Abstract
Activation of glial cells, astrocytes and microglia, has been observed in neurodegenerative diseases including Alzheimer’s disease (AD). Amyloid β (Aβ), which is aggregated and the aggregation is detected as characteristic pathology in AD brain, is known to be produced by neurons and to activate glial cells. Clearance of Aβ from the brain via active transport system is important to prevent the accumulation and aggregation. Low density lipoprotein receptor-related protein 2 (LRP2/megalin) is an Aβ transporter. However, expression and contribution of LRP2 in astrocytes and microglia remain to be clarified. In the present study, we examined the expression of LRP2 and its roles in cultured astrocytes prepared from rat embryonic brain cortex and mouse microglial cell line BV-2. Both cultured rat astrocytes and BV-2 cells expressed LRP2 mRNA detected by RT-PCR. When lipopolysaccharide (LPS) or all-trans retinoic acid (ATRA) were added to BV-2 cells, LRP2 mRNA expression and uptake of microbeads, Aβ and insulin were increased. On the other hand, LPS decreased LRP2 expression and uptake of Aβ and insulin in cultured astrocytes. Knockdown of LRP2 using siRNA attenuated the LPS- or ATRA-increased uptake of microbeads, Aβ and insulin in BV-2 cells. These results suggest that LRP2 was expressed in both astrocytes and microglia and might be involved in endocytosis activities. Adequate control of LRP2 expression and function in astrocytes and microglia might regulate Aβ and insulin levels in brain and would be a potential target in AD pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low density lipoprotein (LDL) receptor-related protein (LRP) is a single transmembrane glycoprotein (gp) belonging to the LDL receptor family, and generally recognized as cell surface endocytic receptors [1, 2]. LRPs bind and internalize extracellular ligands for degradation in lysosomes [3]. The LDL receptor (LDLR) family consists of more than 11 receptors that function in receptor-mediated endocytosis and cellular signaling [4]. Among them, the most important in central nervous system (CNS) are LRP1 and LRP2, and they are multifunctional cell surface receptors that are widely expressed in several tissues including neurons and astrocytes [3].
LRP1, also known as CD91 or α2 macroglobulin receptor, is a multifunctional scavenger and signaling receptor that belongs to the LRP family [5, 6]. LRP1 is a massive protein (600 kDa) that is proteolytically nicked during biosynthesis to give two stably associated polypeptides: an 85-kDa membrane-spanning C-terminal fragment and a 515-kDa extracellular N-terminal chain. LRP1 binds more than 30 ligands extracellularly, including apolipoprotein E (ApoE), blood coagulation factors, amyloid β (Aβ), and prion protein [3]. LRP1 is abundantly expressed in neurons [7], astrocytes [8], and microglia [9]. LRP1 is also expressed in the CNS including vascular cells such as brain endothelial cells and pericytes, and in the choroid plexus of the blood brain barrier (BBB) [4]. LRP1 in neurons plays fundamental roles to uptake cholesterol and fatty acids required for synapse formation [10], and interacts with amyloid precursor protein (APP) and regulates proteolytic processing to increase the production of Aβ peptide [11].
LRP2 was originally identified as the major antigen in Heymann nephritis, a rat model of nephropathy, and named gp330 [12]. LRP2, also called megalin, is structurally very similar to LRP1 and many common ligands were known [3]. LRP2 was reported to be expressed in neurons [13] and astrocytes [14]. In astrocytes, LRP2 is required for albumin binding and internalization into astrocytes including synthesis of neurotrophic factors by the neighboring neurons [15]. However, other reports showed that LRP2 mRNA could not be detected in human cultured astrocytes [16], mouse astrocytes [17], and adult rat brain [18]. Therefore, LRP2 expression in astrocytes needs more studies. Moreover, there were few studies for LRP2 expression in microglia.
Alzheimer’s disease (AD) is a progressive neurodegenerative disease characterized by the progressive declines of memory and cognitive functions, and changes in behavior and personality [19]. The most accepted hypothesis for the mechanism of brain injury in AD is the “amyloid cascade”, comprising amyloid accumulation in the brain, the formation of toxic oligomeric and intermediate forms of Aβ peptides, amyloid plaques, inflammation and the induction of neurofibrillary tangles [20]. There is accumulation of Aβ in both the normal aging brain and the AD brain, and defective Aβ clearance rather than increased Aβ production is thought to be related to the accumulation [21]. Clearance of Aβ from the brain occurs via active transport at the interfaces separating the CNS from the peripheral circulation. Through the BBB, LRP1 and LRP2 facilitate the clearance of the Aβ peptide that is produced by amyloidogenic processing of APP, which can form complexes with different LRPs’ ligands such as ApoE [22, 23]. In the brain cells, astrocytes and microglia can uptake Aβ for eliminating and contribute to Aβ clearance [24].
Several lines of evidence have implicated various LRPs and LRP ligands in the pathogenesis of AD [25]. LRP1 and LRP2 also might be linked to AD. LRP1 antisense or endothelial-specific LRP2 null in BBB caused AD-like impaired cognition in mice [26, 27]. On the other hand, it was reported that LRP1 increased but LRP2 decreased in choroid plexus epithelium in aged rat [28]. Further, in brain tissues from patients with AD, LRP2 expression has been immunohistochemically detected in neurons, even being up-regulated in damaged neurons [13]. Therefore, LRP1 and LRP2 might have different speculative effects in the brain function and AD pathology. LRP1 have been well investigated whereas LRP2 is less studied. Thus, we assessed LRP2 expression in astrocytes and microglia in the present study.
LRP1 and LRP2 can bind to Aβ and facilitate the clearance of the Aβ as described above. In addition, LRP1 and LRP2 can bind to insulin and endocytose, but none is known about the roles of LRP1 and LRP2 in insulin transport into brain [29]. Insulin elongates neuronal axon, potentiates protein synthesis in neurons, and increases synapse formation [30]. It is known that insulin signaling is important in normal neuronal and brain functions and that neurons produce and release insulin by exocytotic mechanism and use it by themselves [30]. In AD pathology, Aβ has been reported to impair insulin signaling in neurons and increase phosphorylation of tau protein causing neurofibrillary tangles [31, 32]. Furthermore, it was reported that insulin concentration in cerebrospinal fluid decreased in severe AD patients [33] and that Aβ reduced insulin synthesis in cultured neurons [30]. In the previous study, we found that insulin was expressed also in astrocytes and released to extracellular medium [34]. On the other hand, it was reported that brain insulin was partly derived from pancreatic β cells to be permeated BBB [35, 36], but the involvement of LRP2 in the process was not identified.
