Abstract
Beta-amyloid peptide (Aβ) induced neurotoxicity is considered as a hallmark of the pathogenesis of Alzheimer’s disease (AD). The present study demonstrated the neuroprotective role of 5-HT6 receptors against Aβ-induced neurotoxicity in PC-12 cells. The 5-HT6 receptor agonist EMD-386088 and antagonist SB-399885 were used as pharmacological tools. The NMDA receptor antagonist, memantine, was used as reference standard. The Aβ25−35 (50 µM) induced apoptosis, increased reactive oxygen species (ROS) generation and impaired neurite outgrowth in PC-12 cells. Pre-treatment with 10 µM EMD-386088 and SB-399885 had significantly protected neuronal cell death by maintaining higher cell viability through attenuation of intracellular ROS. Further, both compounds significantly prevented Aβ25−35-induced impairment in neurite outgrowth in PC-12 cells. Similarly, memantine prevented Aβ25−35-induced neurotoxicity in PC-12 cells. These findings suggest that 5-HT6 receptor ligands have protected neurons from Aβ25−35 induced toxicity by reducing ROS and through prevention of impairment in neurite outgrowth. Therefore, 5-HT6 receptor could be an important disease-modifying therapeutic target for AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by progressive loss of memory and impairment of cognitive functions [1]. With the progression of the disease, patients develop other symptoms like irritability, aggression, problems with speaking and writing. The pathophysiology of AD is complex and not completely understood. Deposition of extracellular b-amyloid (Aβ) plaques and highly phosphorylated microtubule-linked tau proteins forming intracellular neurofibrillary tangles (NFTs) are the important pathological hallmarks of AD [1, 2].
The in vitro Aβ25−35 induced toxicity in PC-12 neuronal cells is a well-established model to study the pathophysiology of AD and to evaluate therapeutic activity of potential agents [3, 4]. PC-12 cells originally obtained from rat pheochromocytoma of rats, and these cells have their origin of embryonic neural crest cells. Though the detailed mechanism of Aβ-induced neurotoxicity is not known, several reports suggest the role of oxidative stress or excessive production of ROS and its consequences such as lipid peroxidation [3, 5]. Further, disruption of neuronal ionic homeostasis and up-regulation of pro-apoptotic Bax with simultaneous down-regulation of neuroprotective Bcl-2 contribute to neuronal death [4]. Studies on PC-12 cells, also showed that Aβ-induced toxicity increases protein expression and activity of acetylcholinesterase (AChE) enzyme [6].
Currently, AChE inhibitors such as donepezil and NMDA antagonist memantine are standard therapeutic medications used to treat patients with AD. However, these drugs provide only symptomatic relief and do not cure the underlying disease pathology or halt the disease progression [7, 8]. In spite of the progress in our understanding the AD pathophysiology, a large number of new chemical entities have failed to show therapeutic benefits in the clinical trials. Therefore, the scientific community and the AD-related sufferers including patients and caregivers are desperately seeking the new therapeutic agent [8]. Among drug targets to treat AD, the 5-HT6 receptor is widely studied [9, 10].
The 5-HT6 receptors are expressed in central nervous system (CNS) in areas such as cortex and hippocampus which are associated with learning and memory functions [11]. Evidence support the role of the 5-HT system in learning and memory processes [12]. Although the significance is not fully understood, extensive serotonergic denervation has been reported in AD. Studies in the AD patients showed a significant reduction in 5-HT6 receptor density in the cortical areas. Further, the dysregulation of 5-HT6 receptor activation by 5-HT in the temporal cortex may be related to behavioral symptoms in AD [12]. Preclinical studies have reported modulation of learning and memory in rodent models of amnesia through 5-HT6 receptors [9, 11]. Further, the 5-HT6 receptor agonists and antagonists both may have similar pro-cognitive activities through activation and inhibition [13]. In rodent models, 5-HT6 receptor agonist and antagonist enhanced memory and prevented memory impairment through the modulation of cholinergic and glutamatergic function [13–15]. Further, a recent study has reported that selective 5-HT6 receptor antagonist, Idalopirdine improve cognitive function in donepezil-treated patients with moderate AD [16].
