Abstract
This study investigated the effects of (−)-sesamin on chronic electric footshock (EF) stress-induced anxiety disorders in mice. Mice were treated with (−)-sesamin (25 and 50 mg/kg) orally once a day for 21 days prior to exposure to EF stress (0.6 mA, 1 s every 5 s, 3 min). Mice treated with (−)-sesamin (25 and 50 mg/kg) exhibited less severe decreases in the number of open arm entries and time spent on open arms in the elevated plus-maze test and the distance traveled in the open field test following exposure to chronic EF stress. Similarly, mice treated with (−)-sesamin exhibited significantly less severe decreases in brain levels of dopamine, norepinephrine, and serotonin following exposure to chronic EF stress. Increases in serum levels of corticosterone and expression of c-Fos were also less pronounced in mice treated with (−)-sesamin (25 and 50 mg/kg). These results suggest that (−)-sesamin may protect against the effects of chronic EF stress-induced anxiety disorders by modulating dopamine, norepinephrine, and serotonin levels, c-Fos expression, and corticosterone levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Repeated or chronic exposure to stress is associated with physiological changes that occur in response to novel or threatening stimuli. Chronic stress has been linked to a number of psychiatric conditions, including anxiety disorders and depression [1].
Research has indicated that disorders of anxiety and depression secondary to chronic stress are associated with decreased levels of dopamine, norepinephrine, and serotonin in the brain [2, 3]. Chronic stress also results in an elevation of serum glucocorticoids such as corticosterone and cortisol due to the involvement of the hypothalamic–pituitary–adrenal (HPA) axis [3], which significantly influences dopamine activity [3], thereby leading to clinical levels of anxiety and depression [4]. In addition, expression of c-Fos protein, which is the product of an immediate early gene, increases in various brain regions of mice and rats exposed to chronic stress, especially in the paraventricular nuclei (PVN) of the hypothalamus [5, 6].
The PVN region plays a vital role in the synthesis of corticortropin-releasing hormone (CRH) responsible for the control of acute and chronic stress-induced HPA activation [5, 6]. The levels of dopamine, norepinephrine, and serotonin may implicate in the acute and chronic stress responses in the PVN region [7]. The changes of dopamine and serotonin lead to anxiety-like behaviors in the elevated plus-maze and open field tests [8–10].
(−)-Sesamin, which is an epimeric isomer lignans with (+)-sesamin, is a major lignan constituent of Asiasari Radix (Asiasarum heterotropoides F. Maekawa var. mandshuricum F. Maekawa, Aristolochiaceae) and has beneficial effects on nitric oxide production, and levels of cholesterol and triglycerides [11, 12]. The water extract of A. Radix has inhibitory effects on hyperalgesia in mice [13]. The methanol extract of A. Radix also ameliorates memory deficits in rats [14]. In addition, (−)-sesamin exerts protective effects against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity in PC12 and dopaminergic neuronal cells in a rat model of Parkinson’s disease (PD) [15]. (−)-Sesamin reduces anxiety-like behaviors induced by chronic pain in mice [16]. Sesamol, a lignin derivative of sesamin and a major compound of Sesame seeds, also has ameliorating effects on depression by modulating central oxidative stress and inflammation in mice [17].
The present study aims to investigate the pharmacological effects of (−)-sesamin on chronic electric footshock (EF) stress-induced anxiety as assessed using the elevated plus-maze, as well as to examine the influence of such treatment on levels of dopamine, norepinephrine, and serotonin, c-Fos expression, and corticosterone levels in mice.
Materials and Methods
Materials
(−)-Sesamin was isolated from A. heteropoides and identified according to a previously described protocol [18]. A voucher specimen was deposited in the herbarium of the College of Pharmacy at Chungbuk National University.
