Abstract
Oxidative stress mediates the pathogenesis of neurodegenerative disorders. Gartanin, a natural xanthone of mangosteen, possesses multipharmacological activities. Herein, the neuroprotection capacity of gartanin against glutamate-induced damage in HT22 cells and its possible mechanism(s) were investigated for the first time. Glutamate resulted in cell death in a dose-dependent manner and supplementation of 1–10 µM gartanin prevented the detrimental effects of glutamate on cell survival. Additional investigations on the underlying mechanisms suggested that gartanin could effectively reduce glutamate-induced intracellular ROS generation and mitochondrial depolarization. We further found that gartanin induced HO-1 expression independent of nuclear factor erythroid-derived 2-like 2 (Nrf2). Subsequent studies revealed that the inhibitory effects of gartanin on glutamate-induced apoptosis were partially blocked by small interfering RNA-mediated knockdown of HO-1. Finally, the protein expression of phosphorylation of AMP-activated protein kinase (AMPK) and its downstream signal molecules, Sirtuin activator (SIRT1) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), increased after gartanin treatment. Taken together, these findings suggest gartanin is a potential neuroprotective agent against glutamate-induced oxidative injury partially through increasing Nrf-2-independed HO-1 and AMPK/SIRT1/PGC-1α signaling pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress has been implicated in a variety of chronic neurodegenerative disorders, such as Parkinson’s disease (PD), Alzheimer’s disease (AD) and amyotrophic lateral sclerosis (ALS) [1]. Excessive reactive oxygen and nitrogen species (ROS and RNS) production damage redox balance, oxidate DNA, especially mitochondrial DNA, protein as well as lipid, and eventually lead to the impairment of biological function [2]. Sustained oxidative stress results in axonal degeneration and neuronal apoptosis and finally induces damage to brain structures [1, 3]. Glutamate is the principal excitatory amino acid neurotransmitter with complex biological activities [4, 5]. However, high concentration of extracellular glutamate is toxic to nerve cells and is thought to be a key contributor in the pathogenesis of AD and PD [6–8]. The pathway of glutamate-induced cell death in the nervous system can be activated by either excitotoxicity or oxidative toxicity (glutamate-induced oxytosis). HT22 cell line is derived from the hippocampus neuronal precursor cells and lacks functional ionotropic glutamate receptors. Glutamate can block glutamate-cystine antiporters, deplete cellular glutathione (GSH), and finally induce necrosis and apoptosis instead of excitocytoxicity in HT22 cells. In addition, glutamate toxicity induces the intracellular ROS accumulation, commonly accompanied by the collapse of the mitochondrial membrane potential (ΔΨm). This makes the HT22 cell line serve as an excellent model of glutamate-induced oxidative neurotoxicity [9–12]. Therefore, the glutamate-induced cell death in HT22 cells has been widely used as an in vitro assay to screen neuroprotective compounds and to elucidate its neuroprotective mechanisms [13–15].
Heme oxygenase (HO) is an enzyme that catalyzes the degradation of heme [16]. HO isoforms catalyze the conversion of heme to carbon monoxide (CO) and bilirubin with a concurrent release of iron, which can drive the synthesis of ferritin for iron sequestration [17]. HO is the sole physiological pathway of heme degradation and, consequently, plays a critical role in the regulation of cellular heme-dependent enzyme levels [18]. HO-1, the inducible isoform, is found ubiquitously in all organs with the exception of the adult brain and is rapidly and transiently expressed after various stressor stimuli [19]. Although initial interest in HO-1 focused on its role in heme metabolism, recent studies hat HO-1 is highly induced by agents that cause oxidative stress have renewed interest in the regulation and function of HO-1 [20]. Being one of the most representative vitagenes, HO-1 plays a crucial role in oxidative stress processes, apoptosis and cell differentiation [21]. Now lots of reports have revealed that small molecules, such as neolignans [22], acerogenin A [23], sanguinarine [24], piceatannol [25] could antagonize glutamate-mediated oxidative injury in HT22 neuronal cells through the induction of HO-1.
Herbaceous plants are rich sources of biologically active compounds. Now, to explore natural antioxidants from herbs has become an alternative strategy for devastating degenerative pathologies [26–28]. Mangosteen (Garcinia mangostana L), ‘‘the queen of fruits’’, is native to Thailand, Malaysia and other tropical countries [29, 30]. The purple pericarp of mangosteen contains rich healthy nutrients and pharmacologically active compounds including prenylated xanthones, triterpenes, tannins and benzophenones [31, 32]. According to the reported work, more than 68 distinct xanthones have been identified in different parts of the mangosteen plant, and among them, 50 xanthones have a higher abundance in pericarp than in other parts [33, 34]. Those xanthones possess a wide range of pharmacological activities, including anti-inflammatory, neuroprotective, cardioprotective, antioxidative, antimicrobial, antiretroviral, antimalarial, analgesic and anticomplement activities, and so on [30, 31, 35–37].
