglia might use MFG-E8 released from astrocytes as well as from microglia. The direct binding between MFG-E8 and TG2 was confirmed in microglial cell lysate. Altogether, Aβ/MFG-E8/TG2/VR might be a novel combination of the mechanism for aggregated Aβ uptake by microglia.
Abbreviations
- Aβ:
-
Amyloid beta
- ACM:
-
Astrocytes conditioned medium;
- AD:
-
Alzheimer’s disease
- BSA:
-
Bovine serum albumin
- CBB:
-
Coomassie brilliant blue
- CNS:
-
Central nervous system
- DMEM:
-
Dulbecco’s modified Eagle medium
- DMSO:
-
Dimethyl sulfoxide
- EGF:
-
Epidermal growth factor
- FBS:
-
Fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- GFAP:
-
Glial fibrillary acidic protein
- HRP:
-
Horseradish peroxidase
- MFG-E8:
-
Milk fat globule EGF factor 8 protein
- LPS:
-
Lipopolysaccharide
- NO:
-
Nitric oxide
- PBS:
-
Phosphate-buffered saline
- PS:
-
Phosphatidylserine
- TG:
-
Transglutaminase
- VR:
-
Vitronectin receptor.
References
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318
Nakamura Y (2002) Regulating factors for microglia activation. Biol Pharm Bull 25:945–953
Neniskyte U, Neher JJ, Brown GC (2011) Neuronal death induced by nanomolar amyloid β is mediated by primary phagocytosis of neurons by microglia. J Biol Chem 286(46):39904–39913
Anderson CM, Swanson RA (2000) Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32:1–14
Abbott NJ (2002) Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat 200:629–638
Correale J, Villa A (2009) Cellular elements of the blood-brain barrier. Neurochem Res 34:2067–2077
Pratten MK, Lloyd JB (1986) Pinocytosis and phagocytosis: the effect of size of a particulate substrate on its mode of capture by rat peritoneal macrophages cultured in vitro. Biochim Biophys Acta 881:307–313
Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT (1994) Macrophage/particle interactions: effect of size, composition and surface area. J Biomed Mat Res 28:81–90
Aukunuru JV, Kompella UB (2002) In vitro delivery of nano- and micro-particles to human retinal pigment epithelial (ARPE-19) cells. Drug Del Technol 2:50–57
Fadok VA, Chimini G (2001) The phagocytosis of apoptotic cells. Semin Immunol 13:365–372
Ravichandran KS (2011) Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity 35(4):445–455
Oshima K, Aoki N, Kato T, Kitajima K, Matsuda T (2002) Secretion of a peripheral membrane protein, MFG-E8, as a complex with membrane vesicles. Eur J Biochem 269:1209–1218
Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417(6885):182–187
Hanayama R, Nagata S (2005) Impaired involution of mammary glands in the absence milk fat globule EGF factor 8. Proc Natl Acad Sci USA 102(46):16886–16891
Neher JJ, Neniskyte U, Zhao JW, Bal-Price A, Tolkovsky AM, Brown GC (2011) Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J Immunol 186:4973–4983
Fricker M, Neher JJ, Zhao JW, Théry C, Tolkovsky AM, Brown GC (2012) MFG-E8 mediates primary phagocytosis of viable neurons during neuroinflammation. J Neurosci 32(8):2657–2666
Griffin M, Casadio R, Bergamini CM (2002) Transglutaminases: Nature’s biological glues. Biochem J 368:377–396
Jeitner TM, Muma NA, Battaile KP, Cooper, AJL (2009) Transglutaminase activation in neurodegenerative diseases. Future Neurol 4:449–467
Basso M, Berlin J, Xia L, Sleiman SF, Ko B, Haskew-Layton R, Kim E, Antonyak MA, Cerione RA, Iismaa S, Willis D, Cho S, Ratan RR (2012) Transglutaminase inhibition protects against oxidative stress-induced neuronal death downstream of pathological ERK activation. J Neurosci 32(19):6561–6569
Belkin AM (2011) Extracellular TG2: emerging functions and regulation. FEBS J 278:4704–4716
Grosso H, Mouradian MM (2012) Transglutaminase 2: biology, relevance to neurodegenerative diseases and therapeutic implications. Pharmacol Ther 133:392–410
Szondy Z, Sarang Z, Molnár P, Németh T, Piacentini M, Mastroberardino PG, Falasca L, Aeschlimann D, Kovács J, Kiss I, Syegeydi E, Lakos G, Rajnavölgyi É, Birckbichler PJ, Melino G, Fésüs L (2003) Tranglutaminase 2-/-mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc Natl Acad Sci USA 100(13):7812–7817
Tóth B, Garabuczi, É., Sarang Z, Vereb G, Vámosi G, Aeschimann D, Blaskó B, Bécsi B, Erdõdi B, Lacy-Hulbert A, Zhang A, Falasca L, Birge RB, Balajthy Z, Melino G, Fésüs L, Szondy Z (2009) Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophage engulfing apoptosis cells. J Immunol 182(4):2084–2092
Kawabe K, Takano K, Moriyama M, Nakamura Y (2015) Lipopolysaccharide-stimulated transglutaminase 2 expression enhances endocytosis activity in mouse microglial cell line BV-2. Neuroimmunomodulation 22(4):243–249
Kawabe K, Takano K, Moriyama M, Nakamura Y (2017) Amphotericin B increases transglutaminase 2 expression associated with upregulation of endocytotic activity in mouse microglial cell line BV-2. Neurochem Res 42:1488–1495
Takano K, Shiraiwa M, Moriyama M, Nakamura Y (2010) Transglutaminase 2 expression induced by lipopolysaccharide stimulation together with NO synthase induction in cultured astrocytes. Neurochem Int 57(7):812–818
Nakamura Y, Si QS, Kataoka K (1999) Lipopolysaccharide-induced microglial activation in culture: temporal profiles of morphological change and release of cytokines and nitric oxide. Neurosci Res 35:95–100
Watanabe T, Totsuka R, Miyatani S, Kurata S, Sato S, Katoh I, Kobayashi S, Ikawa Y (2005) Production of the long and short forms of MFG-E8 by epidermal keratinocytes. Cell Tissue Res 321(2):185–193
Akiyama H, Mori M, Saido T (1999) Occurrence of the diffuse amyloid β-protein (Aβ) deposits with numerous Aβ-containing glial cells in the cerebral cortex of patients with Alzheimer’s disease. Glia 25:324–331
Mitrasinovic OM, Murphy GM Jr (2003) Microglial overexpression of the M-CSF receptor augments phagocytosis of opsonized Abeta. Neurobiol Aging 24(6):807–815
Wang Z, Collighan RJ, Gross SR, Danen EH, Orend G, Telci D, Griffin M (2010) RGD-independent cell adhesion via a tissue transglutaminase-fibronectin matrix promotes fibronectin fibril deposition and requires syndecan-4/2 and α5β1 integrin co-signaling. J Biol Chem 285(51):40212–40229
Acknowledgements
This work was supported in part by JSPS KAKENHI Grant No. JP15J12259 to K.K., JP26850209 to K.T., JP26450447 to M.M., and JP15K07768 to Y.N.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawabe, K., Takano, K., Moriyama, M. et al. Microglia Endocytose Amyloid β Through the Binding of Transglutaminase 2 and Milk Fat Globule EGF Factor 8 Protein. Neurochem Res 43, 41–49 (2018). https://doi.org/10.1007/s11064-017-2284-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2284-y