Dopamine, norepinephrine, serotonin, isoproterenol, and 5-hydroxyindoleacetic acid (HIAA) were purchased from Sigma (St. Louis, MO, USA). c-Fos antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). A corticosterone assay kit was obtained from USCN Life Sciences (E0504m, Wuhan, China). All other chemicals were of analytical grade.
Animals
Male ICR mice (weight: 20–25 g) were obtained from Samtako Co. (Osan, Korea). Mice were housed two per cage in a temperature and humidity controlled environment (12-h light/dark cycle, 23 ± 2 °C, 50 ± 2% humidity), with ad libitum access to standard mouse food and water. This study was approved by the Animal Ethics Committee of Chungbuk National University (Approval No., CBNU-728-14-01), and all experiments were performed in accordance with the guidelines of the NIH for Care and Use of Laboratory Animals and Chungbuk National University Laboratory Animal Research Center.
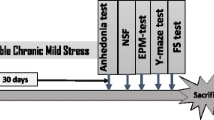
Experimental Design and Chronic EF Stress
Mice were randomly divided into three subsets and each subset has four groups (each group, n = 8–10) for determining (1) dopamine, norepinephrine, and corticosterone levels including the elevated plus-maze test, (2) serotonin levels including the open field test, and (3) c-Fos immunohistochemical analysis. Animals in the control group received saline (0.9%). Mice in the chronic EF stress group (chronic EF stress) received saline (0.9%) and were subjected to unavoidable EF stress alone (intensity: 0.6 mA; shock duration: 1 s every 5 s; period: 3 min) once a day (at 14:00) for 21 days to induce anxiety disorders using an Electric Shock Generator (Seil Electric Co., Taejeon, Korea) [19]. Mice in the chronic EF stress plus (−)-sesamin groups received (−)-sesamin (25 or 50 mg/kg, p.o., feeding needle, length 50 mm, tip 2.0 mm, Fuchigami, Japan) once a day for 21 days [15], in addition to receiving the same EF stress [Chronic EF stress + (−)-Sesamin]. (−)-Sesamin administered 4 h prior to EF stress exposure on each testing day. On day 21, all experimental groups were subjected to elevated plus-maze testing, following which the mice were sacrificed using Zoletil 50 (100 mg/kg, i.p.; Virbac, Carros, France) in order to obtain brain tissue and serum for biochemical and immunohistochemical analyses [15, 20]. The injection of Zoletil 50 showed no influence with the brain levels of dopamine, norepinephrine, and serotonin.
The Elevated Plus-Maze Test
The elevated plus-maze test was used to evaluate the anxiety behaviors [21]. The elevated plus-maze apparatus consisted of four arms: two open arms (30 × 5 cm) and two closed arms of the same size, with 16-cm-high black walls elevated 45 cm above the floor. The open and closed arms were connected via a central square (5 × 5 cm) to form a plus sign. Following a 1-min adaptation period, the number of open arm entries and the time spent on the open arms during a 5-min test period were recorded using a video camera connected to a SMART video-tracking system (Panlab S.I., Barcelona, Spain) [21]. In addition, the distances traveled in 5 min were recorded to measure the locomotion [20].
The Open Field Test
The open field test was used to test distance traveled. The apparatus (ENV-520, Med Associates Inc., St. Albans, VT, USA) consists of one white floor and four transparent walls (30 × 30 × 15 cm). The mice were removed to experimental room at least 24 h prior to the test to adapt the novel environment. Each mouse was put in the center of the open field box and was tested for 5 min to record the distance traveled [20].
Levels of Dopamine, Norepinephrine, Serotonin, and Corticosterone
For the determination of dopamine and norepinephrine levels, whole mouse brains were homogenized in perchloric acid (300 µl, 1 M) and isoproterenol (100 pmol, internal standard), and dopamine levels were measured using an HPLC system [2]. For the determination of serotonin levels, brain samples were homogenized in trichloroacetic acid (500 µl, 0.3 M) and HIAA (300 pmol, internal standard), and serotonin levels were measured using an HPLC system [19].