In the present study, we assessed the expression of LRP2 and its function in endocytosis activity of microbeads, Aβ and insulin using cultured astrocytes and mouse microglial cell line BV-2. Further, we also investigated the effects of lipopolysaccharide (LPS) and all-trans retinoic acid (ATRA) on LRP2 expression and the endocytosis activity. LPS is a commonly used activator of astrocytes and microglia in experiments [37, 38]. ATRA is known to induce proliferation and differentiation of various cells [39, 40] and decrease the activation of astrocytes and microglia [41].
Experimental Procedures
Materials
Deoxyribonuclease I (DNase I), trypsin, anti-Aβ antibody, anti-glial fibrillary acidic protein (GFAP) antibody, and fluorescein isothiocyanate (FITC)-conjugated insulin were purchased from Sigma Chemical Co. (St. Louis, MA, USA). Dulbecco’s modified Eagle medium (DMEM) and horse serum were from Gibco BRL (Grand Island, NY, USA). Fetal bovine serum (FBS) was from GE Healthcare Inc. (Little Chalfont, Bucks, UK). Aβ1− 42 was from Peptide Institute, Inc. (Osaka, Japan). Rat and mouse LRP2 primers and 18S rRNA primer were from Eurofins Genomics Inc. (Tokyo, Japan). Control siRNA and LRP2 siRNA were from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). HilyMax® was from Dojindo (Kumamoto, Japan). FITC-conjugated anti-mouse IgG antibody and rhodamine-conjugated anti-rabbit IgG antibody (Jackson ImmunoResearch, PA, USA).
Preparation of Astrocytes Culture
This study was carried out in compliance with the Guideline for Animal Experimentation at Osaka Prefecture University, with an effort to minimize the number of animals used and their suffering. Astrocytes were prepared as described previously [37]. In brief, cortex from 20-day-old embryos, which were taken out from pregnant Wistar rats deeply anesthetized, were cleared of meninges, cut into about 1-mm3 blocks, and treated with 0.25% trypsin in Ca2+, Mg2+-free phosphate-buffered saline (PBS) containing 5.5 mM glucose for 20 min at 37˚C with gentle shaking. An equal volume of horse serum supplemented with 0.1 mg/mL of DNase I was added to the medium to inactivate the trypsin. Then, the tissues were centrifuged at 350 × g for 5 min. The tissue sediments were triturated through a pipette with DMEM containing 10% FBS, 50,000 units/L penicillin and 100 mg/L streptomycin. After filtering cell suspensions through a lens-cleaning paper (Fujifilm Co., Tokyo, Japan), the cells were plated on polyethyleneimine-coated 100-mm diameter plastic dishes (Iwaki, Asahi Glass Co., Tokyo, Japan) at a density of 0.8–1.3 × 105 cells/cm2. Cultures were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37˚C with changing medium every 3 days. After one week, astrocytes were replated to remove neurons. On days 14–21, they were replated onto adequate plates or dishes using an ordinary trypsin-treatment technique at a density of 0.8–1.3 × 105 cells cells/cm2, and stabilized for 1 day, then we used for experiments.
More than 90% of the cells were immunoreactively positive to an astrocyte marker, glial fibrillary acidic protein (GFAP), using the antibody (Biosensis Pty Ltd., Thebarton, Australia) and FITC-conjugated anti-mouse IgG antibody. Less than 10% of the cells were positive to a microglial marker, Iba-1; using the antibody (Wako) and rhodamine-conjugated anti-rabbit IgG antibody.
BV-2 Cell Culture
Mouse microglial cell line BV-2 cells were grown and maintained in DMEM supplemented with 10% FBS and penicillin (50,000 units/L)/streptomycin (100 mg/L) at 37 °C in a humidified incubator under 5% CO2. Cells were subcultured once a week. They were replated onto adequate plates or dishes using an ordinary trypsin-treatment technique at a density of 0.8–1.3 × 105 cells/cm2, and stabilized for 1 day, then we used for experiments. BV-2 cells were kindly provided from Laboratory of Molecular Pharmacology, Kanazawa University Graduate School.
Realtime Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
For realtime RT-PCR assays, the cells were replated onto 12-well plate (Iwaki, Japan) and stabilized for 1 day in medium containing 10% FBS. And then, the cells were incubated for 24 h and stimulated for 24 h in serum-free medium. Total RNA was isolated using FavorPrep™ Tissue Total RNA Purification Mini Kit (Favorgen, Taiwan). Complementary DNA was prepared using Omniscript Reverse Transcription kit (Qiagen, Germany) according to the manufacturer’s protocol. PCR experiments were done using SYBR® Green Realtime PCR Master Mix (Toyobo, Japan) for LRP2 and 18S rRNA. Table 1 shows the list of the sequence of the primers (Eurofins Genomics, Japan) and cycling protocols used in this study. Detection of the fluorescent product was carried out at the end of the extension period. To compare mRNA levels among samples, mRNA for each gene of interest was normalized to the expression of a housekeeping gene, 18S rRNA using the comparative Ct method. The changes in the levels of each PCR product were calculated as ratio of control.
Immunostaining
The cells were fixed by 4% paraformaldehyde for 30 min at room temperature, and permeabilized with 0.5% Triton X-100 in PBS for 30 min at room temperature. After blocking with 1% bovine serum albumin (BSA) and 4% normal goat serum in PBS for 30 min, the cells were incubated with antibodies against Aβ (1:100), and GFAP (1:750) in blocking buffer at 4ºC overnight followed by an incubation with FITC-conjugated anti-mouse IgG antibody (1:50) or rhodamine-conjugated anti-rabbit IgG antibody (1:50) at room temperature for 3 h. Then, nuclear stain was performed using DAPI for 10 min, and the cells were mounted with SlowFade® Diamond (Molecular Probes). To visualize fluorescent immunostaining, Olympus IX70 microscope was used.
Endocytosis Assay
Endocytosis was assessed by uptake of carboxylate modified fluorescent microspheres (diameter = 1 μm) (Molecular Probes, OR, U.S.A.). 10 µL of fluorescent microspheres was mixed with 5 µL FBS, and diluted 100 times in medium containing 10% FBS (microbeads solution). After the BV-2 cells were stimulated, 50 µL/well of microbeads solution (final concentration; 0.013% solid) was added to the cells and incubated for 1 h. Then the cells were washed by DMEM three times to remove non-endocytosed beads, and observed under a fluorescent microscopy (Olympus IX70). The photographs of phase contrast and fluorescent images were taken at each same site. More than 20 endocytotic cells in each photograph were picked up to measure cellular fluorescence intensity by Image J software, and average of the intensity was evaluated.
