Abstract
Silibinin, a flavonoid derived from the herb milk thistle (Silybum marianum), has been used as a hepato-protectant in the clinical treatment of liver disease. In the present study, the effect of silibinin on lipopolysaccharide (LPS)-induced neuroinflammatory impairment in rats is investigated. Injection of LPS into lateral ventricle caused learning and memory impairment. Rats were treated with silibinin to see the effect in comparison with resveratrol as a positive control. Y-maze and Morris water maze tests showed that silibinin significantly attenuated memory damage caused by LPS treatment. At the molecular analysis, the levels of IL-1β and of IL-4 in the hippocampus were decreased and enhanced, respectively, by the treatment with silibinin. NF-κB expression was attenuated by silibinin treatment. Furthermore, generation of total reactive oxygen species (ROS) in the hippocampus was elevated in silibinin-treated groups, and so were the expressions of brain-derived neurotrophic factor (BDNF) and tyrosine receptor kinase B (TrkB). At the same time, LPS-induced reduction of neurons in hippocampus was reversed by silibinin. In conclusion, silibinin ameliorated the impairment of learning and memory of LPS-injection rats, possibly due to the activation of ROS–BDNF–TrkB pathway in the hippocampus as well as the suppression of inflammatory response. This study gives an insight on the beneficial consequences of ROS in central nervous system. Silibinin might be a potential candidate drug for neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silibinin has been reported to exert hepatoprotective, cardioprotective and neuroprotective effects [1–3]. However, the mechanism of the neuroprotective effect is still unclear. Therefore, we evaluated the potential role of silibinin in modulating neuroinflammation triggered by LPS.
Neuroinflammation has been reported as a pathological hallmark of Alzheimer’s disease and other neurodegenerative diseases [4, 5]. However, the detailed mechanism underlying the effect of neuroinflammation on cognitive function is not completely clarified yet. LPS is a major bacterial Toll-like receptor 4 (TLR4) ligand that activates the innate immune response to infections. Administration of LPS by systemic injection [6], intracerebral microinjection or chronic infusion [7, 8] caused cognitive impairment in animal models. Studies showed that LPS administration resulted in cognitive impairment through the release of pro-inflammatory cytokines [9].
ROS exert diverse biological consequences in the mammalian central nervous system (CNS). It is reported that cytotoxic effects of ROS contribute to the death of neurons of the mammalian CNS in chronic neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease [10, 11]. Interestingly, ROS, as signaling molecules, regulate various physiological processes including cell proliferation, differentiation, migration and survival [12, 13].
Glutathione peroxidase, GSH-PX, a selenium-containing enzyme, plays a principle function to reduce, in presence of GSH, the peroxides of organic compounds within membranes and lipoproteins.
BDNF is widely expressed in the mammalian brain. Declined level of BDNF was implicated in the pathophysiology of various CNS diseases [10]. LPS-administration decreased BDNF expression in both tissue level and plasma concentration [14]. TrkB receptor tyrosine kinase, a BDNF receptor, is widely detected in neurons of the mammalian CNS, and TrkB contributes to diverse biological processes, including neuronal survival and differentiation as well as synaptic structure, function and plasticity [15]. Therefore, in this study, pharmacological effects of silibinin on learning and memory impairment induced by LPS-injection in rats were examined. Furthermore, we investigated its effect on the inflammatory response and ROS–BDNF–TrkB pathway in the hippocampus.
Materials and Methods
Animals
Male Sprague–Dawley rats weighting 240–260 g were obtained from Experimental Animal Center of Shenyang Pharmaceutical University (Shenyang, China). They were housed under conventional conditions with appropriate temperature (22 ± 0.5 °C) and humidity (50–60 %) control and a 12/12 h light/dark cycle (lights on from 8:00 a.m. to 8:00 p.m.) and allowed free access to food and water. All experiments and procedures were carried out according to the Regulations of Experimental Animal Administration issued by State Committee of Science and Technology of China.
Drugs and Reagents
Silibinin (Fig. 1a) was purchased from Jurong Best Medicine Material (Jiangsu, China). The purity was more than 99 % as determined by HPLC. LPS (Escherichia coli; 055:B5), trypsin, 2′,7′-dichlorofluorescein diacetate (H2DCF-DA) were purchased from Sigma Chemical (St. Louis, Mo, USA). Resveratrol was purchased from Aladdin Industrial (L.A., CA, USA). All other chemicals were commercially available and of reagent grade.