Gartanin is a xanthone isolated from the mangosteen pericarp and was first reported in 1971 [38]. Increasing studies have reported that gartanin exert several biological actions, such as anti-bacterial, anti-cancer, anti-inflammatory and anti-oxidant activity [39–41], but the underlying mechanisms of neuroprotective effects have not yet been clarified. As gartanin contains xanthone nuclear structure, featured phenolic hydroxy and isoprene moieties, which are the characteristics of an antioxidant, its antioxidative activity triggers extensive research interest. Herein, it was the first time to investigate the neuroprotection of gartanin against glutamate-induced cell death in HT22 cells and its possible mechanism(s). Our data indicated that gartanin protected neurons against glutamate-induced cell death in HT22 cells independence of nuclear factor erythroid-derived 2-like 2 (Nrf-2), but involvement of HO-1 and AMP-activated protein kinase (AMPK).
Materials and Methods
Chemicals and Reagents
Gartanin was purified from the pericarps of mangosteen as previously described [39]. HT22 cells were received as a generous gift from the Second Affiliated Hospital of Sun Yat-Sen University (Guangzhou, China). 2,7-dichlorofluoroescin diacetate (H2DCF-DA) and DHE were purchased from Molecular Probes Co. (agency in China). Annexin V-FITC/PI kit was purchased from Keygen Biotech (Nanjing, China), GSH assay kits was purchased from Jiancheng Biochemical (Nanjing, China). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco-BRL (NY, USA). The following antibodies were used: Bcl-2, SIRT1, phospho-AMPKα (Thr172), Nrf-2 and AMPK (all from Cell Signaling Technologies), Bax (from Abclonal), HO-1 (from Bioworld Technology), and β-actin, PGC-1α (all from Sigma-Aldrich). Others unless stated elsewhere were purchased from Sigma-Aldrich.
Cell Culture
HT22 cells were maintained in DMEM supplemented with 10 % (v/v) FBS and incubated at 37 °C under 5 % CO2. Cell morphology was observed by an inverse phase contrast microscopy (Olympus, Japan).
Assessment of Cell Viability by 3-(4.5-Dimethylthiazol-2-yl) 2,5-diphenyl-tetrazolium bromide (MTT) Assay
To study the neuroprotective effects of gartanin against glutamate-induced neuronal death and cytotoxicity, HT22 cells were seeded into 96-well plates with a density of 4 × 103 cells/well and incubated overnight. Cells were treated with gartanin or the vehicle control DMSO for 30 min followed with/without 2 mM glutamate for 24 h. 10 µL of 5 mg/mL MTT were added to each well and cells were incubated for 2 h at 37 °C. To dissolved purple formazan crystal, 100 µL DMSO was added to replace the medium. After vigorously shaking for 15 min at 37 °C, the absorbance was measured at 570-nm wavelength using a microculture plate reader (Bio-Tek, USA). The test was repeated three times. All data were represented as folds over control.
Apoptosis Analysis by Flow Cytometry
The apoptotic ratios of cells were determined by the Annexin V-FITC apoptosis detection kit according to the supplier’s protocols. HT22 cells were harvested, centrifuged, and resuspended in 200 μL of binding buffer, incubated with 5 μL of AnnexinV-FITC for 10 min, added with 10 μL Propidium Iodide (PI) for 15 min at room temperature, and analyzed by flow cytometry (Molecular Devices, Sunnyvale, CA, USA) immediately. Values were expressed as a percentage of the fluorescence relative to the non-injured control.
Measurement of Intracellular ROS
HT22 Cells were grown in Corning 48-well plates at a cell density 2 × 104 cells/well. After overnight attachment, cells were pretreated with gartanin or the vehicle control DMSO for 30 min and then incubated with/without 2 mM glutamate for 12 h. Cells were washed twice with phosphate-buffered saline (PBS) and then incubated with 10 μM non-fluorescent dye H2DCF-DA or DHE in serum-free medium for 30 min at 37 °C in the dark. Cells were subsequently washed twice with PBS and photographed using a (200×) fluorescence microscope or analyzed by flow cytometry. Values were expressed as a percentage of the fluorescence relative to the vehicle control.
Determination of ΔΨm
Rhodamine 123 (R123) was used to determine the ΔΨm. R123 is a fluorescent aromatic monovalent cation that accumulates in the matrix of energized mitochondria. After treatment, cells were incubated with 5 μM R123 in PBS for 15 min at 37 °C in the dark, then washed with PBS and incubated at 37 °C for the indicated time before cellular levels of R123 were measured by flow cytometry. Values were expressed as a percentage of the fluorescence relative to the non-injured control.
Western Blot Analysis
HT22 cells were plated in 6-well plates and grown overnight. After treatment, protein was lysed, quantified, and denatured. First, the 20 μg protein were loaded in each well, separated by 10 % SDS-PAGE, and transferred electrophoretically onto the PVDF membranes. Following 2 h blocking with 5 % skim milk, the membranes were incubated of primary antibodies for overnight at 4 °C. Next, the TBST buffer (PBS with 0.01 % Tween 20, pH 7.4) washed membranes three times and then incubated with appropriate horseradish peroxidase-linked anti-mouse or anti-rabbit IgG (1:1000) for 1 h at room temperature. Finally, protein bands were detected using enhanced chemiluminescence (ECL) system (Thermo Scientific) and imaged on BioRad image Quant LAS 4000mini. The intensities of bands were performed using Quantity One Software (Bio-Rad, Hercules, CA, USA).