Uptake of Aβ or Insulin
Uptake of Aβ: Aβ1−42 was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 1 mM as a stock solution and kept at − 80˚C before using. The serum-free DMEM was added with Aβ stock solution at the concentration of 10 µM and incubated for 14 days at 37˚C (aggregated Aβ), then used for Aβ uptake. After the cell stimulation, 0.1 µM Aβ was added to the cells and incubated for 6 h. Then the cells were washed by PBS and immunostained as descried above. In cultured astrocytes, the total numbers of GFAP-positive cells and Aβ-positive cells were counted and the ratio of Aβ and GFAP double-positive cells per GFAP-positive cells was calculated. In BV-2 cells, because almost every cells endocytosed Aβ, more than 20 cells in each photograph were picked up to measure cellular fluorescence intensity by Image J software, and average of the intensity was evaluated.
Uptake of insulin: After stimulation, 50 nM FITC-conjugated insulin was added to the cells and incubated for 30 min. Then the cells were washed by PBS, and fixed by 4% paraformaldehyde. After washing by PBS, the cells were observed under a fluorescent microscope (Olympus IX70). Total cells and FITC fluorescence superposed cells were counted.
Transfection
For knockdown of LRP2 expression, siRNA was transfected using HilyMax® (Dojindo) according to the manufactures’ protocol. In brief, 20 pmol LRP2 siRNA or negative control (CTL) siRNA was mixed with serum-free DMEM, and HilyMax solution was added and incubated for 15 min at room temperature to form siRNA-HilyMax complex.
BV-2 cells were replated onto 24 well plate (Iwaki) and stabilized for 1 day. siRNA-HilyMax complex was added to the cells in serum-free DMEM and incubated for 24 h. And then, the stimulation for 24 h in each experiment was performed.
Data Analysis
For statistical analysis of the data, one-way ANOVA followed by Tukey-Kramer or Dunnet multiple comparison procedure or Student’s t test was used. Differences between treatments were considered statistically significant when p < 0.05.
Results
Changes of LRP2 mRNA Expression in Cultured Astrocytes
To assess the changes of LRP2 expression with various stimulation in cultured astrocytes, the cells were incubated with LPS (0.1 and 1.0 µg/mL) and ATRA (10 µM) for 24 h in serum-free medium, and the expression of LRP2 mRNA was evaluated by realtime RT-PCR. The expression of LRP2 was not significantly changed by ATRA (Fig. 1B), but it was significantly decreased by LPS in a dose-dependent manner (Fig. 1A).
Expression of LRP2 mRNA in cultured astrocytes treated with LPS or ATRA. Cultured astrocytes were treated with 0.1, 1.0 µg/mL LPS (A) and 10 µM ATRA (B) for 24 h. The expression of mRNAs of LRP2 and 18S rRNA were assessed by realtime RT-PCR procedure. Data are mean ± S.D. of repeated 3–10 experiments. **p < 0.01, significantly different from control (CTL) by Dunnet multiple comparison procedure
Uptake of Aβ and Insulin was Decreased by LPS Stimulation in Cultured Astrocytes
We examined the uptake of Aβ and insulin in cultured astrocytes to assess the effect of LRP2 expression changed by LPS. After the stimulation by 1.0 µg/mL LPS for 24 h, the cells were incubated with 0.1 µM Aβ for 6 h and the uptake of Aβ was assessed by immunostaining. The ratio of Aβ and GFAP double-positive cells per GFAP-positive cells was decreased by LPS stimulation (Fig. 2A). Similarly, after the stimulation by 1.0 µg/mL LPS for 24 h, the cells were incubated with 50 nM FITC-conjugated insulin for 30 min and the uptake of insulin was assessed under a fluorescent microscope. The ratio of FITC-conjugated insulin-positive cells per total cells was decreased by LPS stimulation (Fig. 2B).
Uptake of Aβ and insulin in cultured astrocytes treated with LPS. Cultured astrocytes were treated with 1.0 µg/mL LPS for 24 h. (A) The uptake of Aβ by cultured astrocytes for 6 h was evaluated by immunostaining. The numbers of Aβ-positive cells and GFAP-positive astrocytes were counted (n = 25–289). The graph shows the ratio of Aβ and GFAP double-positive cells per GFAP-positive cells. The photograph shows representative fluorescent images at each same site. (B) The uptake of insulin by cultured astrocytes for 30 min was evaluated. The numbers of FITC-conjugated insulin-positive cells and total cells were counted (n = 81–748). The graph shows the ratio of FITC-positive cells per total cells. The photograph shows representative phase contrast (PhC) and fluorescent images at each same site. Scale bar = 30 μm. Data are mean ± S.D. of repeated three experiments: ##p < 0.01, significantly different from control (CTL) by Student’s t test
Changes of LRP2 mRNA Expression in BV-2 Cells
Next, we assessed in BV-2 cells the changes of LRP2 expression with various stimulation. The cells were incubated with LPS (0.001-1.0 µg/mL) and ATRA (1.0 and 10 µM) for 24 h in serum-free medium, and the expression of LRP2 mRNA was evaluated by realtime RT-PCR. The expression of LRP2 was significantly increased by LPS at 0.1 and 1.0 µg/mL (Fig. 3A), and it was also significantly increased by 1.0 and 10 µM ATRA dose-dependently (Fig. 3B).
Expression of LRP2 mRNA in BV-2 cells treated with LPS or ATRA. BV-2 cells were treated with various concentrations of LPS (A) and ATRA (B) for 24 h. The expression of mRNAs of LRP2 and 18S rRNA were assessed by realtime RT-PCR procedure. Data are mean ± S.D. of repeated 3–9 experiments. **p < 0.01, significantly different from control (CTL) by Dunnet multiple comparison procedure. ##p < 0.01, significantly different from control (CTL) by Student’s t test
Uptake of Aβ and Insulin was Increased by LPS and ATRA Stimulation in BV-2 Cells
As described above, the expression of LRP2 mRNA was increased by LPS and ATRA treatments. Then, we examined the endocytosis activity of microbeads and the uptake of Aβ and insulin in BV-2 cells. After the cells were treated with 0.1 µg/mL LPS or 1.0 µM ATRA for 24 h, the endocytosis of fluorescent microbeads was assessed. The endocytosis activity was significantly increased by LPS and ATRA (Fig. 4A). Similarly, after the stimulation by LPS or ATRA for 24 h, the cells were incubated with 0.1 µM Aβ for 6 h and the uptake of Aβ was assessed by immunostaining. The cellular fluorescence of Aβ-positive cells was increased by LPS and ATRA (Fig. 4B). Moreover, after stimulation, the cells were incubated with 50 nM FITC-conjugated insulin for 30 min and the uptake of insulin was also assessed under a fluorescent microscope. The ratio of FITC-conjugated insulin-positive cells per total cells was increased by LPS and ATRA stimulation (Fig. 4C).