Treatment
All rats were first screened using the Morris water maze test to exclude those held still or gained extremely scores in escape latency in the maze. 66 rats with similar scores in escape latency and searching distance were then divided randomly into six groups: control group, model group, three silibinin-treated groups (25, 50, and 100 mg/kg) and resveratrol-treated group (30 mg/kg). Rats were then anesthetized with chloral hydrate (350 mg/kg body wt., i.p.) and placed in stereotaxic apparatus (Kiel, Wl, USA) where they were received an injection of 50 μg of LPS in 5 μl into the lateral ventricle. The injection coordinates: anteroposterior, −0.9 mm from the bregma; lateral, 1.5 mm from the bregma; ventral, −3.8 mm from the skull. The control group received an injection of 5 μl of sterile saline into the lateral ventricle. Silibinin and resveratrol were suspended in a 0.3 % carboxymethylcellulose (CMC) solution and administrated by oral gavage once a day. All compounds were administrated systemically in a volume of 10 ml/kg body weight.
Y-Maze Test
The working memory in terms of spontaneous alteration behavior in Y-maze test was assessed according to the experimental protocol (Fig. 1b) [16]. The Y-maze test was a horizontal maze (40 cm long and 14 cm wide, with walls 22 cm high) made of polyvinyl chloride (PVC) material with three arms (labeled A, B, and C) disposed at 120° to each other. Each rat was placed at the center of the apparatus and allowed to move freely through the maze for 8 min. The number of alternations (i.e., consecutive entry sequences of ABC, CAB or BCA, but not BAB) and the numbers of arm entries were recorded. Maze arms were thoroughly cleaned between tests with water spray to remove residual odors. The percentage alternation was calculated according to the following equation: percentage alternation (%) = [(number of alternations)/(total arm entries − 2)] × 100.
Morris Water Maze Test
All rats were tested in the Morris water maze using a pool 150 cm in diameter filled with water at 20 ± 2 °C [17, 18]. A hidden platform 13 cm in diameter was placed 1–2 cm under the water surface in the southwestern quadrant (the target quadrant) of the pool. Rats were trained twice a day for six consecutive days with an inter-trial interval of 3 h and each trial lasted for 90 s. Rats were placed in the pool facing the wall in the northeast, southeast, or northwest direction, and escape latency, swimming speed and distance travelled were recorded with a video camera attached to the ceiling. On the seventh day of the test, rats performed a probe test for 90 s with the platform removed. Swimming speed, platform-site crossings, time spent and distance travelled in the target quadrant were recorded and analyzed by SLY-ETS type software (Beijing Sunny Instruments, Beijing, China).
ELISA Assay
Whole blood was collected from the eye venous plexus 1 h later after the administration of silibinin on 8:00 a.m. The samples were naturally coagulated in room temperature for 15 min, then centrifuged at 2500×g for 20 min, and the supernatant, serum, was collected. The hippocampus samples were removed on an ice-cold glass plate. Tissues were homogenized in PBS. After centrifuging at 2500×g for 20 min, the protein concentration of the supernatant was quantified using the BCA Protein Assay Kit (Beyotime, Jiangsu, China). The concentrations of IL-1β, TNFα and IL-4 were determined with ELISA kits (Dakewe Biotech, Shenzhen, China) according to the manufacturer’s protocol.
Western Blotting Analysis
The rats received the final administration of silibinin 1 h before sacrificed. The hippocampus samples were removed on an ice-cold glass plate and stored at −80 °C. Western blotting was performed according to standard protocols. Tissues were cut into small pieces then homogenized in lysis buffer [50 mM Hepes (pH 7.4), 1 % Triton-X 100, 2 mM sodium orthovanadate, 100 mM sodium fluoride, 1 mM edetic acid, 1 mM PMSF, 10 μg/mL aprotinin and 10 μg/mL leupeptin] on ice for 1 h. After centrifugation at 13,000×g for 15 min, the protein concentration of the supernatant was quantified using the BCA Protein Assay Kit. Samples were separated by 12 % sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5 % skim milk in phosphate buffer solution and Tween-20 (PBST) at room temperature for 2 h, then incubated overnight at 4 °C with primary antibodies against NF-κB (1:1000), BDNF (1:1000), TrkB (1:500), IL-1β (1:100), IL-4 (1:100) and β-actin (1:2000) (Santa Cruz, CA, USA) serving as a loading control. Membranes were washed three times for 10 min with PBST and incubated with the respective peroxidase-conjugated secondary antibodies at room temperature for 3 h. After three times washing for 10 min, the proteins were visualized by enhanced chemiluminescent ECL reagents (Thermo Scientific, Rockford, IL, USA). Densities of the protein bands were determined with Bio-Rad Quantity One 4.6.2 imaging software (Hercules, CA, USA).