Transfection and Luciferase Reporter Assay
HT22 cells planted in 48-well plates (1 × 105 cells/well) were transfected with either pARE-luc or pGL6-luc cis reporter plasmids along with pRL-TK renilla. Transfections were performed using 200 ng DNA, 0.3 μL Lipofectamine™ 3000 (Invitrogen) and 0.4 μL P3000/well according to the manufacturer’s instructions. After transfection 24 h, cells were treated with gartanin or tert-butyl hydroquinone (t-BHQ). Luciferase activity was detected at 24 h using the dual luciferase assay system (Promega, Madison, WI, USA). The Renilla luciferase activity was normalized to the firefly luciferase activity.
HO-1 Small Interfering RNA (siRNA) Transfection
siRNA duplexes and nontargeting control siRNA were purchased from Jima Biotechnology (Shanghai, China). The sense and antisense strands of murine HO-1 siRNA were as follows: sequence 1, 5′-CCACACAGCACUAUGUAAATT-3′ (sense) and 5′-UUUACAUAGUGCUGUGUGGTT-3′ (antisense); sequence 2, 5′-CCGAGAAUGCUGAGUUCAUTT-3′ (sense) and 5′-AUGAACUCAGCAUUCUCGGTT-3′ (antisense); sequence 3, 5′-GCUGACAGAGGAACACAAATT-3′ (sense) and 5′-UUUGUGUUCCUCUGUCAGCTT-3′ (antisense). The transfection was mediated by Lipofectamine™ 3000 according to the manufacturer’s instructions. The cells were transfected at a density of ~50 % confluence with siRNA duplex (100 nM). After 6 h, media were changed, and the cells were incubated for another 48 h and processed for MTT assay or western blotting analysis.
Statistical Analysis
All analyses were repeated at least in duplicate. Representative experiments are shown and statistical analyses among group were performed by either one- or two-way analysis of variance (ANOVA) with Tukey’s post hoc test. Differences were considered statistically significant at P < 0.05.
Results
Gartanin Reduced Glutamate-Induced Oxidative Toxicity in HT22 Cells
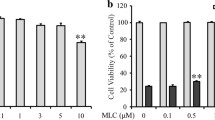
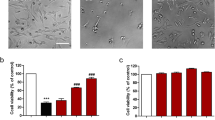
To determine the effect of gartanin on oxidative stress, cells were exposed to glutamate and cell survival was measured using MTT assay. Glutamate induced cell death in a concentration-dependent manner (Fig. 1a). Incubation with glutamate (2 mM) for 24 h markedly reduced the HT22 neuronal cell viability, and resulted in chromatin condensation and blebbing of cell membrane, like apoptotic cell death (Fig. 1b). These changes were significantly attenuated when cells were pretreated with gartanin (1–10 μM) for 30 min (Fig. 1b, c). The highest concentration (10 μM) of gartanin was not more effective than the concentrations of 3 μM (3 μM gartanin 98.20 ± 2.07 %, VS 10 μM gartanin, 92.57 ± 1.39 %). The reason might be that 10 μM gartanin slightly inhibited cell MTT reduction (Fig. 1d). Therefore, the concentration of 3 μM gartanin was selected as the optimal concentration for further experiments.
Gartanin protected HT22 cells against glutamate-induced cell death. a Cell viability was measured by MTT assay after exposed to 0–4 mM glutamate for 24 h. b HT22 cells were photographed using a photomicroscope (×200) after cells were pretreated with 3 μM gartanin for 30 min and then incubated with 2 mM glutamate for 24 h. c HT22 cells were pretreated with 0.3, 1, 3, and 10 μM gartanin for 30 min and then incubated with 2 mM glutamate for 24 h, cells viability was determined by MTT assay. d MTT assay was used to measure cell viability after treatment with 0.3–100 μM gartanin for 24 h. Data are presented as mean ± SEM (n = 3). ***P < 0.001; **P < 0.01; *P < 0.05 versus control group. ### P < 0.001 versus glutamate-treated group. CT control, Glu glutamate. Scale bar 50 μM
Gartanin Decreased Glutamate-Induced Apoptotic Death and Increased the Ratio of Bcl-2/Bax Expression
We examined the effects of gartanin on glutamate-induced apoptosis by Annexin V-FITC/PI double staining analyzed by flow cytometry. Early apoptotic stage is marked by FITC+/PI−. As shown in Fig. 2a, after 12 h stimulation with glutamate (2 mM), the proportion of the fraction marked by FITC+/PI− increased from 1.03 to 16.03 %,whereas cells pretreated with gartanin showed a lower proportion (1.19 %).