Uptake of microbeads, Aβ and insulin in BV-2 cells treated with LPS or ATRA. BV-2 cells were treated with 0.1 µg/mL LPS or 1.0 µM ATRA for 24 h. (A) The uptake of fluorescent microbeads for 1 h was evaluated. The graph shows level of fluorescence per cell. The photograph shows representative phase contrast and fluorescent images at each same site. (B) The uptake of Aβ for 6 h was evaluated by immunostaining. The graph shows level of fluorescence per cell. The photograph shows representative phase contrast and fluorescent images at each same site. (C) The uptake of insulin for 30 min was evaluated. The numbers of FITC-conjugated insulin-positive cells and total cells were counted (n = 179–1433). The graph shows the ratio of FITC-positive cells per total cells. The photograph shows representative phase contrast and fluorescent images at each same site. Scale bar = 30 μm. Data are mean ± S.D. of repeated 3–5 experiments: **p < 0.01, significantly different from control (CTL) by Tukey-Kramer multiple comparison procedure. ##p < 0.01, significantly different from control (CTL) by Student’s t test
LRP2 was Associated with Endocytosis Activity and Uptake of Aβ and Insulin in BV-2 Cells
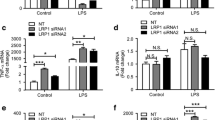
To evaluate whether LRP2 might play roles in endocytosis and uptake of Aβ and insulin we studied using LRP2 knockdown in BV-2 cells. After LRP2 siRNA and negative control (CTL) siRNA were transfected in BV-2 cells for 24 h, the cells were treated with 0.1 µg/mL LPS or 1.0 µM ATRA for 24 h, and the endocytosis of fluorescent microbeads and the uptake of Aβ and insulin were assessed. To confirm the reduction of LRP2 by siRNA, the expression of LRP2 mRNA was evaluated by realtime RT-PCR. Figure 5A showed in control group that LRP2 siRNA decreased LRP2 mRNA to about 60% compared with CTL siRNA (Fig. 5A). The endocytosis activity was significantly increased by LPS and ATRA in BV-2 cells transfected with control siRNA, while the increased endocytosis activity was completely suppressed in BV-2 cells transfected with LRP2 siRNA (Fig. 5B). The uptake of Aβ and insulin was also significantly increased by LPS and ATRA stimulation in BV-2 cells with control siRNA, and that was absolutely inhibited in BV-2 cells with LRP2 siRNA (Fig. 5C, D).
Uptake of microbeads, Aβ and insulin in BV-2 cells transfected with LRP2 siRNA. BV-2 cells were transfected with negative control (CTL) and LRP2 siRNA for 24 h and treated with 0.1 µg/mL LPS or 1.0 µM ATRA for 24 h. (A) To confirm the knockdown of LRP2, the expression of mRNAs of LRP2 and 18S rRNA were assessed by realtime RT-PCR procedure. Data are mean ± S.D. of repeated five samples. (B) The uptake of fluorescent microbeads for 1 h was evaluated. The graph shows level of fluorescence per cell. The photograph shows representative phase contrast and fluorescent images at each same site. (C) The uptake of Aβ for 6 h was evaluated by immunostaining. The graph shows level of fluorescence per cell. The photograph shows representative phase contrast and fluorescent images at each same site. (D) The uptake of insulin for 30 min was evaluated. The numbers of FITC-conjugated insulin-positive cells and total cells were counted (n = 105–1507). The graph shows the ratio of FITC-positive cells per total cells. The photograph shows representative phase contrast and fluorescent images at each same site. Scale bar = 30 μm. Data are mean ± S.D. of repeated 3–4 experiments: **p < 0.01, significantly different from control (CTL) and $$p < 0.01, significantly different from CTL siRNA by Tukey-Kramer multiple comparison procedure
Discussion
In the present study, we demonstrated that LPS decreased LRP2 expression and the uptake of Aβ and insulin in cultured astrocytes. On the other hand, LPS increased LRP2 expression and the uptake of microbeads, Aβ and insulin in BV-2 microglial cell line. ATRA also increased LRP2 expression and the uptake activity in BV-2 cells. LPS- and ATRA-increased the uptake activity in BV-2 cells was reduced by knockdown of LRP2. These results suggest that LRP2 expressed in glial cells might be involved in the uptake of microbeads, Aβ and insulin.
In the present study, we found that LRP2 mRNA expressions in cultured astrocytes (Fig. 1) and BV-2 cells (Fig. 3). Also in cultured microglia prepared from neonatal rat whole brain, LRP2 mRNA expression was confirmed (data not shown); therefore, at least mRNA levels of LRP2 expression in astrocytes and microglia were clarified. Unfortunately, we could not detect protein levels of LRP2 expression in these cells by western blot and immunocytochemistry analysis using commercial LRP2 antibodies. It was reported in mouse cultured astrocytes that LRP2 expression could not be detected using semi-quantitative PCR and immunocytochemistry [17]. The expression of LRP2 in glial cells might be limited and further investigation for LRP2 protein expression was needed.
LPS decreased LRP2 mRNA expression in cultured astrocytes (Fig. 1) and LPS and ATRA increased LRP2 expression in BV-2 cells (Fig. 3). Although transcription factor binding sites that are important for LRP2 expression have been identified [42], little is known about regulation of LRP2 mRNA expression [1]. Increased LRP2 expression was observed in neurons associated with AD that accumulate ApoE [13]. It was reported that oxidative stress increased LRP2 expression in renal proximal tubular cells [43] and LPS stimulation increased intracellular reactive oxygen species (ROS) production in microglia [44]. Therefore, oxidative stress might be involved in LPS-increased LRP2 expression in BV-2 cells. LPS stimulation has been commonly used to activate glial cells in vitro experiments [37] and it has been reported that LPS binds to toll-like receptor (TLR) and activates both microglia and astrocytes through nuclear factor-kappa B (NF-κB) signaling pathway [45, 46]. However, LRP2 mRNA expression in cultured astrocytes was decreased and that in BV-2 cells was increased by LPS in the present study. The downstream signaling of LPS for regulation of LRP2 mRNA expression would be considered in our future study.