Blood was collected from the hearts of sacrificed mice and subsequently centrifuged to obtain a serum (13,000×g, 15 min, 4 °C). Corticosterone levels were assessed using an enzyme-linked immunosorbent assay kit [19].
Immunohistochemistry of c-Fos
Mice were intracardially perfused with a paraformaldehyde solution (4% in 0.1 M phosphate buffered saline, pH 7.4), following which the brains were removed. Coronal section (35 μm) at the PVN regions were processed for c-Fos immunocytochemistry using polyclonal rabbit anti-c-Fos antibody (1:500) and biotinylated goat anti-rabbit antibody (1:1000; Vector Laboratories, Burlingame, CA) according to a previously described protocol [2]. Cells positive for c-Fos in the PVN were counted using an image analysis system (Axiovision software, Carl Zeiss MicroImaging, GmbH, Jena, Germany) and microscope (×100 magnification) (Zeiss Axiophot, Carl Zeiss MicroImaging).
Statistical Analysis
Data were analyzed using one-way analyses of variance (ANOVA) followed by Tukey’s test. All data are represented as means ± SEM with p values <0.05 being considered statistically significant.
Results
Effects of (−)-Sesamin in the Elevated Plus-Maze Test
Following 21 days of exposure to chronic EF stress, the number of open arm entries and time spent on the open arms were decreased to 64.2% (df = 19, F = 30.2, p < 0.01, n = 10) and 63.0% (df = 19, F = 36.5, p < 0.01, n = 10), respectively, in the chronic EF stress group compared to the control group (Fig. 1a, b). However, less severe decreases in the number of open arm entries were observed in the chronic EF stress + (−)-sesamin (25 mg/kg) (76.8, df = 19, F = 9.08, p < 0.05, n = 10) and chronic EF stress + (−)-sesamin (50 mg/kg) (81.8%, df = 19, F = 15.7, p < 0.05, n = 10) groups (Fig. 1a) when compared to the chronic EF stress group. Similarly, less severe decreases were also observed in the amount of time spent on open arms in both the chronic EF stress + (−)-sesamin (25 mg/kg) (76.1%, df = 19, F = 4.65, p < 0.05, n = 10) and chronic EF stress + (−)-sesamin (50 mg/kg) (77.4%, df = 19, F = 5.00, p < 0.05, n = 10) groups (Fig. 1b). In addition, the number of open arm entries (df = 29, F = 17.9, p < 0.05. n = 10) and time spent on the open arms (df = 29, F = 16.1, p < 0.05, n = 10) in chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups were significant compared to the control group following exposure to chronic EF stress for 21 days (Fig. 1a, b).
Effects of (−)-sesamin on the number of open arm entries (a), the time spent on open arms (b), and distance traveled (c) in the elevated plus-maze test. Control group (I), chronic EF stress (II), chronic EF stress + (−)-sesamin (25 mg/kg) (III), chronic EF stress + (−)-sesamin (50 mg/kg) (IV). The results are expressed as the mean ± SEM for ten animals per group. *p < 0.01 compared to the control group; # p < 0.05 compared to the chronic EF stress group; § p < 0.05, compared between the control group and chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups
The distance traveled in the elevated plus-maze test was significantly decreased to 68.6% (df = 19, F = 45.2, p < 0.01, n = 10) in the chronic EF stress group compared to the control group following 21 days of exposure to chronic EF stress (Fig. 1c). However, the decreases of distance traveled were significantly less pronounced in the chronic EF stress + (−)-sesamin (25 mg/kg) (82.1%, df = 19, F = 7.70, p < 0.05, n = 10) and chronic EF stress + (−)-sesamin (50 mg/kg) (85.9%, df = 19, F = 16.9, p < 0.05, n = 10) groups (Fig. 1c), compared to the chronic EF stress group. The distance traveled in chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups (df = 29, F = 15.8, p < 0.05, n = 10) was also significant compared to the control group following exposure to chronic EF stress for 21 days (Fig. 1c).