Flow Cytometric Analysis of Intracellular ROS
The rats were sacrificed 1 h later after received administration of silibinin. The hippocampus tissues were removed on an ice-cold glass plate, then cut into small pieces with small scissors, and digested with 4 ml trypsin (0.125 %) in the constant temperature box at 37 °C for 30 min [19]. The hippocampus samples were centrifuged at 1200×g for 15 min, and then the pellets were harvested and suspended in 1 ml PBS. To measure the hippocampal intracellular ROS levels, the cells were stained with 10 μM 2′,7′-dichlorofluorescein diacetate (H2DCF-DA) (Sigma Chemical, St. Louis, MO, USA) at 37 °C for 30 min. DCFH-DA is a stable non-polar compound that readily diffuses into cells and is hydrolysed by intracellular esterase to yield H2DCF, which is trapped within the cells. ROS produced by the cells oxidizes H2DCF to the highly fluorescent compound DCF; thus, the fluorescence intensity is proportional to the amount of ROS produced by the cells. The samples were analyzed by a FACScan flow cytometer (Becton–Dickinson, Franklin Lakes, NJ, USA) to process the image.
GSH-PX Assay
The hippocampus samples were homogenized and centrifuged at 2500×g for 20 min. the protein concentration of the supernatant was quantified using the BCA Protein Assay Kit. Subsequently, the supernatant was used to measure the activity of GSH-PX by using commercial reagent kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocol.
Nissl Staining
Rats were perfused with 4 % paraformaldehyde (pH 7.3) after anesthesia with chloral hydrate (350 mg/kg, i.p.). Briefly, after post-fixation in the same fixative at 37 °C for 1 h, the brains were cut into 30–40 μm-thick coronal sections. The sections were mounted on poly-lysine coated slides, rehydrated in distilled water overnight, and then hydrated in graded ethanol, paraffin-embedded. The sections were cut into 5 μm thick coronal sections using paraffin slicing machine (Heidelberger StraBe, NuBloch, Germany). The samples were submerged in 1 % cresyl violet for about 7 min until the desired depth of staining was achieved. After being rinsed in distilled water and dehydrated in graded ethanol, sections were immersed in xylene, mounted in neutral balsam and cover slipped. Nissl-positive cells in the pyramidal layer of medial CA1 region were examined to assess neuronal loss.
Statistical Analysis
The results were expressed as mean ± SEM. Statistical significance was determined with the one- or two-way ANOVA followed by Fisher’s LSD multiple comparisons test using Statistics Package for Social Science software (version 13.0; SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Silibinin Reversed Working Memory Impairment Induced by LPS in Y-Maze Test
LPS-injected rats showed significantly reduced spontaneous alternation behavior compared with saline-injected rats [F(5, 14) = 4.722, P < 0.05, post hoc, P < 0.05, Fig. 2a]. Silibinin (25, 50 and 100 mg/kg) markedly attenuated the impairment of spontaneous alternation behavior in LPS-injected rats [F(5, 14) = 4.722, P < 0.05, Fig. 2a]. There was no obvious difference in the number of arm entries among the groups [F(5,20) = 0.918, P = 0.49, Fig. 2b]. Resveratrol-treated group (30 mg/kg) also showed similar improvement in spontaneous alternation behavior (P < 0.01, Fig. 2a). These data suggested that silibinin ameliorated working memory deficits in LPS-injected rats.