It was reported that LRP2 expression in vitro was stimulated by retinoic acid (RA), dibutyryl cyclic AMP, and vitamin D [47, 48]. ATRA increased LRP2 expression and induced differentiation in prostate and colon epithelial cells [49]. Consistent with these reports, ATRA increased LRP2 expression in BV-2 cells in the present study (Fig. 3). On the other hand, LRP2 expression in cultured astrocytes was not significantly changed by ATRA treatments (Fig. 1). RA, ATRA and 9-cis RA, are metabolites of vitamin A that can modify the proliferation and differentiation of a variety of cells [39]. ATRA and 9-cis RA bind to the RA receptors (RARα, β, and γ isotypes), whereas 9-cis RA binds to the retinoid receptors (RXRα, β, and γ isotypes), to regulate the expressions of various target genes [40]. RAR was thought to bind to RA-response elements (RAREs) in response to ATRA and promote transcription through interaction with coactivator [40]. It was known that astrocytes and microglia recognized ATRA via RAR/RXR causing functional changes [50, 51]. It was also reported that astrocytes might be a regulated source of RA for the brain because the expression of retinal dehydrogenase, an enzyme for RA synthesis, in astrocytes changed according to the RA concentration [52]. Different changes of LRP2 expression in astrocytes and microglia by ATRA might be associated with ATRA concentration containing in the medium or using as the treatments in the present study. Further investigation was needed for the mechanisms of changes in LRP2 expression by ATRA.
ATRA had an anti-inflammatory function, decreasing the activation of astrocytes and microglia to inhibit the production of nitric oxide (NO) and inflammatory cytokines [53]. It was also known that ATRA had direct effects on neurogenesis to induce the differentiation of neuronal stem cell [50]. Therefore, ATRA might be a putative candidate drug for the treatment of patients with AD. The concentration of RA was reported as about 15 µM in human serum, 5 µM in rat serum, and 0.04–0.75 µM in rat tissues [54]. Therefore, the concentration of ATRA used in the present study could occur in vivo. In our preliminary experiments, 1–10 µM ATRA decreased LPS-induced NO production in cultured astrocytes and BV-2 cells consistent with previous research [41]. It was suggested that ATRA might inhibit LPS-induced NF-κB activation [55]. However, both LPS and ATRA increased LRP2 expression in BV-2 cells in the present study (Fig. 3). The regulation of LRP2 mRNA might be associated with transcriptional factor other than NF-κB and/or other mechanisms might be involved.
In the endocytosis assay of microbeads, Aβ and insulin, LPS decreased the uptake of Aβ and insulin in cultured astrocytes and LPS and ATRA increased the endocytosis activity in BV-2 cells correlated with LRP2 expression (Figs. 1, 2, 3 and 4). In addition, LRP2 knockdown by siRNA in BV-2 cells abolished the LPS- and ATRA-increased endocytosis of microbeads, Aβ and insulin (Fig. 5). Therefore, LRP2 might be involved in the endocytosis activity in microglia and related in astrocytes.
In the endocytosis of microbeads, no reports indicated direct interaction with LRP2. It has been reported that LRP2 binds to retinol binding protein and might be associated with the metabolism of vitamin A [56]. LRP2 was reported in macrophages to be a scavenger receptor endocytosing LDL [57]. Intracellular domain of LRP2 was cleaved and translocated into nuclear, and might interact with some transcription factors and regulate the expression of target genes [3]. Therefore, LPS- and ATRA-increased LRP2 expression in BV-2 cells might be involved in endocytosis through other related molecules. In our previous study, LPS and ATRA increased transglutaminase 2 expression and endocytosis of microbeads in BV-2 cells [in press]. It was suggested that LPS-increased transglutaminase 2 expression might be involved in endocytosis of microbeads [38].
Abnormal accumulation and aggregation of Aβ in brain is a characteristic pathology in AD, and it is reported in sporadic AD that Aβ production rate is not different from healthy brain while Aβ clearance is impaired [58]. Therefore, to elucidate the mechanism of Aβ uptake in astrocytes and microglia would be important for inhibition of Aβ accumulation. Several candidate molecules for Aβ uptake were reported; LRP, receptor of advanced glycation end product (RAGE), CD36 and CD47 [59]. LRP1 in astrocytes and microglia is known to mediate the uptake of Aβ [60, 61]. In the present study, the changes in LPR2 expressions after LPS and ATRA treatments had same direction of changes in the uptake of Aβ in both cultured astrocytes and BV-2 cells. LRP2 knockdown by siRNA in BV-2 cells abolished the LPS- and ATRA-increased endocytosis of Aβ. Therefore, not only LRP1 but also LRP2 might be associated with Aβ transport and clearance by microglia.
It was reported in kidney that LRP2 might play a significant role as a renal reabsorption receptor for the uptake of insulin [29]. However, none was known about the roles of LRP1 and LRP2 in insulin transport into brain. The results of present study suggested that LRP2 might be associated with endocytosis of insulin in astrocytes and microglia, similar to Aβ uptake. It has been reported that LRP1 in neurons interacts with insulin receptor and is involved in glucose transport [62]. The concentration of insulin in human cerebrospinal fluid was about 1 pM [63]. Increase of insulin levels augmented insulin resistance [64] and insulin resistance might be associated with the pathogenesis of AD [65]. Endocytosis of insulin via LRP2 in microglia might control the extracellular insulin levels in brain.