Although, previous studies showed improvement in cognitive function following modulation of 5-HT6 receptor function in animal models and AD patients. The exact role of the 5-HT6 receptor in neurodegeneration is not elucidated so far. Therefore, we investigated the role of 5-HT6 receptor agonist and antagonist in neurodegeneration induced by Aβ25−35 in vitro. In the present study, PC-12 cells were used as the model system.
Materials and Methods
Materials
PC-12 cells were purchased from ATCC (Rockville, MD, USA). Fetal bovine serum (FBS), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT), nerve growth factor (NGF), Poly-d-lysine hydrobromide (PDL), amyloid-b protein 25–35 (Aβ25−35), dimethyl sulfoxide (DMSO) and memantine hydrochloride were procured from Sigma-Aldrich (St. Louis, MO, USA). Pen-Streep and Horse serum (HS) were obtained from Gibco (Grand Island, NY, USA). 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA), Dulbecco’s Modified EagleMedium (DMEM), Hoechst 33258 were procured from Life Technologies (Delhi, India). The lactate dehydrogenase (LDH) assay kit was purchased from Abcam (MA, USA). Dulbecco’s Phosphate Buffered Saline (DPBS) was purchased from Lonza (MA, USA). SB-399885 and EMD-386088 were synthesized in-house at Lupin Research Park, Lupin Limited, Pune, India.
Preparation of Aggregated Aβ25−35
Aβ25−35 was dissolved in sterile (deionized water) milli-Q water (1 mM) and aggregated by incubation at 37 °C for 4 days and stored at −20 °C until use [17].
Cell Culture and Drug Treatments
The PC-12 were cultured in DMEM supplemented with 15% (v/v) heat-inactivated horse serum (HS) and 5% (v/v) fetal bovine serum (FBS). PC-12 cells were differentiated in DMEM medium containing 50 ng/mL NGF and 1% FBS by 4 days [18]. The flocculant PC-12 cells were seeded in poly-d-lysine-coated 96-well culture plates at a density of 2 × 104 cells/well and treated with the test compounds SB-399885 and EMD-386088 at a concentration of 10 µM and the reference standard memantine at 20 µM concentration (standardized in-house, data not shown). After 2 h of incubation with test compounds, the cells were treated with aggregated Aβ25−35 (50 µM) and incubated at 37 °C for 24 h. The per se effect of drugs on cells was evaluated by treating at various concentrations for 2 h and then treated with sterile milli-Q water (vehicle of Aβ25−35) for 24 h.
MTT Assay
The PC-12 cells were pretreated with the SB-399885 and EMD-386088 at 10 µM concentration and the reference standard memantine at 20 µM concentrations for 2 h and then intoxicated with Aβ25−35 (50 µM) for 24 h at 37 °C. Cell viability was estimated by a quantitative colorimetric assay using MTT. After 24 h of treatment, cells were incubated with 5 mg/mL MTT (10 µL/well) for 4 h at 37 °C, and then 100 µL of DMSO was added to dissolve the formazan crystals. The absorbance was measured at 570 nm using a UV–Vis ELISA plate reader (Synergy 2 BioTek’s USA).
Lactate Dehydrogenase (LDH) Assay
The PC-12 cells were plated at the same density as mentioned in MTT assay. The cells were pretreated with 10 µM solutions of SB-399885, EMD-386088 (each), and memantine at 20 µM concentrations for 2 h and then intoxicated with Aβ25−35 (50 µM) for 24 h at 37 °C. The supernatant was then transferred to a 96-well plate, and the amount of LDH-release were determined by using LDH-cytotoxicity assay kit protocol (Abcam USA). The absorbance was measured at 450 nm using a UV–Vis ELISA plate reader (Synergy 2 BioTek’s USA).
Hoechst 33258 Nuclear Staining
The PC-12 cells were pretreated with the SB-399885 and EMD-386088 at 10 µM concentration and the reference standard memantine at 20 µM concentrations for 2 h and then intoxicated with Aβ25−35 (50 µM) for 24 h at 37 °C. The characteristic features of apoptotic nuclei were assessed using the Hoechst 33258 fluorescent dye. For Hoechst 33258 staining, the cells were fixed with 4% paraformaldehyde and stained with 5 μg/mL Hoechst 33258 solution in DPBS for 20 min at room temperature in a dark chamber. The cells were viewed under a fluorescence microscope (Olympus IX51, USA) to check the changes in nuclear morphology such as reduced size and condensations.