Protective effects of silibinin against LPS-induced impairment of working memory in Y-maze test. a Alternation behavior measured during 8 min session. Treatment with silibinin reversed LPS-induced decrease in percentage of alternation. b Number of arm entries was measured during 8 min session. No significant difference was observed among the groups. sili silibinin, rev resveratrol. All of the results are expressed as the mean ± SEM n = 4–5; # P < 0.05 versus saline-injected rats; *P < 0.05, **P < 0.01 versus LPS-injected rats
Silibinin Reversed LPS-Induced Learning and Memory Impairment in MWM Test
The group difference in mean escape latencies was illustrated in Fig. 3a. During the training period, there was a significant difference in the performance of six different rat groups [F group(5,321) = 5.012, P < 0.001; F day(5,321) = 39.711, P < 0.001; F group×day (25,321) = 0.843, P = 0.686, Fig. 3a]. From day 3 in the training test, rats in model group took a longer time to find the platform compared to the control group, and treatment with silibinin (25, 50 and 100 mg/kg) decreased the escape latencies (P < 0.05). In the probe test, the distance travelled and the time spent in the target quadrant were significantly less in the LPS-injected group compared to the saline-injected group [distance: F(5,52) = 2.724, P < 0.05, post hoc, P < 0.05; time: F(5,49) = 5.472, P < 0.001, post hoc, P < 0.01, Fig. 3b, c]. Silibinin (100 mg/kg) increased the distance travelled [F(5,52) = 2.724, P < 0.05, post hoc, P < 0.01, Fig. 3b] and the time [F(5,49) = 5.472, P < 0.001, post hoc, P < 0.01, Fig. 3c] spent in the target quadrant. Silibinin-treated rats showed improvement in their search accuracy as indicated by higher number of platform crossings compared to the LPS-injected group [F(5,50) = 14.572, P < 0.001, Fig. 3d], and silibinin (50, 100 mg/kg) showed a marked improvement effect [P < 0.05; P < 0.001, Fig. 3d]. There was no difference in the swimming speed among the groups [F(5,57) = 0.21, P = 0.957, Fig. 3e]. The resveratrol was used as positive control. These data indicated that silibinin improved the learning and memory impairment in LPS-injected rats.
Effects of silibinin on learning and memory deficits induced with LPS in the Morris water maze test. a Changes in the latency to reach the platform during the training period. Changes in the time spent (b) and distance travelled (c) in the target quadrant in the probe trail. LPS-treated group exhibited a decrease in time spent and distance travelled in the target quadrant compared to saline-injected group. These effects were reversed by silibinin treatment. d The number of platform crossings during the probe trail. e No significant difference in swimming speed was observed among the groups. All of the results are expressed as the mean ± SEM n = 9–11; # P < 0.05, ## P < 0.01, ### P < 0.001 versus saline-injected rats; *P < 0.05, **P < 0.01, ***P < 0.001 versus LPS-injected rats
Silibinin Suppressed Neuronal Loss Induced by LPS in Hippocampal CA1 Region
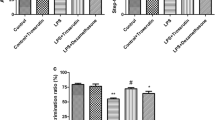
As shown in Fig. 4, LPS induced an obvious neuronal loss and injury of neuron structure in the CA1 region, and treatment with silibinin (25, 50, 100 mg/kg) reduced this loss and reversed the structural injury, as reflected by density and morphology of the cells with Nissl bodies.
Hippocampal CA1 region was observed with Nissl staining. Silibinin reduced LPS-induced neuron reduction in the CA1 region, as reflected by the density of the cells with Nissl bodies. The number of neurons in the CA1 subfield was statistically analyzed with Image-Pro Plus 6.0 analysis software. The bar graph showed the relative number of neurons of silibinin-treated groups relative to saline-injected group. ## P < 0.01 versus saline-injected rats; **P < 0.01 versus LPS-injected rats. Scale bar = 50 μm
Silibinin Inhibited the Expression of IL-1β and TNFα Induced by LPS in the Serum
As shown in Fig. 5a, b, treatment of rats with LPS resulted in increased expression of IL-1β and TNFα in the serum [IL-1β: F(5,28) = 2.797, P < 0.05, post hoc, P < 0.01; TNFα: F(5,24) = 10.135, P < 0.001, post hoc, P < 0.01], which was significantly decreased by silibinin (25, 50 and 100 mg/kg) [IL-1β: F(5,28) = 2.797, P < 0.05, Fig. 5a; TNFα: [F(5,24) = 10.135, P < 0.001, Fig. 5b] in a dose-dependent manner. Resveratrol prevented the elevation of IL-1β and TNFα expression induced with LPS (P < 0.05 and P < 0.05).