Gartanin decreased glutamate-induced HT22 cell apoptosis and increased the ratio of Bcl-2/Bax expression. a HT22 cells were treated with 3 μM gartanin for 30 min, before exposed to 2 mM glutamate. After 12 h, Annexin V-FITC/PI staining was analyzed by flow cytometry and the percentage of early apoptotic stage demonstrated by FITC+/PI− was analyzed. b Bcl-2 and Bax protein level was detected by western blot in HT22 cells treated with 3 μM gartanin for indicated times. The loading control was β-actin. Quantitative analysis was expressed as Bcl-2 relative to Bax following exposure to vehicle control. Data are presented as mean ± SEM (n = 3). *** P < 0.001; **P < 0.01, versus control group. ### P < 0.001; ## P < 0.01 versus glutamate-treated group
A crucial amplification event in the apoptotic cascades is the nearly complete release of cytochrome C from mitochondria [42]. This is regulated by Bcl-2 family proteins, which include anti- and pro-apoptotic members. HT22 cells were treated with 3 μM gartanin for indicated times, and Bcl-2 and Bax protein levels were determined by western blot analysis. Gartanin increased the expression of Bcl-2 but not Bax (Fig. 2b). Glutamate treatment decreased the expression of Bcl-2, whereas the level of Bax was not markedly altered. Gartanin effectively maintained the Bcl-2 protein level down-regulated by glutamate (Fig. 2c).
Gartanin Reduced Glutamate-Induced ROS Accumulation and Mitochondrial Membrane Depolarization
A number of studies have indicated that glutamate-mediated neuronal cell death is closely associated with oxidative stress including excessive ROS production and mitochondrial damage [8, 43]. To test whether gartanin could protect mitochondria against glutamate, we examined glutamate-induced alterations in ROS level and ΔΨm in the presence of 3 μM gartanin in HT22 cells. On the one hand, H2DCF-DA and DHE staining were performed to measure intracellular ROS production. DCF and DHE fluorescence were analyzed by visual observation of cell morphology through fluorescence microscopy (Fig. 3a) and flow cytometry analysis (Fig. 3b). On the other hand, ΔΨm was measured using R123 dye (Fig. 3c). Data showed glutamate induced intracellular ROS accumulation and mitochondrial membrane depolarization in HT22 cells, which was significantly reduced by pretreatment with gartanin for 30 min prior to glutamate treatment.
Gartanin significantly decreased glutamate-induced ROS and stabilized mitochondrial membrane potential. HT22 cells were pretreated with 3 μM gartanin for 30 min and then incubated with 2 mM glutamate for 12 h. a DCF and DHE fluorescence were analyzed by visual observation of cell morphology through fluorescence microscopy (×200). b The cellular fluorescence intensity was monitored by flow cytometry. c R123 fluorescence intensity was detected by flow cytometry. Data are presented as mean ± SEM (n = 3). ***P < 0.001; **P < 0.01, versus control group. ### P < 0.001 versus glutamate-treated group. Scale bar 50 μM
Gartanin Promoted HO-1 Protein Level Independent of Nrf-2 Activation and Mediates it Protection
As we all know, Nrf-2 is an inducible transcription factor and binds to the antioxidant responsive element (ARE) to activate a battery of genes encoding antioxidant proteins and phase II enzymes in response to oxidative stress [44]. We firstly speculated that gartanin could promote Nrf-2-ARE activation, and ultimately protect from oxidative stress-induced cell death. However, gartanin did not show a significant difference on both the protein level and transcriptional activity of Nrf-2 (Fig. 4a–c). Nrf-2/ARE targeting genes didn’t change the glutamate-cysteine ligase, modifier subunit (GCLM) and NADPH: quinone oxidoreductase 1 (NQO-1) protein expression (data not shown), while HO-1 protein level increased in a time- and concentration-dependent manners (Fig. 4a, b).
Gartanin elevated the protein expression of HO-1 and mediated its protection, but had no effects on Nrf-2. a, b Gartanin elevated HO-1 but not Nrf-2 in a time- and concentration-dependent. Quantitative analysis of the bands was shown. c Gartanin had no effect on Nrf-2 transcriptional activity. tBHQ (50 μM) was used as positive control. d Western blot analysis of HO-1 protein expression after HO-1 siRNA treated. Lanes 2, 3 and 4 represented three HO-1 siRNA sequences respectively. NC represented nonspecific control siRNA. Quantitative analysis of the bands was shown. e Cells were transfected with HO-1 siRNA sequence 1. After 48 h, 3 μM gartanin was added 30 min before the treatment with 2 mM glutamate for 24 h. Cell viability was determined by the MTT assay. Data are presented as mean ± SEM (n = 3). ***P < 0.001; **P < 0.01; *P < 0.05 versus control group. ### P < 0.001 versus glutamate-treated group. $$$ P < 0.001 versus gartanin combined with glutamate-treated cells
Next, we screened the effective siRNA sequences, HO-1 siRNA sequence 1 and 3 significantly reduced HO-1 protein level (Fig. 4d). MTT assay showed that the protective effect of gartanin was partially blocked by the HO-1 siRNA sequence 1 in HT22, indicating the involvement of HO-1 activation (Fig. 4e).
AMPK Signaling Pathways May Be Involved in the Protection of Gartanin
To further explore the mechanism responsible for the protective effects of gartanin, we measured a series of signal pathways, which are closely related to neuronal cell survival and death. As shown in Fig. 5a, compared with control, phosphorylated AMPKα at Thr172 was significantly increased at 3–12 h without any change in total AMPK level after gartanin treatment. In addition, SIRT1 Sirtuin activator 1 (SIRT1) and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) protein levels were significantly increased in our culture system, confirming activation of the AMPK pathway.