Estimation of ROS
The PC-12 cells were pretreated with the SB-399885 and EMD-386088 at 10 µM concentration and the reference standard memantine at 20 µM concentrations for 2 h and then intoxicated with Aβ25−35 (50 µM) and incubated for 24 h at 37 °C. Later the medium was removed, and the cells were incubated with 100 µM of DCFH-DA solution for 30 min at room temperature. Further, the cells were washed three times to remove the extracellular DCFH–DA. Then 100 µL of DPBS was added, and the fluorescence intensity of the cells was measured using flow cytometry (BD FACSCanto™, USA) at the excitation wavelength of 485 nm and an emission wavelength of 550 nm.
Neurite Outgrowth Assay
The PC-12 cells were plated at a density of 5 × 103 cells in poly-d-lysine-coated six-well plates in DMEM medium supplemented with 50 ng/mL NGF and 1% (v/v) FBS. After 48 h of incubation, the medium was replaced with fresh DMEM medium containing 1% FBS and 50 ng/mL NGF and cells were allowed to differentiated for four days. Later, the PC-12 cells were pretreated with the SB-399885 and EMD-386088 at 10 µM concentration and the reference standard memantine at 20 µM concentrations for 2 h and then intoxicated with Aβ25−35 (50 µM) for 24 h. Images were captured in the presence of DMEM medium using inverted microscope at 40× magnification. All images were analysis using ImageJ 1.43u software and using length-tool neurite outgrowth length was measured [18].
Results
Protective Effect of SB-399885 and EMD-386088 Against Aβ-Induced Toxicity in PC-12 Cells
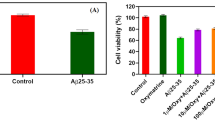
The MTT cell viability assay investigated the possible neuroprotective effects of 5-HT6 receptor agonist and antagonist. The results were expressed as % cell viability in medium control. The PC-12 cells were pretreated with the SB-399885 and EMD-386088 at 10 µM concentration and the reference standard memantine at 20 µM concentrations for 2 h and then intoxicated with Aβ25−35 (50 µM) and incubated for 24 h. Aggregated Aβ25−35 (50 µM) significantly (P < 0.05) killed the cells in comparison to control indicating neurotoxicity in PC-12 cells. The 5-HT6 receptor antagonist SB-399885 (10 µM) significantly increased (P < 0.01) PC-12 cell viability. Similarly, 5-HT6 receptor agonist, EMD-386,088 (10 µM) significantly prevented Aβ25−35 induced PC-12 cell death. The reference standard, memantine (20 µM) also significantly (P < 0.01) increased cell viability in Aβ25−35 treated PC-12 cells (Fig. 1a). Treatment of PC-12 cells with SB-399885, EMD-386088, and memantine had no significant effect on cell viability (Fig. 1b).
a Effects of SB-399885, EMD-386,088, and Memantine on Aβ25−35 induced cytotoxicity on PC-12 cells. #P < 0.05 compared with the control group; **P < 0.01 and *P < 0.05 compared with the Aβ25 − 35 treated group; b effects of SB-399885, EMD-386088, and Memantine on PC-12 cell viability. Values given are the mean ± SEM (n = 3)
To further study the effect of SB-399885 and EMD-386088 on Aβ25−35 induced PC-12 cell damage, LDH release assay was performed. As shown in Fig. 2, when PC-12 cells were incubated with 50 µM of Aβ25−35 for 24 h, there was a significant increase in LDH leakage compared to control. In contrast, when the cells were pretreated with SB-399885 or EMD-386088 (10 µM), the percentage of LDH leakage was significantly decreased (P < 0.01) compared with the Aβ25−35 treated cells group. Memantine also significantly decreased LDH release induced by Aβ25−35 in PC-12 cells.