Silibinin ameliorates inflammatory response in LPS-treated rats. a, b Effects of silibinin on LPS induced IL-1β and TNFα productions in the serum. c–e Effects of silibinin on LPS induced IL-1β and IL-4 productions in the hippocampus, as analyzed by c, d ELISA and e Western blotting. f Silibinin down-regulated the expression level of NF-κB in the hippocampus. Hippocampus homogenates were prepared and analyzed by Western blotting. All of the results are expressed as the mean ± SEM ## P < 0.01, ### P < 0.001 versus saline-injected rats; *P < 0.05, **P < 0.01, ***P < 0.001 versus LPS-injected rats
Silibinin Inhibited the Production of IL-1β and Promoted the Production of IL-4 Induced by LPS in the Hippocampus
The expression of cytokines levels (IL-1β and IL-4) in the hippocampus were shown in Fig. 5c, d, and LPS injection led to the increase in IL-1β expression [F(5,15) = 13.036, P < 0.001, post hoc, P < 0.001, Fig. 5c] and its production was inhibited by silibinin-treatment (50 and 100 mg/kg) [F(5,15) = 13.036, P < 0.001]. LPS-injection led to the decrease in the expression of IL-4 [F(5,24) = 3.967, P < 0.01, post hoc, P < 0.01, Fig. 5d] and this effect was reversed by silibinin (50 and 100 mg/kg) administration [F(5,24) = 3.967, P < 0.01, Fig. 5d]. Resveratrol prevented the increase in IL-1β expression and had no influence on the level of IL-4 (P < 0.001 and P > 0.05). Data of the Western blotting analysis of IL-1β and IL-4 showed the same effects (Fig. 5e).
Silibinin Suppressed LPS-Induced NF-κB Expression in the Hippocampus
Nuclear factor kappa B (NF-κB) is a transcription factor involved in immune and inflammatory response especially induction of proinflammatory cytokines [20]. Our study showed that an increase in the level of NF-κB was attenuated by silibinin treatment (Fig. 5f).
Silibinin Reversed the Reduction of Total ROS in the Hippocampus of LPS-Treated Rats
The silibinin’s effect on the production of ROS was examined by flow cytometry analysis. As shown in Fig. 6a, treatment of rats with LPS resulted in decreased production of total ROS [F(5,14) = 9.419, P < 0.001, post hoc, P < 0.001, Fig. 6a] which was reversed by silibinin (25, 50 and 100 mg/kg) [F(5,14) = 9.419, P < 0.001, Fig. 6a]. Resveratrol also markedly increased the ROS production (P < 0.001, Fig. 6a).
Silibinin reversed the decrease of ROS level in LPS-injected rats. a Effects of silibinin on ROS generation in the hippocampus. Silibinin promoted ROS production. ROS level was measured by flow cytometry analysis with H2DCF-DA staining. b Effects of silibinin on the activity of GSH-PX in the hippocampus. All of the results are expressed as the mean ± SEM n = 3–4; ### P < 0.001 versus saline-injected rats; *P < 0.05, **P < 0.01, ***P < 0.001 versus LPS-injected rats
Silibinin Inhibited LPS-Induced GSH-PX Activity in the Hippocampus
These results showed that the activity of GSH-PX was significantly increased in the LPS-injected group compared to the saline-injected group [F(5,17) = 4.803, P < 0.01, post hoc, P < 0.01, Fig. 6b], and silibinin-treatment decreased this up-regulation of GSH-PX activity [F(5,17) = 4.803, P < 0.01, Fig. 6b]. Resveratrol also decreased the GSX-PX activity [P < 0.05, Fig. 6b].
Silibinin Increased BDNF and TrkB Expression in the Hippocampus of LPS-Treated Rats
The expression levels of BDNF and TrkB were obviously down-regulated in the LPS-injected rats compared to the saline-injected rats, and silibinin-treated groups showed higher expression levels compared to the LPS-injected group (Fig. 7). Resveratrol also increased the expression of BDNF and TrkB (Fig. 7).