The neuroprotective effects of gartanin was partially mediated by activating AMPK pathway. a Cells were treated with gartanin of 3 μM for different hours, representative western blots showed that gartanin increased pAMPKα (Thr172), SIRT1 and PGC-1α protein level. Quantitative analysis of the bands is shown. b, c HT22 cells were pretreated with Compound C (10 μM, 1 h) and glutamate (0.5 h), and then were maintained with gartanin for 12 h, western blots analysis of AMPK phosphorylation and MTT assay analysis of cell viability Data are presented as mean ± SEM (n = 3). ***P < 0.001; **P < 0.01 versus control group. ### P < 0.001; # P < 0.05 versus glutamate-treated group. $$ P < 0.01 versus gartanin combined with glutamate-treated cells
We investigated whether gartanin acted through AMPK signaling to confer neuroprotection under oxidative toxicity. As shown in Fig. 5b, glutamate induced moderate AMPK activation in HT22 cells, which was dramatically enhanced by gartanin and didn’t blocked by Compound C, a well-established AMPK inhibitor. Glutamate-induced HT22 cells damages evidenced by decreased cell viability were further inhibited by gartanin. Compound C robustly strengthened the glutamate tolerance and had no effect on cell viability when applied alone to control cells (10 μM) (Fig. 5c). Furthermore, under glutamate oxidative stress, the compound C didn’t inhibit cyto-protection by gartanin, instead increased survival of HT22 cells (Fig. 5c).
Discussion
It is widely known mangosteen peel is one of the most important sources of natural antioxidants [45, 46]. Mangosteen-based functional beverage can enhance plasma antioxidant capacity in healthy adults, which may be due to the enhancement of endogenous antioxidant activity [47]. However, precise mechanisms are still unclear. In neurodegenerative diseases, persistent oxidative stress is a major disastrous element that causes living cells’ death. Therefore, antioxidant therapy should be an attractive strategy against neuronal loss. The treatment of HT22 cells with high dose of glutamate resulted in a significant increase in intracellular ROS. In this study, the results of H2DCF-DA and DHE fluorescence intensity supported that gartanin could decrease the levels of ROS accumulated in cells treated with glutamate.
So far, it is the first time to reveal that gartanin significantly attenuated glutamate-induced oxidative cytotoxicity in vitro. High concentrations of glutamate inhibit glutamate/cystine antiporter and result in reducing intracellular GSH levels. Unfortunately, we found gartanin did not reverse the glutamate-induced intracellular GSH depletion (data not shown). It might quench ROS through a GSH-independent way. It had been previously claimed the Bax expression is not required for oxidative stress-induced HT22 cell death and Bcl-2 can protect cells from oxidative stress as an anti-oxidant protein, so the ratio of the Bax/Bcl-2 determines the cells’ resistance to apoptosis triggered by different stimulus [48, 49]. Our findings showed that gartanin could elevated the level of Bcl-2 in time-dependent manner and effectively preserving the Bcl-2 protein level down-regulated by glutamate, whereas it did not affect the expression of Bax, the Bcl-2/Bax protein ratio increased (Fig. 2b, c). Meanwhile, we found gartanin preserved ΔΨm in glutamate treated cells. All these indicated that the neuroprotective effects of gartanin against glutamate-induced cell death, to some extent, might exert through increasing mitochondrial anti-apoptotic protein.
Accumulating evidence suggests that Nrf-2 binds to the ARE promoter and gives rise to general antioxidant responses, which represents a promising therapeutic approach to restore the CNS redox balance of neurodegenerative disorders [50]. Among the redox-sensitive inducible enzymes, HO-1 protects neurons against acute insults under stress conditions due to its antioxidant abilities and anti-inflammatory properties [51]. To better understand the neuroprotective mechanism of gartanin, we focused on the stress-sensing transcription factor Nrf-2 and its downstream proteins. We initially speculated that gartanin could activate Nrf-2/ARE pathway, which ultimately leaded to a powerful antioxidant response. The current result demonstrated gartanin could induce the expression of HO-1 in a time- and concentration-dependent manner (Fig. 2a, b), while the protein level and transcriptional activity of Nrf-2 did not show significant change (Fig. 2a–c). Additionally, we found that siRNA mediated knock-down of HO-1 significantly inhibited gartanin-induced neuroprotective response (Fig. 2e), which suggested that the neuroprotective effects of gartanin partially resulted from the HO-1 in HT22 cells. It has been reported that HO-1 is regulated by various moleculars. Jione Kang’s study showed that forkhead box O1 (FoxO1) could directly bind to FRE within the HO-1 gene promoter and stimulate HO-1 gene transcription [52]. Several investigations identified peroxisome proliferator-activated receptor (PPAR) α and γ are important modulators of HO-1 [53]. Further studies are needed to elucidate how HO-1 is modulated by gartanin.