Hoechst 33258 staining also showed Aβ25−35 (50 μM) could induce PC-12 cell apoptosis (P < 0.001) when compared to control group. As shown in Fig. 3a, b, both SB-399885 and EMD-386088 (10 µM) and significantly prevented (P < 0.01) Aβ25−35 induced PC-12 cell apoptosis. Memantine (20 µM) also significantly reduced (P < 0.01) Aβ25−35 induced PC-12 cell apoptosis.
Effects of Aβ25–35 on cell apoptosis. a Hoechst 33258 staining was performed to detect the cell apoptosis, nuclear condensation and fragmentation (shown by the arrow marks: Green arrow indicates normal cells; Red arrow indicates nuclear condensation/apoptotic cells). Aβ25−35 (50 μM) could effectively induce PC-12 cell apoptosis; b the data shown represent three independent experiments to determine apoptotic index (%); ###P < 0.001 versus the controls and **P < 0.01 compared with the Aβ25−35 induced cells. (Color figure online)
Protective Effect of SB-399885 and EMD-386088 Against Aβ-Induced Oxidative Stress in PC-12 Cells
Oxidative stress was assessed by measuring the levels of intracellular ROS by using DCFH-DA. The representative graphs were shown in Fig. 4a. The PC-12 cells were pretreated with the SB-399885 and EMD-386088 at 10 µM concentration and the reference standard memantine at 20 µM concentrations for 2 h and then intoxicated with Aβ25−35 (50 µM) and incubated for 24 h. After exposure of PC-12 cells to 50 µM of Aβ25−35 for 24 h, intracellular ROS level was significantly elevated as compared to control, suggesting that Aβ25−35 induces marked oxidative stress. SB-399885 and EMD-386088 at the concentrations of 10 µM significantly (P < 0.001) reduced Aβ25−35 induced ROS production in PC-12 cells. The reference standard memantine protected PC-12 cells from Aβ25−35 induced oxidative stress as indicated by significant (P < 0.001) reduction in ROS generation (Fig. 4b).
Effects of SB-399885, EMD-386088, and memantine on the Aβ25−35 induced ROS generation in PC-12 cells. a The representative fluorescent graphs from flow cytometry; b ROS generation (% of control). Values given are the mean ± SEM (n = 6). ###P < 0.001 compared with the control group; ***P < 0.001 compared with the Aβ25−35 intoxicated cells
Protective Effect of SB-399885 and EMD-386088 Against Aβ-Induced Impairment of Neurite Outgrowth in PC-12 Cells
The treatment with aggregated Aβ25−35 to differentiated PC-12 cells resulted in a reduction of neurite length significantly (Fig. 5a, b). The PC-12 cells were pretreated with the SB-399885 and EMD-386088 at 10 µM concentration and the reference standard memantine at 20 µM concentrations for 2 h and then intoxicated with Aβ25−35 (50 µM) and incubated for 24 h. SB-399885 and EMD-386088 significantly (P < 0.001) protected Aβ25−35 induced impairment of neurite outgrowth in PC-12 cells. The reference standard memantine prevented significantly impairment of neurite outgrowth in PC-12 cells induced by Aβ25−35.
Effects of SB-399885, EMD-386088, and Memantine on the Aβ25−35 induced impairment of neurite outgrowth. a Representative images from cells treated with NGF 50 ng/mL and different treatments; b Neurite length of PC-12 cells. Data was expressed as means ± SEM (n = 6–7); ###P < 0.001 compared with the control group; **P < 0.01 and *P < 0.05 compared with the Aβ25−35 intoxicated cells
Discussion
The present study investigated the effect of 5-HT6 receptor agonist and antagonist on aggregated Aβ25−35 peptide-induced neurotoxicity in PC-12 cells. The results of this study showed that modulation of 5-HT6 receptor function exerted a protective effect against Aβ25−35 induced cytotoxicity in PC-12 cells through inhibition of intracellular oxidative stress and preventing impairment of neurite outgrowth. Memantine is a NMDA receptor antagonist and is approved for the symptomatic treatment of AD (10 mg tablet twice a day). Further, in animal models, memantine has been reported to prevent Aβ25−35 induced memory impairment. Therefore, to validate the Aβ25−35 induced in vitro neurotoxicity model in PC-12 cell lines, the present study used memantine as the reference standard.