In conclusions, LRP2 expression in astrocytes and microglia might be related to endocytosis activity. Interestingly, activation by LPS caused opposite effects on LRP2 expression and endocytosis activities between astrocytes and microglia. In addition, both LPS, an activator, and ATRA, an inhibitor, of inflammatory responses of glial cells increased LRP2 expression and endocytosis in BV-2 cells. LRP2 expression and its regulations in glial functions would be complicated. LRP2 expression might be associated with AD pathology through the regulation of Aβ and insulin levels in brain and glial function; however, further investigation was needed to enclose the roles of LRP2 in AD pathology.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- Aβ:
-
Amyloid beta
- AD:
-
Alzheimer’s disease
- ApoE:
-
Apolipoprotein E
- APP:
-
Amyloid precursor protein
- ATRA:
-
All-trans retinoic acid
- BBB:
-
Blood brain barrier
- CNS:
-
Central nervous system
- DMEM:
-
Dulbecco’s modified Eagle medium
- FBS:
-
Fetal bovine serum
- FITC:
-
Fluorescein isothiocyanate
- GFAP:
-
Glial fibrillary acidic protein
- gp:
-
Glycoprotein
- iNOS:
-
Inducible nitric oxide synthase
- LDL:
-
Low density lipoprotein
- LDLR:
-
Low density lipoprotein receptor
- LPS:
-
Lipopolysaccharide
- LRP:
-
Low density lipoprotein receptor-related protein
- NF-κB:
-
Nuclear factor-kappa B
- NO:
-
Nitric oxide
- PBS:
-
Phosphate buffered saline
- RA:
-
Retinoic acid
- RAGE:
-
Receptor of advanced glycation end product
- RAR:
-
Retinoic acid receptor
- RARE:
-
Retinoic acid response element
- ROS:
-
Reactive oxygen species
- RXR:
-
Retinoid receptor
- TLR:
-
Toll-like receptor
References
Christensen EI, Birn H (2002) Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol 3(4):256–266. https://doi.org/10.1038/nrm778
Beenken A, Gabriele Cerutti G, Brasch J, Guo Y, Sheng Z, Erdjument-Bromage H, Aziz Z, Robbins-Juarez SY, Chavez EY, Ahlsen G, Katsamba PS, Neubert TA, Fitzpatrick AWP, Barasch J, Shapiro L (2023) Structures of LRP2 reveal a molecular machine for endocytosis. Cell 186(4):821–836. https://doi.org/10.1016/j.cell.2023.01.016
Spuch C, Ortolano S, Navarro C (2012) LRP-1 and LRP-2 receptors function in the membrane neuron. Trafficking mechanisms and proteolytic processing in Alzheimer’s disease. Front Physiol 3:Article number 269. https://doi.org/10.3389/fphys.2012.00269
Herz J, Bock HH (2002) Lipoprotein receptors in the nervous system. Ann Rev Biochem 71:405–434. https://doi.org/10.1146/annurev.biochem.71.110601.135342
Bruno E, Quattrocchi G, Nicoletti A, Le Pira F, Maci T, Mostile G, Andreoli V, Quattrone A, Zappia M (2010) Lack of interaction between LRP1 and A2M polymorphisms for the risk of Alzheimer’s disease. Neurosci Lett 482:112–116. https://doi.org/10.1016/j.neulet.2010.07.012
Boucher P, Herz J (2011) Signaling through LRP1, protection from atherosclerosis and beyond. Biochem Pharmacol 81:1–5. https://doi.org/10.1016/j.bcp.2010.09.018
Andersen OM, Willnow TE (2006) Lipoprotein receptors in Alzheimer’s disease. Trends Neurosci 29:687–694. https://doi.org/10.1016/j.tins.2006.09.002
Rebeck GW, Harr SD, Strickland DK, Hyman BT (1995) Multiple, diverse senile plaque-associated proteins are ligands of an apolipoprotein E receptor, the alpha 2-macroglobulin receptor/low-density-lipoprotein receptor related protein. Ann Neurol 37:211–217. https://doi.org/10.1002/ana.410370212
Marzolo MP, von Bernhardi R, Bu G, Inestrosa NC (2000) Expression of α2-macroglobulin receptor/low density lipoprotein receptor-related protein (LRP) in rat microglial cells. J Neurosci Res 60(3):401–411 doi: 10.1002/(SICI)1097-4547(20000501)60:3<401::AID-JNR15>3.0.CO;2-L
Mauch DH, Nägier K, Schumacher S, Göritz C, Müller EC, Otto A, Pfrieger FW (2001) CNS synaptogenesis promoted by glia-derived cholesterol. Science 294(5545):1354–1357. https://doi.org/10.1126/science.294.5545.1354
Pietrzik CU, Busse T, Merriam DE, Weggen S, Koo EH (2002) The cytoplasmic domain of the LDL receptor-related protein regulates multiple steps in APP processing. EMBO J 21(21):5691–5700. https://doi.org/10.1093/emboj/cdf568
Kuhbandner K, Herz J, Pohlkamp T (2021) Chap. 18 - lipoprotein receptors. Biochemistry of lipids, lipoproteins and membranes. (Seventh Edition) 583–622. https://doi.org/10.1016/B978-0-12-824048-9.00018-3
LaFerla FM, Troncoso JC, Strickland DK, Kawas CH, Jay G (1997) Neuronal cell death in Alzheimer’s disease correlates with apoE uptake and intracellular Aβ stabilization. J Clin Invest 100(2):310–320. https://doi.org/10.1172/JCI119536
Bento-Abreu A, Velasco A, Polo-Hernández E, Pérez-Reyes PL, Tabernero A, Medina JM (2008) Megalin is a receptor for albumin in astrocytes and is required for the synthesis of the neurotrophic factor oleic acid. J Neurochem 106(3):1149–1159. https://doi.org/10.1111/j.1471-4159.2008.05462.x
Bento-Abreu A, Velasco A, Polo-Hernández E, Lillo C, Kozyraki R, Tabernero A, Medina JM (2009) Albumin endocytosis via megalin in astrocytes is caveola- and dab-1 dependent and is required for the synthesis of the neurotrophic factor oleic acid. J Neurochem 111(1):49–60. https://doi.org/10.1111/j.1471-4159.2009.06304.x
Mulder SD, Veerhuis R, Blankenstein MA, Nielsen HM (2012) The effect of amyloid associated proteins on the expression of genes involved in amyloid-β clearance by adult human astrocytes. Exp Neurol 233(1):373–379. https://doi.org/10.1016/j.expneurol.2011.11.001
Alvira-Botero X, Pérez-Gonzalez R, Spuch C, Vargas T, Antequera D, Garzón M, Bermejo-Pareja F, Carro E (2010) Megalin interacts with APP and the intracellular adapter protein FE65 in neurons. Mol Cell Neurosci 45(3):306–315. https://doi.org/10.1016/j.mcn.2010.07.005