AMPK was initially characterized as a “fuel gauge” and sensitive to both physiological and pathophysiological stimuli in various cells and tissues [54]. Mitochondrial respiratory chain function and glucose metabolism are closely associated with AMPK-SIRT1-PGC-1α signaling axis [55, 56]. Meanwhile, modest AMPK activation has also been documented to induce neurogenesis and improves cognition [57]. It has also been reported that hydroxytyrosol could activate AMPK with subsequent activation of forkhead transcription factor 3a (FoxO3a) and catalase to reduce intracellular ROS levels in vascular endothelial cells [58]. In this study, the levels of pAMPKα (Thr172) and two other regulators of AMPK signals, SIRT1 and PGC-1α, were significantly increased in the HT22 cells after gartanin treatment. Indeed, it is well-accepted that AMPK activation plays a dual role in the regulation of neuronal survival resulting from difference in downstream targets of AMPK (as a crucial signaling node, AMPK is connected to many downstream effectors), the type of stress, type of cells, and duration of exposure [59–61]. The MTT assay result showed that Compound C remarkably protected the HT22 cells from glutamate toxicity, but the phosphorylation status of AMPKα in cells exposed to gartanin and glutamate was dramatically increased and wasn’t inhibited by Compound C. Of note, gartanin also elevated basal AMPK activation levels (Fig. 5b, c). Thus, we suggested that AMPK activation might play a major role in gartanin protected against glutamate neurotoxicity. The reason that the existing researches failed to consist with result may be that Compound C has off-target effects or gartanin activate AMPK by noncanonical mechanisms that may not involve increases in AMP, ADP or Ca2+ levels. Of course, it should also be noted that excessive activation of AMPK would in turn trigger detrimental effects that augment neurodegenerative diseases pathogenesis [62]. Possibly, gartanin specifically induced other activators of AMPK, which are responsible for the neuroprotective activity by unknown mechanisms.
Conclusions
In summary, our research indicated that gartanin exerted strikingly protective effects against glutamate-induced cytotoxicity on HT22 cells, partly through reduced glutamate-induced ROS production, prevented mitochondrial hyperpolarization and activated HO-1 and AMPK. Our results underscored a potential role for gartanin in the prevention of glutamate-induced oxidative stress-associated diseases, such as PD and AD.
Abbreviations
- PD:
-
Parkinson’s disease
- AD:
-
Alzheimer’s disease
- ROS:
-
Reactive oxygen species
- ΔΨm:
-
Mitochondrial membrane potential
- HO-1:
-
Heme oxygenase 1
- Nrf-2:
-
Nuclear factor erythroid-derived 2-like 2
- AMPK:
-
AMP-activated protein kinase
- H2DCF-DA:
-
H2DCF-DA dichlorodihydrofluorescein diacetate
- DHE:
-
Dihydroethidium
- R123:
-
Rhodamine 123
- siRNA:
-
Small interfering RNA
- LC3:
-
Microtubule-associated protein light chain 3
- PPARα:
-
Peroxisome proliferator-activated receptor α
- SIRT1:
-
Sirtuin activator 1
- PGC-1α:
-
Peroxisome proliferator-activated receptor-γ coactivator-1α
References
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795
Forman HJ, Fukuto JM, Torres M (2004) Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am J Physiol Cell Physiol 287(2):C246–C256
Radi E, Formichi P, Battisti C, Federico A (2014) Apoptosis and oxidative stress in neurodegenerative diseases. JAD 42(Suppl 3):S125–S152
Pita-Almenar JD, Collado MS, Colbert CM, Eskin A (2006) Different mechanisms exist for the plasticity of glutamate reuptake during early long-term potentiation (LTP) and late LTP. J Neurosci 26(41):10461–10471
Paoletti P (2011) Molecular basis of NMDA receptor functional diversity. Eur J Neurosci 33(8):1351–1365
Rudy CC, Hunsberger HC, Weitzner DS, Reed MN (2015) The role of the tripartite glutamatergic synapse in the pathophysiology of Alzheimer’s disease. Aging Dis 6(2):131–148
Gardoni F, Bellone C (2015) Modulation of the glutamatergic transmission by dopamine: a focus on Parkinson, Huntington and Addiction diseases. Front Cell Neurosci 9:25
Kritis AA, Stamoula EG, Paniskaki KA, Vavilis TD (2015) Researching glutamate-induced cytotoxicity in different cell lines: a comparative/collective analysis/study. Front Cell Neurosci 9:91
Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT (1989) Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2(6):1547–1558
Davis JB, Maher P (1994) Protein kinase C activation inhibits glutamate-induced cytotoxicity in a neuronal cell line. Brain Res 652(1):169–173
Tan S, Wood M, Maher P (1998) Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J Neurochem 71(1):95–105
van Leyen K, Siddiq A, Ratan RR, Lo EH (2005) Proteasome inhibition protects HT22 neuronal cells from oxidative glutamate toxicity. J Neurochem 92(4):824–830