A large number of animal and human studies confirmed the central role played by beta-amyloid peptides in the pathophysiology of AD [19]. The differentiated PC-12 cells were reported to be more vulnerable to Aβ-peptide insult and have been used extensively in neurotoxicity studies. In the represent study, Aβ25−35 induced cytotoxicity on PC-12 cells as indicated by enhanced cell death in MTT assay. Further, the cytotoxic effect of Aβ25−35 was confirmed by LDH-release assay and Hoechst 33258 staining. The Aβ25−35 induces apoptosis and neuronal cell death by increasing intracellular ROS, resulting into membrane lipids peroxidation and oxidative stress. Our data suggest that Aβ25−35 (50 µM) triggered cytotoxicity as indicated by loss of MTT reducing activity, increased LDH release and increased apoptotic index in comparison to the control.
To investigate the protective role of the 5-HT6 receptor in Aβ25−35 induces neurotoxicity, the 5-HT6 receptor agonist EMD-386088, and antagonist SB-399885 were used as experimental pharmacological tools. Recently, the 5-HT6 receptor has received much attention for its role in the neuronal disorders including AD [9–11, 14, 15]. Several preclinical studies have shown that modulation of 5-HT6 receptor function exerts pro-cognitive and anti-amnesic activity through modulation of central cholinergic and glutamatergic function [13]. The beneficial effects of 5-HT6 receptor antagonist were also observed in patients with moderate AD [16]. In the present study, we have demonstrated that the 5-HT6 receptor agonist and antagonist will also prevent neuronal death upon Aβ25−35 induces neurotoxicity.
Both SB-399885 and EMD-386088 protected PC-12 cells from Aβ25−35 induced cytotoxicity as indicated by increased cell viability and reduced apoptosis. Similarly, the reference standard memantine prevented the cytotoxic effect of Aβ25−35 in PC-12 cells. We further studied the effect of 5-HT6 receptor ligands on Aβ25−35 induced oxidative stress and impairment of neurite outgrowth. Aβ25−35 stimulates the production of ROS which is implicated in the apoptotic mechanism of Aβ25−35 mediated neurotoxicity and may also involve in the apoptotic processes observed in AD. The ROS generated by Aβ-toxicity causes neuronal damage through mechanisms including membrane lipid peroxidation, receptor-mediated mechanisms, disruption of cellular calcium homeostasis. Pretreatment of SB-399885 in PC-12 cells significantly reduced Aβ25−35 induced oxidative stress as shown by significant reduction in ROS-generation. Similarly, the 5-HT6 receptor agonist EMD-386088 and NMDA receptor antagonist memantine reduced Aβ25−35 induced ROS generation in PC-12 cells.
One of the functionally most relevant histopathological lesions of AD brain is a loss of neurites and synapses and Aβ-peptides have been reported to reduce neurite outgrowth in different neuronal cell lines including PC-12 cells [20, 21]. In agreement with these observations, the addition of aggregated Aβ25−35 to NGF-treated PC-12 cells reduced neurite length significantly. This neurotoxic effect of Aβ25−35 was significantly inhibited when cells are pretreated with SB-399885, EMD-386088, and memantine. Neurite outgrowth is an essential part of synaptic plasticity which in turn is one of the important neurochemical foundations of learning and memory [22]. Therefore, the prevention of Aβ25−35 induced impairment in neurite outgrowth by 5-HT6 receptor agonist and antagonist could be an important mechanism responsible for pro-cognitive, anti-amnesic and neuroprotective effects shown by these agents.