Page KJ, Hollister RD, Hyman BT (1998) Dissociation of apolipoprotein and apolipoprotein receptor response to lesion in the rat brain: an in situ hybridization study. Neuroscience 85(4):1161–1171. https://doi.org/10.1016/s0306-4522(97)00661-1
Kandimalla R, Thirumala V, Reddy PH (2016) Is Alzheimer’s disease a type 3 diabetes? A critical appraisal. Biochim Biophys Acta 1863(5):1078–1089. https://doi.org/10.1016/j.bbadis.2016.08.018
Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe D (2011) Soluble amyloid beta protein dimers isolated from Alzheimer cortex directly induce tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci USA 108:5819–5824. https://doi.org/10.1073/pnas.1017033108
Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA (2010) Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid β peptide elimination from the brain. J Neurochem 115(5):1077–1089. https://doi.org/10.1111/j.1471-4159.2010.07002.x
Zlokovic BV (2004) Clearing amyloid through the blood-brain barrier. J Neurochem 89(4):807–811. https://doi.org/10.1111/j.1471-4159.2004.02385.x
Nuutinen T, Suuronen T, Kauppinen A, Salminen A (2009) Clusterin: a forgotten player in Alzheimer’s disease. Brain Res Rev 61(2):89–104. https://doi.org/10.1016/j.brainresrev.2009.05.007
Liu RX, Huang C, Benett DA, Li H, Wang R (2016) The characteristics of astrocyte on Aβ clearance altered in Alzheimer’s disease were reversed by anti-inflammatory agent (+)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeate. Am J Transl Res 8(10):4082–4094. PMID: 27829994, PMCID: PMC5095303, Free PMC article
Nieoullon A (2011) Neurodegenerative diseases and neuroprotection: current views and prospects. J Appl Biomed 9:173–183. https://doi.org/10.2478/v10136-011-0013-4
Jaeger LB, Dohgu S, Hwang MC, Farr SA, Murphy MP, Fleegal-Demotta MA, Lynch JL, Robinson SM, Niehoff ML, Johnson SN, Kumar VB, Banks WA (2009) Testing the neurovascular hypothesis of Alzheimer’s disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-β protein, and impairs cognition. J Alzheimer’s Dis 17(3):553–570. https://doi.org/10.3233/JAD-2009-1074
Dietrich M, Antequera D, Pascual C, Castro N, Bolos M, Carro E (2014) Alzheimer’s disease-like impaired cognition in endothelial-specific megalin-null mice. J Alzheimer’s Dis 39(4):711–717. https://doi.org/10.3233/JAD-131604
Pascale CL, Miller MC, Chiu C, Boylan M, Caralopoulos IN, Gonzalez L, Johanson CE, Silverberg GD (2011) Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent. Fluids Barriers CNS 8(1): Article number 21. https://doi.org/10.1186/2045-8118-8-21
Orlando RA, Rader K, Authier F, Yamazaki H, Posner BI, Bergeron JJM, Farquhar MG (1998) Megalin is an endocytic receptor for insulin. J Am Soc Nephrol 9(10):1759–1766. https://doi.org/10.1681/ASN.V9101759
Nemoto T, Toyoshima-Aoyama F, Yanagita T, Maruta T, Fujita H, Koshida T, Yonaha T, Wada A, Sawaguchi A, Murakami M (2013) New insights concerning insulin synthesis and its secretion in rat hippocampus and cerebral cortex: amyloid β1–42-induced reduction of proinsulin level via glycogen synthase kinase-3β. Cell Signal 26:253–259. https://doi.org/10.1016/j.cellsig.2013.11.017
Zhao WQ, Fernanda G, Sara F, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL (2008) Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J 22:246–260. https://doi.org/10.1096/fj.06-7703com
Tokutake T, Kasuga K, Yajima R, Sekine Y, Tezuka T, Nishizawa M, Ikeuchi T (2012) Hyperphosphorylation of tau induced by naturally secreted amyloid-β at nanomolar concentrations is modulated by insulin-dependent Akt-GSK3β signaling pathway. J Biol Chem 287(42):35222–35233. https://doi.org/10.1074/jbc.M112.348300
Craft S, Peskind E, Schwartz W, Gerard D, Raskind M, Porte D Jr (1998) Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease. Neurology 50(1):164–168. https://doi.org/10.1212/wnl.50.1.164
Takano K, Koarashi K, Kawabe K, Itakura M, Nakajima H, Moriyama M, Nakamura Y (2018) Insulin expression in cultured astrocytes and the decrease by amyloid β. Neurochem Int 119:171–177. https://doi.org/10.1016/j.neuint.2017.10.017
Banks WA (2004) The source of cerebral insulin. Eur J Pharmacol 490(1–3):5–12. https://doi.org/10.1016/j.ejphar.2004.02.040
de la Monte SM (2017) Insulin resistance and neurodegeneration: Progress towards the development of new therapeutics for Alzheimer’s disease. Drugs 77(1):47–65. https://doi.org/10.1007/s40265-016-0674-0
Takano K, Shiraiwa M, Moriyama M, Nakamura Y (2010) Transglutaminase 2 expression induced by lipopolysaccharide stimulation together with NO synthase induction in cultured astrocytes. Neurochem Int 57(7):812–818. https://doi.org/10.1016/j.neuint.2010.08.019
Kawabe K, Takano K, Moriyama M, Nakamura Y (2015) Lipopolysaccharide-stimulated transglutaminase 2 expression enhances endocytosis activity in the mouse microglial cell line BV-2. Neuroimmunomodulation 22:243–249. https://doi.org/10.1159/000365484
Kaur C, Sivakumar V, Dheen ST, Ling EA (2006) Insulin-like growth factor I and II expression and modulation in amoeboid microglial cells by lipopolysaccharide and retinoic acid. Neuroscience 138:1233–1244. https://doi.org/10.1016/j.neuroscience.2005.12.025
Lo HM, Wang SW, Chen CL, Wu PH, Wu WB (2014) Effects of all-trans retinoic acid, retinol, and β-carotene on murine macrophage activity. Food Funct 5:140–148. https://doi.org/10.1039/c3fo60309a
Takamura R, Watamura N, Nikkuni M, Ohshima T (2017) All-trans retinoic acid improved impaired proliferation of neural stem cells and suppressed microglial activation in the hippocampus in an Alzheimer’s mouse model. J Neurosci Res 95:897–906. https://doi.org/10.1002/jnr.23843
Zhao J, Oleinikov AV, Oleinikova I, Makker SP (2001) Functional characterization of rat gp600/megalin promoter: combination of proximal Sp1 site and JCV repeat is important in rat gp600/megalin promoter activation. Gene 265:123–131. https://doi.org/10.1016/s0378-1119(01)00351-1