Effects of (−)-Sesamin in the Open Field Test
The distance traveled in the open field test was significantly decreased to 64.4% (df = 19, F = 48.1, p < 0.01, n = 10) in the chronic EF stress group compared to the control group after 21 days of exposure to chronic EF stress (Fig. 2). However, less severe decreases in the distance traveled were observed in the chronic EF stress + (−)-sesamin (25 mg/kg) (80.2%, df = 19, F = 11.2, p < 0.05, n = 10) and chronic EF stress + (−)-sesamin (50 mg/kg) (82.2%, df = 19, F = 12.2, p < 0.05, n = 10) groups when compared to the chronic EF stress group (Fig. 2). In addition, the distance traveled in chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups (df = 29, F = 31.3, p < 0.01, n = 10) was significant compared to the control group following exposure to chronic EF stress for 21 days (Fig. 2).
Effects of (−)-sesamin on distance traveled in the open field test. Control group (I), chronic EF stress (II), chronic EF stress + (−)-sesamin (25 mg/kg) (III), chronic EF stress + (−)-sesamin (50 mg/kg) (IV). The results are expressed as the mean ± SEM for ten animals per group. *p < 0.01 compared to the control group; # p < 0.05 compared to the chronic EF stress group; §§ p < 0.01 compared between the control group and chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups
Effects of (−)-Sesamin on Levels of Dopamine, Norepinephrine, and Serotonin in the Brain
Significantly decreased levels of dopamine were observed in the chronic EF group (66.5%, df = 15, F = 15.0, p < 0.01, n = 8) relative to controls following 21 days of exposure to chronic EF stress (Fig. 3a). In the chronic EF stress + (−)-sesamin (25 mg/kg) and chronic EF stress + (−)-sesamin (50 mg/kg) groups, however, such decreases were significantly less pronounced (78.6%, df = 15, F = 5.75, p < 0.05, n = 8; 81.7%, df = 15, F = 7.53, p < 0.05, n = 8, respectively) than in the chronic EF stress group (Fig. 3a). The levels of dopamine in chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups (df = 23, F = 5.11, p < 0.05, n = 8) were also significant compared to the control group following exposure to chronic EF stress for 21 days (Fig. 3a).
Effects of (−)-sesamin on dopamine (a), norepinephrine (b), and serotonin (c) levels in the brain. Control group (I), chronic EF stress (II), chronic EF stress + (−)-sesamin (25 mg/kg) (III), chronic EF stress + (−)-sesamin (50 mg/kg) (IV). Dopamine, norepinephrine, and serotonin levels in the control group (ng/mg of tissue) were 5.1 ± 1.2, 2.1 ± 0.7, and 3.2 ± 1.1, respectively. The results are expressed as the mean ± SEM for eight animals per group. *p < 0.01 compared to the control group; # p < 0.05 compared to the chronic EF stress group; § p < 0.05, compared between the control group and chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups
In addition, significantly decreased levels of norepinephrine were observed in the chronic EF group (70.2%, df = 15, F = 8.72, p < 0.01, n = 8) relative to controls following 21 days of exposure to chronic EF stress (Fig. 3b). In the chronic EF stress + (−)-sesamin (25 mg/kg) and chronic EF stress + (−)-sesamin (50 mg/kg) groups, the decreases in norepinephrine were significantly less pronounced (85.7%, df = 15, F = 4.71, p < 0.05, n = 8; 87.1%, df = 15, F = 5.33, p < 0.05, n = 8, respectively), than in the chronic EF stress group (Fig. 3b). The levels of norepineprine in chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups (df = 23, F = 1.63, p > 0.05, n = 8) were also not significant compared to the control group, following exposure to chronic EF stress for 21 days (Fig. 3b).