Effects of silibinin on the expression levels of BDNF and TrkB in the hippocampus. Silibinin increased BDNF and TrkB protein expression levels, as determined by Western blotting analysis. All results are represented as mean ± SEM n = 3 or 4. # P < 0.05, ### P < 0.001 versus saline-injected rats; *P < 0.05, **P < 0.01, ***P < 0.001 versus LPS-injected rats
Discussion
We examined the effect on memory impairment induced by LPS in rats. LPS-treatment induced a long-term impairment on memory [21, 22]. Silibinin significantly protected against LPS-induced learning and memory impairment in both Y-maze and MWM tests. This study further verified the protective effect of silibinin focusing on neuroinflammation as a potential mechanism. Furthermore, silibinin ameliorated inflammatory response and activated the ROS–BDNF–TrkB pathway in the rat hippocampus.
Neuroinflammation has been reported as one of the causes of the neuropathogenesis and cognitive impairment. It plays a critical role in the development of Alzheimer’s and other neurodegenerative diseases [4]. It has been reported that LPS-injection causes many behavioral and pathological symptoms including memory impairment [23]. Injection of LPS activates glial cells to synthesize and secrete the proinflammatory cytokines, such as TNFα, IL-1β and IL-6. LPS-injection also results in upregulation of autophagy in the hippocampus [24]. Our study demonstrated that silibinin could reduce the overproduction of serum TNFα and IL-1β induced by LPS. At the same time, the decrease in the expression of IL-4 and the increase in the production of IL-1β in the hippocampus were reversed by silibinin-treatment. IL-4 is an anti-inflammatory cytokine that is produced by Th2 cells. It coupled with anti-inflammatory cytokines such as IL-10 causes diminution of pathological inflammation and mediate numerous beneficial effects on CNS function in animal models of Alzheimer’s disease [25, 26]. Silibinin increased IL-4 production in LPS-treated hippocampus and might play anti-inflammatory role. Since NF-κB induces gene expression of proinflammatory cytokines such as IL-1β and TNFα, inhibition of the NF-κB signaling pathway by silibinin also plays an important role in its beneficial effect against LPS-induced neuroinflammation. It suggested that the anti-inflammatory effect of silibinin contributes to the improvement of memory deficits. To our knowledge resveratrol shows no regulatory effect on production of anti-inflammatory cytokine IL-4. Furthermore, in this study the decrease in the production of IL-4 was not reversed by resveratrol. We speculate that silibinin can ameliorate inflammatory response by regulating cytokine production by macrophage lineage and Th2 cells, but resveratrol may only accommodate macrophage.
The injury effects of excess ROS, such as oxidative stress and cell injury, were well documented [10, 11], but the beneficial consequences of ROS in CNS neurons are not well appreciated. The neurotoxic effects of ROS are paralleled by neuroprotective functions [12, 13]. The major peroxidase enzymes are GSH-PX that is both cytosolic and mitochondrial [27], and catalase that is localized in intracellular peroxisomes [28]. Basal H2O2 levels can be amplified in a concentration-dependent manner by the GSH-PX inhibitor, mercaptosuccinate (MCS) [29]. Inhibition of the activity of GSH-PX results in the accumulation of endogenous H2O2. In this study, LPS-injection increased the activity of GSH-PX and decreased the level of ROS. Silibinin played a protective role by decrease in the GSH-PX activity and gave rise to the increased ROS production. Our results are partially consistent with previous studies [30, 31], demonstrating that scavenging of some superoxide species impairs learning and memory and that protective effect of some ROS is required for learning and memory. There are reports that LPS-injection induces oxidative stress and we suppose that this is due to the short-term effects of administration of LPS [32, 33], the length of time after LPS-administration is important to the regulation of ROS in the hippocampus.
Reports suggest that BDNF is widely expressed in the mammalian brain and reduced level of BDNF is implicated in the pathophysiology of various CNS diseases [10]. As a neurotransmitter modulator, BDNF regulates plasticity processes as well as learning and memory [34, 35]. The interplay between inflammation and BDNF may lead dysfunction of the hippocampus [36]. In our study, the reduced expression of BDNF and TrkB induced by LPS-injection was reversed by silibinin-treatment. It was reported that neuroprotective effects of ROS was due to the ROS-triggered activation of TrkB [37, 38]. Taken together, the protective effect of silibinin appears to be partially due to the activation of ROS–BDNF–TrkB pathway.