Kurosaki Y, Imoto A, Yokoba M, Takenaka T, Katagiri M, Ichikawa T, Ishii T (2017) Oxidative stress increases megalin expression through PI3K/Akt signaling in renal proximal tubular cells. Nephrol Dialysis Transplant 32(3):iii613. https://doi.org/10.1152/ajprenal.00108.2017
Qin L, Liu Y, Hong J-S, Crews FT (2013) NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia 61(6):855–868. https://doi.org/10.1002/glia.22479
Lee SY, Son DJ, Lee YK, Lee JW, Lee HJ, Yun YW, Ha TY, Hong JT (2006) Inhibitory effect of sesaminol glucosides on lipopolysaccharide-induced NF-κB activation and target gene expression in cultured rat astrocytes. Neurosci Res 56:204–212. https://doi.org/10.1016/j.neures.2006.06.005
Zhao J, Bi W, Xiao S, Lan X, Cheng X, Zhang J, Lu D, Wei W, Wang Y, Li H, Fu Y, Zhu L (2019) Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep 9(1):5790. https://doi.org/10.1038/s41598-019-42286-8
Stefansson S, Chappell DA, Argraves KM, Strickland DK, Argraves WS (1995) Glycoprotein 330/low density lipoprotein receptor-related protein-2 mediates endocytosis of low density lipoproteins via interaction with apolipoprotein B100. J Biol Chem 270(33):19417–19421. https://doi.org/10.1074/jbc.270.33.19417
Liu W, Yu WR, Carling T, Juhlin C, Rastad J, Ridefelt P, Akerström G, Hellman P (1998) Regulation of gp330/megalin expression by vitamins a and D. Eur J Clin Invest 28(2):100–107. https://doi.org/10.1046/j.1365-2362.1998.00253.x
Ternes SB, Rowling MJ (2013) Vitamin D transport proteins megalin and disabled-2 are expressed in prostate and colon epithelial cells and are induced and activated by all-trans-retinoic acid. Nutr Cancer 65(6):900–907. https://doi.org/10.1080/01635581.2013.805422
Hellmann-Regen J, Kronenberg G, Uhlemann R, Freyer D, Endres M, Gertz K (2013) Accelerated degradation of retinoic acid by activated microglia. J Neuroimmunol 256(1–2):1–6. https://doi.org/10.1016/j.jneuroim.2012.11.005
Zhao J, Fu Y, Liu CC, Shinohara M, Nielsen HM, Dong Q, Kanekiyo T, Bu G (2014) Retinoic acid isomers facilitate apolipoprotein E production and lipidation in astrocytes through the retinoid X receptor/retinoic acid receptor pathway. J Biol Chem 289(16):11282–11292. https://doi.org/10.1074/jbc.M113.526095
Shearer KD, Fragoso YD, Clagett-Dame M, McCaffery PJ (2012) Astrocytes as a regulated source of retinoic acid for the brain. Glia 60(12):1964–1976. https://doi.org/10.1002/glia.22412
Fukasawa H, Nakagomi M, Yamagata N, Katsuki H, Kawahara K, Kitaoka K, Miki T, Shudo K (2012) Tamibarotene: a candidate retinoid drug for Alzheimer’s disease. Biol Pharm Bull 35(8):1206–1212. https://doi.org/10.1248/bpb.b12-00314
Napoli JL (1986) Quantification of physiological levels of retinoic acid. Methods Enzymol 123(1):112–124. https://doi.org/10.1016/s0076-6879(86)23015-3
Wang R, Chen S, Liu Y, Diao S, Xue Y, You X, Park EA, Liao FF (2015) All-trans-retinoic acid reduces BACE1 expression under inflammatory conditions via modulation of nuclear factor κB (NFκB) signaling. J Biol Chem 290:22532–22542. https://doi.org/10.1074/jbc.M115.662908
Christensen EI, Nielsen R (2007) Role of megalin and cubilin in renal physiology and pathophysiology. Rev Physiol Biochem Pharmacol 158:1–22. https://doi.org/10.1007/112_0604
Herz J, Strickland DK (2001) LRP: a multifunctional scavenger and signaling receptor. J Clin Invest 108(6):779–784. https://doi.org/10.1172/JCI13992
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ (2010) Decreased clearance of CNS amyloid-β in Alzheimer’s disease. Science 330(6012):1774. https://doi.org/10.1126/science.1197623
Jones RS, Minogue AM, Connor TJ, Lynch MA (2013) Amyloid-β-induced astrocytic phagocytosis is mediated by CD36, CD47 and RAGE. J Neuroimmune Pharmacol 8(1):301–311. https://doi.org/10.1007/s11481-012-9427-3
Laporte V, Lombard Y, Levy-Benezra R, Tranchant C, Poindron P, Warter JM (2004) Uptake of Aβ1-40- and Aβ1-42-coated yeast by microglial cells: a role for LRP. J Leukoc Biol 76(2):451–461. https://doi.org/10.1189/jlb.1203620
Liu CC, Hu J, Zhao N, Wang J, Wang N, Cirrito JR, Kanekiyo T, Holtzman DM, Bu G (2017) Astrocytic LRP1 mediates brain Aβ clearance and impacts amyloid deposition. J Neurosci 37(15):4023–4031. https://doi.org/10.1523/JNEUROSCI.3442-16.2017
Liu CC, Hu J, Tsai CW, Yue M, Melrose HL, Kanekiyo T, Bu G (2015) Neuronal LRP1 regulates glucose metabolism and insulin signaling in the brain. J Neurosci 35(14):5851–5859. https://doi.org/10.1523/JNEUROSCI.5180-14.2015
Wallum BJ, Taborsky GJ, Porte D, Figlewicz DP, Jacobson L, Beard JC, Ward WK, Dorsa D (1987) Cerebrospinal fluid insulin levels increase during intravenous insulin infusions in man. J Clin Endocrinol Metab 64(1):190–194. https://doi.org/10.1210/jcem-64-1-190
Wilcox G (2005) Insulin and insulin resistance. Clin Biochem Rev 26(2):19–39. PMID: 16278749, PMCID: PMC1204764, Free PMC article
Watson GS, Craft S (2003) The role of insulin resistance in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs 17(1):27–45. https://doi.org/10.2165/00023210-200317010-00003
Acknowledgements
This work was supported in part by JSPS KAKENHI Grant Number JP17K15390, JP19K06441 to K.T.
Funding
None.
Author information
Authors and Affiliations
Contributions
K. T.-K. wrote the main manuscript text and manage the study. K. M. performed the experiments and prepared the figures. Y. N and M. M. advised the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takano-Kawabe, K., Matoba, K., Nakamura, Y. et al. Low Density Lipoprotein Receptor-related Protein 2 Expression and Function in Cultured Astrocytes and Microglia. Neurochem Res 49, 199–211 (2024). https://doi.org/10.1007/s11064-023-04022-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-04022-7