Furthermore, the significant decreased levels of serotonin were observed in the chronic EF group (64.7%, df = 15, F = 9.63, p < 0.01, n = 8) relative to controls (Fig. 3c). In the chronic EF stress + (−)-sesamin (25 mg/kg) and chronic EF stress + (−)-sesamin (50 mg/kg) groups, however, such decreases were significantly less pronounced (80.5%, df = 15, F = 5.23, p < 0.05, n = 8; 84.2%, df = 15, F = 7.91, p < 0.05, n = 8, respectively) than in the chronic EF stress group (Fig. 3c). In addition, the levels of serotonin in chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups (df = 23, F = 6.30, p < 0.05, n = 8) were significant compared to the control group, following exposure to chronic EF stress for 21 days (Fig. 3c).
Effects of (−)-Sesamin on Serum Levels of Corticosterone
Corticosterone levels in the chronic EF stress group increased to 185.9% (df = 15, F = 79.6, p < 0.01, n = 8) relative to controls (Fig. 4) following exposure to chronic EF stress. However, these increases were less pronounced in the chronic EF stress + (−)-sesamin (25 mg/kg) and chronic EF stress + (−)-sesamin (50 mg/kg) groups (150.8%, df = 15, F = 48.1, p < 0.01, n = 8 and 140.6%, df = 15, F = 58.7, p < 0.01, n = 8, respectively) than in the chronic EF stress group (Fig. 4). In addition, the corticosterone levels in chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups (df = 23, F = 40.5, p < 0.01, n = 8) was significant compared to the control group following exposure to chronic EF stress for 21 days (Fig. 4).
Effects of (−)-sesamin on serum corticosterone levels. Control group (I), chronic EF stress (II), chronic EF stress + (−)-sesamin (25 mg/kg) (III), chronic EF stress + (−)-sesamin (50 mg/kg) (IV). Corticosterone levels in the control group were 196.2 ± 26.8 ng/ml. The results are expressed as the mean ± SEM for eight animals per group. *p < 0.01 compared to the control group; ## p < 0.01 compared to the chronic EF stress group; §§ p < 0.01 compared between the control group and chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups
Effects of (−)-Sesamin on c-Fos Expression in the PVN
Expression of c-Fos positive cells significantly increased to 265.3% (df = 15, F = 95.6, p < 0.001, n = 8) relative to controls in the chronic EF stress group following exposure to chronic EF stress (Fig. 5). However, these increases were less pronounced in the chronic EF stress + (−)-sesamin (25 mg/kg) (173.6%, df = 15, F = 45.1, p < 0.01, n = 8) and chronic EF stress + (−)-sesamin (50 mg/kg) (167.9%, df = 15, F = 51.3, p < 0.01, n = 8) groups (Fig. 5). The expression of c-Fos in chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups (df = 23, F = 46.8, p < 0.01, n = 8) was also significant compared to the control group following exposure to chronic EF stress for 21 days (Fig. 5).
Representative photographs illustrating the effects of (−)-sesamin on c-Fos positive cells in the PVN (a) and the number of c-Fos positive cells (b). Control group (I), chronic EF stress (II), chronic EF stress + (−)-sesamin (25 mg/kg) (III), chronic EF stress + (−)-sesamin (50 mg/kg) (IV). The number of c-Fos positive cells in the control group was 30 ± 6 cells per section. Arrows indicate the nuclei of c-Fos-positive neurons. Scale bar is 100 µm. The results are expressed as the mean ± SEM for ten animals per group. **p < 0.001 compared to the control group; ## p < 0.01 compared to the chronic EF stress group; §§ p < 0.01 compared between the control group and chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups
Discussion
The elevated plus-maze reflects passive avoidance of an animal in response to a potential threat and has become a very popular approach for the evaluation of anxiety behaviors by assessing the number of open arm entries as well as the time spent on open arms [21]. The distance traveled in the elevated plus-maze and open field tests is a common measure of exploratory and spontaneous locomotor activity in rodents [20–22]. The elevated plus-maze test is closely associated with dopamine and serotonin activities [9, 10, 23], and the open field test is also associated with serotonin activity [8]. Chronic EF stress may decrease spontaneous locomotor activity in mice [2]. In this study, the effects of (−)-sesamin on chronic EF stress-induced anxiety disorders in mice were investigated. Exposure to chronic EF stress decreased the number of open arm entries of the elevated plus-maze and the spontaneous locomotor activity by distance traveled of the elevated plus-maze and open field tests. However, these decreases were less severe in the chronic EF stress + (−)-sesamin (25 and 50 mg/kg) groups than in the chronic EF stress group (Figs. 1, 2). In addition, the decreased levels of dopamine, norepinephrine, and serotonin by chronic stress in the brain were recovered by (−)-sesamin treatment (Fig. 3a–c). These results indicate that (−)-sesamin can ameliorate anxiety-like behaviors induced by chronic EF stress.