Although the underlying mechanism of 5-HT6 receptor agonist and antagonist showing a similar protective effect against Aβ25−35 induced neurotoxicity, such type of observation was reported elsewhere in animal models. Woods et al. reported that 5-HT6 receptor antagonists and agonists reverse the memory deficits induced by modulation of cholinergic or glutamatergic neurotransmission in rats. The agonists and antagonists act differentially in different neuronal populations producing similar beneficial effects on learning and memory [10]. The serotonergic signaling was known to lower Aβ-proteins and plaques both in mice and humans [23]. Another mechanism for paradoxically similar effects of agonist/antagonists could be related to the existence of alternative biochemical pathways activated by 5-HT6 receptors. Selective binding of 5-HT to receptor subtypes 5-HT4, 5-HT6 and 5-HT7 which are Gs-protein linked activates protein kinase A (PKA). The binding of serotonin to Gs-linked receptors and activation of PKA is said to be important in the physiological protection of memory loss. The activated PKA then activates extracellular signal-regulated kinases (ERK) and MEK (MAPkinase-ERK kinase) signaling [24]. The ERK-MEK signaling is said to phosphorylate α-secretase and cleaves the amyloid precursor protein (APP) whereby decreases the production of Aβ-protein [25]. It has been reported that apart from coupling to Gas, 5-HT6 receptors are coupled to other Ga protein subunits (Gai/o or Gaq). Also, the coupling of 5-HT6 receptors to Ca2+ signaling has been reported [26].
In the present study, we have seen that the actions of Aβ-proteins can be modulated by both agonist and antagonist to prevent neuronal death by reducing oxidative stress. The mechanism responsible for this paradoxically similar neuroprotective effect of agonist and antagonist in single cell type is still not completely understood. However, further studies are warranted to understand molecular mechanism responsible for this paradoxical effect.
Conclusions
The 5-HT6 receptor agonist SB-399885 and antagonist EMD-386088 prevent neuronal cytotoxicity induced by Aβ25−35. These results provide evidence for the potential use of 5-HT6 receptor ligands to treat neurodegenerative conditions like AD.
Abbreviations
- Aβ:
-
Amyloid beta
- AChE:
-
Acetylcholinesterase
- AD:
-
Alzheimer’s disease
- DCFH-DA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- DMEM:
-
Dulbecco’s modified eagle medium
- DPBS:
-
Dulbecco’s phosphate buffered saline
- FBS:
-
Fetal bovine serum
- HS:
-
Horse serum
- 5-HT:
-
5-Hydroxytryptamine
- LDH:
-
Lactate dehydrogenase assay
- MTT:
-
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide
- NFTs:
-
Neurofibrillary tangles
- NGF:
-
Nerve growth factor
- NMDA:
-
N-methyl-d-aspartate
- PC-12:
-
Pheochromocytoma
- PDL:
-
Poly-d-lysine hydrobromide
- ROS:
-
Reactive oxygen species
References
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. NEJM 362:329–344
Kumar A, Singh A, Ekavali (2015) A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep 67:195–203
Xian YF, Lin ZX, Mao QQ, Ip SP, Su ZR, Lai XP (2012) Protective effect of isorhynchophylline against b-amyloid-induced neurotoxicity in cells. Cell Mol Neurobiol 32:353–360
Sun YL, Tae YH, Dong JS, Sung RK, Jin TH (2005) Effect of sesaminol glucosides on b-amyloid-induced PC12 cell death through antioxidant mechanisms. Neurosci Res 52:330–341
Allan Butterfield D, Castegna A, Lauderback CM, Drake J (2002) Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol Aging 23:655–664
Islam F, Khan A, Vaibhav K, Javed H, Moshahid Khan M, Tabassum R, Ahmed ME, Srivastava P, Khuwaja G, Islam F, Saeed Siddiqui M, Shafi MM (2012) Attenuation of Ab-induced neurotoxicity by thymoquinone via inhibition of mitochondrial dysfunction and oxidative stress. Mol Cell Biochem 369:55–65
Suh WH, Suslick KS, Suh YH (2005) Therapeutic agents for Alzheimer’s disease. Curr Med Chem Cent Nerv Syst Agents 5:259–269
Han SH, Mook-Jung I (2014) Diverse molecular targets for therapeutic strategies in Alzheimer’s disease. J Korean Med Sci 29:893–902