Chen J, Chua KW, Chua CC, Yu H, Pei A, Chua BH, Hamdy RC, Xu X, Liu CF (2011) Antioxidant activity of 7,8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity. Neurosci Lett 499(3):181–185
Poteet E, Winters A, Yan LJ, Shufelt K, Green KN, Simpkins JW, Wen Y, Yang SH (2012) Neuroprotective actions of methylene blue and its derivatives. PLoS ONE 7(10):e48279
Chao XJ, Chen ZW, Liu AM, He XX, Wang SG, Wang YT, Liu PQ, Ramassamy C, Mak SH, Cui W, Kong AN, Yu ZL, Han YF, Pi RB (2014) Effect of tacrine-3-caffeic acid, a novel multifunctional anti-Alzheimer’s dimer, against oxidative-stress-induced cell death in HT22 hippocampal neurons: involvement of Nrf2/HO-1 pathway. CNS Neurosci Ther 20(9):840–850
Bramanti V, Grasso S, Tomassoni D, Traini E, Raciti G, Viola M, Li Volti G, Campisi A, Amenta F, Avola R (2015) Effect of growth factors and steroid hormones on heme oxygenase and cyclin D1 expression in primary astroglial cell cultures. J Neurosci Res 93(3):521–529
Shibahara S, Yoshizawa M, Suzuki H, Takeda K, Meguro K, Endo K (1993) Functional analysis of cDNAs for two types of human heme oxygenase and evidence for their separate regulation. J Biochem 113(2):214–218
Abraham NG, Lin JH, Schwartzman ML, Levere RD, Shibahara S (1988) The physiological significance of heme oxygenase. Int J Biochem 20(6):543–558
Li Volti G, Murabito P (2014) Pharmacologic induction of heme oxygenase-1: it is time to take it seriously*. Crit Care Med 42(8):1967–1968
Kushida T, Li Volti G, Quan S, Goodman A, Abraham NG (2002) Role of human heme oxygenase-1 in attenuating TNF-alpha-mediated inflammation injury in endothelial cells. J Cell Biochem 87(4):377–385
Bramanti V, Tomassoni D, Grasso S, Bronzi D, Napoli M, Campisi A, Li Volti G, Ientile R, Amenta F, Avola R (2012) Cholinergic precursors modulate the expression of heme oxigenase-1, p21 during astroglial cell proliferation and differentiation in culture. Neurochem Res 37(12):2795–2804
Tang GH, Chen ZW, Lin TT, Tan M, Gao XY, Bao JM, Cheng ZB, Sun ZH, Huang G, Yin S (2015) Neolignans from Aristolochia fordiana prevent oxidative stress-induced neuronal death through maintaining the Nrf2/HO-1 pathway in HT22 Cells. J Nat Prod 78(8):1894–1903
Lee DS, Cha BY, Woo JT, Kim YC, Jang JH (2015) Acerogenin A from Acer nikoense maxim prevents oxidative stress-induced neuronal cell death through Nrf2-mediated heme oxygenase-1 expression in mouse hippocampal HT22 cell line. Molecules (Basel, Switzerland) 20(7):12545–12557
Park SY, Jin ML, Kim YH, Kim CM, Lee SJ, Park G (2014) Involvement of heme oxygenase-1 in neuroprotection by sanguinarine against glutamate-triggered apoptosis in HT22 neuronal cells. Environ Toxicol Pharmacol 38(3):701–710
Son Y, Byun SJ, Pae HO (2013) Involvement of heme oxygenase-1 expression in neuroprotection by piceatannol, a natural analog and a metabolite of resveratrol, against glutamate-mediated oxidative injury in HT22 neuronal cells. Amino Acids 45(2):393–401
Wang R, Yan H, Tang XC (2006) Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin 27(1):1–26
Wu TY, Chen CP, Jinn TR (2011) Traditional Chinese medicines and Alzheimer’s disease. Taiwan J Obstet Gynecol 50(2):131–135
Kim HG, Oh MS (2012) Herbal medicines for the prevention and treatment of Alzheimer’s disease. Curr Pharm Des 18(1):57–75
Liu QY, Wang YT, Lin LG (2015) New insights into the anti-obesity activity of xanthones from Garcinia mangostana. Food Funct 6(2):383–393
Chin YW, Kinghorn AD (2008) Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini Rev Org Chem 5(4):355–364
Cui J, Hu W, Cai Z, Liu Y, Li S, Tao W, Xiang H (2010) New medicinal properties of mangostins: analgesic activity and pharmacological characterization of active ingredients from the fruit hull of Garcinia mangostana L. Pharmacol Biochem Behav 95(2):166–172
Li G, Thomas S, Johnson JJ (2013) Polyphenols from the mangosteen (Garcinia mangostana) fruit for breast and prostate cancer. Front Pharmacol 4:80
Obolskiy D, Pischel I, Siriwatanametanon N, Heinrich M (2009) Garcinia mangostana L.: a phytochemical and pharmacological review. PTR 23(8):1047–1065
Gutierrez-Orozco F, Failla ML (2013) Biological activities and bioavailability of mangosteen xanthones: a critical review of the current evidence. Nutrients 5(8):3163–3183
Pedraza-Chaverri J, Cardenas-Rodriguez N, Orozco-Ibarra M, Perez-Rojas JM (2008) Medicinal properties of mangosteen (Garcinia mangostana). Food Chem Toxicol 46(10):3227–3239
Quan GH, Oh SR, Kim JH, Lee HK, Kinghorn AD, Chin YW (2010) Xanthone constituents of the fruits of Garcinia mangostana with anticomplement activity. PTR 24(10):1575–1577
Shan T, Ma Q, Guo K, Liu J, Li W, Wang F, Wu E (2011) Xanthones from mangosteen extracts as natural chemopreventive agents: potential anticancer drugs. Curr Mol Med 11(8):666–677