Under stressed conditions, changes in dopamine, norepinephrine, and serotonin are associated with behavioral disturbances including anxiety, and learning and memory [2, 20]. Chronic EF stress may induce anxiety disorders, which are closely linked to decreased levels of dopamine, norepinephrine, and serotonin in the brain [3]. In this experiment, treatment with (−)-sesamin (25 and 50 mg/kg) significantly attenuated decreases in levels of dopamine, norepinephrine, and serotonin following exposure to chronic stress (Fig. 3a–c).
Corticosteroids such as corticosterone are secreted from the adrenal glands in response to chronic stress, a process mediated by the HPA axis [4]. In chronic stress-induced anxiety-like states, increased levels of corticosterone relative to unstressed states are maintained [3]. However, levels of corticosterone associated with chronic stress may be lower than those induced by acute stress [2, 24], though persistently elevated corticosteroid levels may influence dopamine and serotonin levels [3]. The levels of dopamine, norepinephrine, and serotonin in the PVN are also enhanced by corticosterone levels, which are associated with the release of CRH [7]. Therefore, the levels of dopamine, norepinephrine, and serotonin may be implicated in the acute and chronic stress responses in the PVN [7]. In addition, corticosterone levels have high correlation with elevated plus-maze [25] and chronic corticosterone treatment increases anxiety-like behaviors in the elevated plus maze and open field tests in rats [26]. Valtrate isolated from Valeriana jatamansi may also reduce both anxiety-like behaviors in the elevated plus-maze and open field tests and concurrently corticosterone levels in the serum in rats [27].
In present study, treatment with (−)-sesamin (25 and 50 mg/kg) significantly decreased the serum levels of corticosterone, which were increased due to chronic stress exposure (Fig. 4), and concurrently recovered the decreased levels of dopamine, norepinephrine, and serotonin (Fig. 3). (−)-Sesamin (25 and 50 mg/kg) also recovered decreased in the number of open arm entries and spontaneous locomotor activities in the elevated plus-maze and open field tests (Figs. 1, 2). These results showed that (−)-sesamin has anxiolytic effects by modulating corticosterone levels.
In addition, c-Fos in the PVN is an immediate early gene linked with glucocorticoid regulation whose activation is induced following exposure to chronic and acute stresses in mice and rats [5, 6]. The PVN is essential for the regulation of HPA axis hormone secretion, which may ultimately influence levels of corticosterone [28]. In this study, the increased expression of c-Fos protein following exposure to chronic EF stress was significantly less pronounced in animals treated with (−)-sesamin (25 and 50 mg/kg) (Fig. 4).