In summary, silibinin significantly protected the rats from LPS-induced neuroinflammation and cognitive dysfunction. The protective effect of silibinin appears to be due to the amelioration of inflammatory response as well as the activation of ROS–BDNF–TrkB pathway in the hippocampus. Our study highlights the beneficial consequences of ROS in CNS, as the protective effect of ROS in the brain is associated with curing aging and neurodegenerative diseases, including Alzheimer’ disease and Parkinson’s disease. Taken together, silibinin may be a potential candidate drug for neurodegenerative diseases in which protective effect of ROS is involved.
References
Salamone F, Galvano F, Marino Gammazza A, Paternostro C, Tibullo D, Bucchieri F, Mangiameli A, Parola M, Bugianesi E, Li Volti G (2012) Silibinin improves hepatic and myocardial injury in mice with nonalcoholic steatohepatitis. Dig Liver Dis 44:334–342
Flora K, Hahn M, Rosen H, Benner K (1998) Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol 93:139–143
Lu P, Mamiya T, Lu L, Mouri A, Niwa M, Kim HC, Zou LB, Nagai T, Yamada K, Ikejima T, Nabeshima T (2010) Silibinin attenuates cognitive deficits and decreases of dopamine and serotonin induced by repeated methamphetamine treatment. Behav Brain Res 207:387–393
Pizza V, Agresta A, D’Acunto CW, Festa M, Capasso A (2011) Neuroinflamm-aging and neurodegenerative diseases: an overview. CNS Neurol Disord Drug Targets 10:621–634
Eikelenboom P, van Exel E, Hoozemans JJ, Veerhuis R, Rozemuller AJ, van Gool WA (2010) Neuroinflammation—an early event in both the history and pathogenesis of Alzheimer’s disease. Neuro-degener Dis 7:38–41
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55:453–462
Deng XH, Ai WM, Lei DL, Luo XG, Yan XX, Li Z (2012) Lipopolysaccharide induces paired immunoglobulin-like receptor B (PirB) expression, synaptic alteration, and learning-memory deficit in rats. Neuroscience 209:161–170
Zhu B, Wang ZG, Ding J, Liu N, Wang DM, Ding LC, Yang C (2014) Chronic lipopolysaccharide exposure induces cognitive dysfunction without affecting BDNF expression in the rat hippocampus. Exp Ther Med 7:750–754
Bossu P, Cutuli D, Palladino I, Caporali P, Angelucci F, Laricchiuta D, Gelfo F, De Bartolo P, Caltagirone C, Petrosini L (2012) A single intraperitoneal injection of endotoxin in rats induces long-lasting modifications in behavior and brain protein levels of TNF-alpha and IL-18. J Neuroinflamm 9:101
Hu Y, Russek SJ (2008) BDNF and the diseased nervous system: a delicate balance between adaptive and pathological processes of gene regulation. J Neurochem 105:1–17
Peng S, Garzon DJ, Marchese M, Klein W, Ginsberg SD, Francis BM, Mount HT, Mufson EJ, Salehi A, Fahnestock M (2009) Decreased brain-derived neurotrophic factor depends on amyloid aggregation state in transgenic mouse models of Alzheimer’s disease. J Neurosci 29:9321–9329
Rhee SG, Bae YS, Lee SR, Kwon J (2000) Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE Sig Transduct Knowl Environ 2000:pe1
Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270:296–299
Nowacka MM, Paul-Samojedny M, Bielecka AM, Plewka D, Czekaj P, Obuchowicz E (2015) LPS reduces BDNF and VEGF expression in the structures of the HPA axis of chronic social stressed female rats. Neuropeptides 54:17–27
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736
Ohgidani M, Kato TA, Sagata N, Hayakawa K, Shimokawa N, Sato-Kasai M, Kanba S (2015) TNF-alpha from hippocampal microglia induces working memory deficits by acute stress in mice. Brain Behav Immun. doi:10.1016/j.bbi.2015.08.022
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
Liu P, Zou L, Jiao Q, Chi T, Ji X, Qi Y, Xu Q, Wang L (2013) Xanthoceraside attenuates learning and memory deficits via improving insulin signaling in STZ-induced AD rats. Neurosci Lett 543:115–120
Eruslanov E, Kusmartsev S (2010) Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol 594:57–72