Govindachari TR, Kalyanaraman PS, Muthukumaraswamy N, Pai BR (1971) Xanthones of Garcinia mangostana Linn. Tetrahedron 27(16):3919–3926
Xu Z, Huang L, Chen XH, Zhu XF, Qian XJ, Feng GK, Lan WJ, Li HJ (2014) Cytotoxic prenylated xanthones from the pericarps of Garcinia mangostana. Molecules (Basel, Switzerland) 19(2):1820–1827
Liu Z, Antalek M, Nguyen L, Li X, Tian X, Le A, Zi X (2013) The effect of gartanin, a naturally occurring xanthone in mangosteen juice, on the mTOR pathway, autophagy, apoptosis, and the growth of human urinary bladder cancer cell lines. Nutr Cancer 65(Suppl 1):68–77
Jung HA, Su BN, Keller WJ, Mehta RG, Kinghorn AD (2006) Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J Agric Food Chem 54(6):2077–2082
Cosentino K, Garcia-Saez AJ (2014) Mitochondrial alterations in apoptosis. Chem Phys Lipids 181:62–75
Fukui M, Song JH, Choi J, Choi HJ, Zhu BT (2009) Mechanism of glutamate-induced neurotoxicity in HT22 mouse hippocampal cells. Eur J Pharmacol 617(1–3):1–11
Zhang H, Davies KJ, Forman HJ (2015) Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med 88:314–3336
Aukkanimart R, Boonmars T, Sriraj P, Songsri J, Laummaunwai P, Waraasawapati S, Boonyarat C, Rattanasuwan P, Boonjaraspinyo S (2015) Anthelmintic, anti-inflammatory and antioxidant effects of Garcinia mangostana extract in hamster opisthorchiasis. Exp Parasitol 154:5–13
Suttirak W, Manurakchinakorn S (2014) In vitro antioxidant properties of mangosteen peel extract. J Food Sci Technol 51(12):3546–3558
Xie Z, Sintara M, Chang T, Ou B (2015) Functional beverage of Garcinia mangostana (mangosteen) enhances plasma antioxidant capacity in healthy adults. Food Sci Nutr 3(1):32–38
Cory S, Huang DC, Adams JM (2003) The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene 22(53):8590–8607
Dargusch R, Piasecki D, Tan S, Liu Y, Schubert D (2001) The role of Bax in glutamate-induced nerve cell death. J Neurochem 76(1):295–301
Lim JL, Wilhelmus MM, de Vries HE, Drukarch B, Hoozemans JJ, van Horssen J (2014) Antioxidative defense mechanisms controlled by Nrf2: state-of-the-art and clinical perspectives in neurodegenerative diseases. Arch Toxicol 88(10):1773–1786
Chen J (2014) Heme oxygenase in neuroprotection: from mechanisms to therapeutic implications. Rev Neurosci 25(2):269–280
Kang J, Jeong MG, Oh S, Jang EJ, Kim HK, Hwang ES (2014) A FoxO1-dependent, but NRF2-independent induction of heme oxygenase-1 during muscle atrophy. FEBS Lett 588(1):79–85
Kronke G, Kadl A, Ikonomu E, Bluml S, Furnkranz A, Sarembock IJ, Bochkov VN, Exner M, Binder BR, Leitinger N (2007) Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol 27(6):1276–1282
Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S (2009) AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem 109(Suppl 1):17–23
Feige JN, Auwerx J (2007) Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol 17(6):292–301
Jager S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104(29):12017–12022
Dagon Y, Avraham Y, Magen I, Gertler A, Ben-Hur T, Berry EM (2005) Nutritional status, cognition, and survival: a new role for leptin and AMP kinase. J Biol Chem 280(51):42142–42148
Zrelli H, Matsuoka M, Kitazaki S, Zarrouk M, Miyazaki H (2011) Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK-FOXO3a pathway. Eur J Pharmacol 660(2–3):275–282
Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13(4):251–262
Amato S, Man HY (2011) Bioenergy sensing in the brain: the role of AMP-activated protein kinase in neuronal metabolism, development and neurological diseases. Cell Cycle 10(20):3452–3460
Weisova P, Davila D, Tuffy LP, Ward MW, Concannon CG, Prehn JH (2011) Role of 5′-adenosine monophosphate-activated protein kinase in cell survival and death responses in neurons. Antioxid Redox Signal 14(10):1863–1876
Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M (2011) AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem 118(4):460–474
Acknowledgments
This study was supported by Guangdong Provincial International Cooperation Project of Science & Technology (No. 2013B051000038), National Natural Science Foundation of China (No. 31371070) and the Fundamental Research Funds for the Central Universities (No. 15ykjc08b) to R. Pi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All other authors declare no conflict of interest.
Additional information
Xiao-yun Gao, Sheng-nan Wang, Xiao-hong Yang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gao, Xy., Wang, Sn., Yang, Xh. et al. Gartanin Protects Neurons against Glutamate-Induced Cell Death in HT22 Cells: Independence of Nrf-2 but Involvement of HO-1 and AMPK. Neurochem Res 41, 2267–2277 (2016). https://doi.org/10.1007/s11064-016-1941-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1941-x