(−)-Sesamin has an inhibitory effect on LPS-induced nitric oxide production in BV-2 microglial cells, which is associated with oxidative stress and inflammation [8, 29] and exerts protective effects against 6-OHDA-induced oxidative cell death in 6-OHDA-lesioned rat models of PD during long-term L-DOPA treatment [15]. (−)-Sesamin also reduces anxiety-like behaviors induced by chronic pain via regulating the GABAergic and glutamatergic transmission in the amygdala of mice [16]. In addition, (+)-sesamin possesses reactive oxygen species scavenging activity, allowing it to modulate the ERK1/2, p38MAPK, and JNK1/2 pathways in PC12 cells [30, 31], and can scavenge free radicals and superoxide anions [32]. With respect to these studies, our findings suggest that the protective functions of (−)-sesamin against oxidative stress-induced cell death may play a role in producing the observed anxiolytic effects on chronic stress-induced anxiety-like disorders in the mouse model.
In conclusion, the present study indicate that (−)-sesamin attenuates the behavioral effects of chronic EF stress-induced anxiety disorders by modulating levels of dopamine, norepinephrine, and serotonin as well as c-Fos expression and corticosterone levels. These results suggest that (−)-sesamin may serve as a phytonutrient for the prevention of chronic stress-induced anxiety disorders, though further clinical evaluation is required.
References
Rajkowska G (2000) Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry 48:766–777
Zhao TT, Shin KS, Choi HS, Lee MK (2015) Ameliorating effects of gypenosides on chronic stress-induced anxiety disorders in mice. BMC Complem Altern Med 15(323):1–10
Sheikh N, Ahm A, Siripurapu KB, Kuchibhotla VK, Singh S, Pali G (2007) Effect of Bacopa monniera on stress induced changes in plasma corticosterone and brain monoamines in rats. J Ethnopharmacol 111:671–676
Sheline YI (2000) 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry 48:791–800
Chen X, Herbert J (1995) Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine. Neuroscience 64:675–685
Imaki T, Katsumata H, Konishi SI, Kasagi Y, Minami S (2003) Corticotropin-releasing factor type-1 receptor mRNA is not induced in mouse hypothalamus by either stress or osmotic stimulation. J Neuroendocrinol 15:916–924
Russell JA, Shipston MJ (2015) Neuroendocrinology of stress. In: Hale MW, Lowry CA (eds) Brain m onoaminergic s ystems in s tress n euroendocrinology, Chap. 2. Wiley, New York, pp 19–42
Broderick PA, Phelix CF (1997) Serotonin (5-HT) within dopamine reward circuits signals open-field behavior. II. Basis f or 5-HT-DA interaction in cocaine dysfunctional behavior. Neurosci Biobehav Rev 21:227–260
Espejo EF (1997) Selective dopamine depletion within the medial prefrontal cortex induces anxiogenic-like effects in rats placed on the elevated plus maze. Brain Res 762:281–284
G riebel G, R odgers RJ, Perrault G, S anger DJ (1997) Risk assessment behaviour: evaluation of utility in the study of 5-HT-related drugs in the rate levated plus-maze test. Pharmacol Biochem Behav 57:817–827
Han AR, Kim HJ, Shin M, Hong M, Kim YE, Bae H (2008) Constituents of Asarum sieboldii with inhibitory activity on lipopolysaccharide (LPS)-induced NO production in BV-2 microglial cells. Chem Biodivers 5:346–351
Peñalvo JL, Hopia A, Adlercreutz H (2006) Effect of sesamin on serum cholesterol and triglycerides levels in LDL receptor-deficient mice. Eur J Nutr 45:439–444
Suzuki Y, Yuzurihara M, Hibino T, Yano S, Kase Y (2009) Aqueous extract of Asiasari Radix inhibits formalin-induced hyperalgesia via NMDA receptors. J Ethnopharmacol 123:128–133