Meneses A, Pérez-García G, Ponce-Lopez T, Castillo C (2011) 5-ht6 receptor memory and amnesia: behavioral pharmacology—learning and memory processes. Int Rev Neurobiol 96:27–47
Woods S, Clarke NN, Layfield R, Fone KCF (2012) 5-HT6 receptor agonists and antagonists enhance learning and memory in a conditioned emotion response paradigm by modulation of cholinergic and glutamatergic mechanisms. Br J Pharmacol 167:436–449
Rossé G, Schaffhauser H (2010) 5-HT6 receptor antagonists as potential therapeutics for cognitive impairment. Curr Top Med Chem 10:207–221
Štrac DŠ, Pivac N, Mück-Šeler D (2016) The serotonergic system and cognitive function. Transl Neurosci 7:35–49
Fone KCF (2008) An update on the role of the 5-hydroxytryptamine 6 receptor in cognitive function. Neuropharmacology 55:1015–1022
Arnt J, Bang-Andersen B, Grayson B, Bymaster FP, Cohen MP, Delapp NW, Giethlen B, Kreilgaard M, McKinzie DL, Neill JC, Nelson DL, Nielsen SM, Poulsen MN, Schaus JM, Witten LM (2010) Lu AE58054, a 5-HT 6 antagonist, reverses cognitive impairment induced by subchronic phencyclidine in a novel object recognition test in rats. Int J Neuropsychopharmacol 13:1021–1033
De Bruin NMWJ, Kruse CG (2015) 5-HT6 receptor antagonists: potential efficacy for the treatment of cognitive impairment in schizophrenia. Curr Pharm Des 21:3739–3759
Wilkinson DD, Windfeld K, Colding-Jørgensen E (2014) Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer’s disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 13:1092–1099
Wei H, Leeds PR, Qian Y, Wei W, Chen R, Chuang DM (2000) β-Amyloid peptide-induced death of PC 12 cells and cerebellar granule cell neurons is inhibited by long-term lithium treatment. Eur J Pharmacol 392:117–123
Kurz C, Ungerer I, Lipka U, Kirr S, Schütt T, Eckert A, Leuner K, Müller WE (2010) The metabolic enhancer piracetam ameliorates the impairment of mitochondrial function and neurite outgrowth induced by ß-amyloid peptide. Br J Pharmacol 160:246–257
Mufson EJ, Ikonomovic MD, Counts SE, Perez SE, Malek-Ahmadi M, Scheff SW, Ginsberg SD (2016) Molecular and cellular pathophysiology of preclinical Alzheimer’s disease. Behav Brain Res 311:54–69
Petratos S, Li QX, George AJ, Hou X, Kerr ML, Unabia SE, Hatzinisiriou I, Maksel D, Aguilar MI, Small DH (2008) The β-amyloid protein of Alzheimer’s disease increases neuronal CRMP-2 phosphorylation by a Rho-GTP mechanism. Brain 131:90–108
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298:789–791
Mattson MP (2007) Mitochondrial regulation of neuronal plasticity. Neurochem Res 32:707–715
Cirrito JR, Disabato BM, Restivo JL, Verges DK, Goebel WD, Sathyan A, Hayreh D, D’Angelo G, Benzinger T, Yoon H, Kim J, Morris JC, Mintun MA, Sheline YI (2011) Serotonin signaling is associated with lower amyloid-βlevels and plaques in transgenic mice and humans. Proc Natl Acad Sci USA 108:14968–14973
Fisher JR, Wallace CE, Tripoli DL, Sheline YI, Cirrito JR (2016) Redundant Gs-coupled serotonin receptors regulate amyloid-β metabolism in vivo. Mol Neurodegener. doi:10.1186/s13024-016-0112-5
Panza F, Solfrizzi V, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Vendemiale G, Capurso A, Imbimbo BP (2009) Disease-modifying approach to the treatment of Alzheimer’s disease: from α-secretase activators to γ-secretase inhibitors and modulators. Drugs Aging 26:537–555
Ramírez M (2013) 5-HT6 receptors and Alzheimer’s disease. Alzheimers Res Ther 5:15
Acknowledgements
The authors express their gratitude to Lupin Ltd, Lupin Research Park, Pune and Manipal University, Manipal for their support and help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they do not have any conflict in publishing the data.
Rights and permissions
About this article
Cite this article
Bokare, A.M., Praveenkumar, A.K., Bhonde, M. et al. 5-HT6 Receptor Agonist and Antagonist Against β-Amyloid-Peptide-Induced Neurotoxicity in PC-12 Cells. Neurochem Res 42, 1571–1579 (2017). https://doi.org/10.1007/s11064-017-2217-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2217-9