Makarov SS (2000) NF-kappaB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today 6:441–448
Valero J, Mastrella G, Neiva I, Sanchez S, Malva JO (2014) Long-term effects of an acute and systemic administration of LPS on adult neurogenesis and spatial memory. Front Neurosci 8:83
Hopp SC, D’Angelo HM, Royer SE, Kaercher RM, Crockett AM, Adzovic L, Wenk GL (2015) Calcium dysregulation via L-type voltage-dependent calcium channels and ryanodine receptors underlies memory deficits and synaptic dysfunction during chronic neuroinflammation. J Neuroinflamm 12:56
Zhang XY, Cao JB, Zhang LM, Li YF, Mi WD (2015) Deferoxamine attenuates lipopolysaccharide-induced neuroinflammation and memory impairment in mice. J Neuroinflamm 12:20
Francois A, Terro F, Quellard N, Fernandez B, Chassaing D, Janet T, Rioux Bilan A, Paccalin M, Page G (2014) Impairment of autophagy in the central nervous system during lipopolysaccharide-induced inflammatory stress in mice. Mol Brain 7:56
Xie M, Hu A, Luo Y, Sun W, Hu X, Tang S (2014) Interleukin-4 and melatonin ameliorate high glucose and interleukin-1beta stimulated inflammatory reaction in human retinal endothelial cells and retinal pigment epithelial cells. Mol Vis 20:921–928
Yang S, Gao L, Lu F, Wang B, Gao F, Zhu G, Cai Z, Lai J, Yang Q (2015) Transcription factor myocyte enhancer factor 2D regulates interleukin-10 production in microglia to protect neuronal cells from inflammation-induced death. J Neuroinflamm 12:33
Stults FH, Forstrom JW, Chiu DT, Tappel AL (1977) Rat liver glutathione peroxidase: purification and study of multiple forms. Arch Biochem Biophys 183:490–497
Dringen R, Pawlowski PG, Hirrlinger J (2005) Peroxide detoxification by brain cells. J Neurosci Res 79:157–165
Avshalumov MV, Chen BT, Koos T, Tepper JM, Rice ME (2005) Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci 25:4222–4231
Tsien JZ, Huerta PT, Tonegawa S (1996) The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87:1327–1338
Gahtan E, Auerbach JM, Groner Y, Segal M (1998) Reversible impairment of long-term potentiation in transgenic Cu/Zn-SOD mice. Eur J Neurosci 10:538–544
Chung ES, Chung YC, Bok E, Baik HH, Park ES, Park JY, Yoon SH, Jin BK (2010) Fluoxetine prevents LPS-induced degeneration of nigral dopaminergic neurons by inhibiting microglia-mediated oxidative stress. Brain Res 1363:143–150
Noworyta-Sokolowska K, Gorska A, Golembiowska K (2013) LPS-induced oxidative stress and inflammatory reaction in the rat striatum. Pharmacol Rep PR 65:863–869
Bekinschtein P, Cammarota M, Medina JH (2014) BDNF and memory processing. Neuropharmacology 76(Pt C):677–683
Yamada K, Mizuno M, Nabeshima T (2002) Role for brain-derived neurotrophic factor in learning and memory. Life Sci 70:735–744
Nowacka MM, Paul-Samojedny M, Bielecka AM, Obuchowicz E (2014) Chronic social instability stress enhances vulnerability of BDNF response to LPS in the limbic structures of female rats: a protective role of antidepressants. Neurosci Res 88:74–83
Puttaparthi K, Gitomer WL, Krishnan U, Son M, Rajendran B, Elliott JL (2002) Disease progression in a transgenic model of familial amyotrophic lateral sclerosis is dependent on both neuronal and non-neuronal zinc binding proteins. J Neurosci 22:8790–8796
Huang YZ, McNamara JO (2012) Neuroprotective effects of reactive oxygen species mediated by BDNF-independent activation of TrkB. J Neurosci 32:15521–15532
Acknowledgments
This research was supported by National Natural Science Foundation of China (No. 81273517).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Rights and permissions
About this article
Cite this article
Song, X., Zhou, B., Zhang, P. et al. Protective Effect of Silibinin on Learning and Memory Impairment in LPS-Treated Rats via ROS–BDNF–TrkB Pathway. Neurochem Res 41, 1662–1672 (2016). https://doi.org/10.1007/s11064-016-1881-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1881-5