Han Y, Kim SJ (2003) Memory enhancing actions of Asiasari Radix extracts via activation of insulin receptor and extracellular signal regulated kinase (ERK) I/II in rat hippocampus. Brain Res 974:193–201
Park HJ, Zhao TT, Lee KS, Lee SH, Shin KS, Park KH et al (2015) Effects of (−)-sesamin on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and dopaminergic neuronal cells of Parkinson’s disease rat models. Neurochem Int 83–84:19–27
Guo HL, Xiao Y, Tian Z, Li XB, Wang DS, Wang XS, Zhang ZW, Zhao MG, Liu SB (2016) Anxiolytic effects of sesamin in mice with chronic inflammatory pain. Nutr Neurosci 19:231–236
Kumar B, Kuhad A, Chopra K (2011) Neuropsychopharmacological effect of sesamol in unpredictable chronic mild stress model of depression: behavioral and biochemical evidences. Psychopharmacology (Berl) 214:819–828
Li CY, Chow TJ, Wu TS (2005) The epimerization of sesamin and asarinin. J Nat Prod 68:1622–1624
Choi HS, Zhao TT, Shin KS, Kim SH, Hwang BY, Lee CK (2013) Anxiolytic effects of herbal ethanol extract from Gynostemma pentaphyllum after exposure to chronic stress in mice. Molecules 18:4342–4356
Zhao TT, Shin KS, Kim KS, Park HJ, Kim HJ, Lee KE, Lee MK (2016) Effects of (−)-sesamin on motor and memory deficits in an MPTP-lesioned mouse model of Parkinson’ s disease treated with L-DOPA. Neuroscience 339:644–654
Lister RG (1987) The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 92:180–185
Verma P, Hellemans KGC, Choi FY, Yu W, Weinberg J (2010) Circadian phase and sex effects on depressive/anxiety-like behaviors and HPA axis responses to acute stress. Physiol Behav 99:276–285
Vallone D, Picetti R, Borrelli E (2000) Structure and function of dopamine receptors. Neurosci Biobehav Rev 24:125–132
Zhao TT, Shin KS, Choi HS, Lee MK (2013) Effects of gypenosides on acute stress in mice. Nat Prod Sci 19:337–341
Rodgers RJ, Haller J, Holmes A, Halasz J, Walton TJ, Brain PF (1999) Corticosterone response to the plus-maze: high correlation with risk assessment in rats and mice. Physiol Behav 68:47–53
Skórzewska A, Lehner M, Wisłowska-Stanek A, Krząścik P, Ziemba A, Płaźnik A (2014) The effect of chronic administration of corticosterone on anxiety- and depression-like behavior and the expression of GABA-A receptor alpha-2 subunits in brain structures of low- and high-anxiety rats. Horm Behav 65:6–13
Shi SN, Shi JL, Liu Y, Wang YL, Wang CG, Hou WH, Guo JY (2014) The anxiolytic effects of valtrate in rats involves changes of corticosterone levels. Evid-Based Compl Alt 325948:1–8
Smith SM, Vale WW (2006) The role of the hypothalamic–pituitary–adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 8:383–395
Ohshima H, Bartsch H (1994) Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res 305:253–264
Hou RCW, Huang HM, Tzen JTC, Jeng KCG (2003) Protective effects of sesamin and sesamolin on hypoxic neuronal and PC12 cells. J Neurosci Res 74:123–133
Hamada N, Fujita Y, Tanaka A, Naoi M, Nozawa Y, Ono Y et al (2009) Metabolites of sesamin, a major lignan in sesame seeds, induce neuronal differentiation in PC12 cells through activation of ERK1/2 signaling pathway. J Neural Transm 116:841–852
Kuo PC, Lin MC, Chen GF, Yiu TJ, Tzen JTC (2011) Identification of methanol-soluble compounds in sesame and evaluation of antioxidant potential of its lignans. J Agric Food Chem 59:3214–3219
Acknowledgements
This research was financially supported by the National Research Foundation of Korea (Grant No. 2013R1A1A2058230, 2015–2016), Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhao, T.T., Shin, K.S., Park, H.J. et al. Effects of (−)-Sesamin on Chronic Stress-Induced Anxiety Disorders in Mice. Neurochem Res 42, 1123–1129 (2017). https://doi.org/10.1007/s11064-016-2146-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2146-z