Abstract
This study was conducted to clarify the potential mechanisms of Troxerutin neuroprotection against Lipopolysaccharide (LPS) induced oxidative stress and neuroinflammation through targeting the SIRT1/SIRT3 signaling pathway. To establish a model, a single dose of LPS (500μg/kg body weight) was injected to male Wistar rats intraperitoneally. Troxerutin (100 mg/kg body weight) was injected intraperitoneally for 5 days after induction of the model. Cognitive and behavioral evaluations were performed using Y-maze, single-trial passive avoidance, and novel object recognition tests. The expression of inflammatory mediators, SIRT1/SIRT3, and P53 was measured using the ELISA assay. Likewise, the expression levels of SIRT1/SIRT3 and NF-κB were determined using Western blot assay. Brain acetyl-cholinesterase activity was determined by utilizing the method of Ellman. Reactive oxygen species (ROS) was detected using Fluorescent probe 2, 7-dichlorofluorescein diacetate (DCFH-DA). Furthermore, malondialdehyde (MDA) levels were determined. A single intraperitoneal injection of LPS was led to ROS production, acute neuroinflammation, apoptotic cell death, and inactivation of the SIRT1/SIRT3 signaling pathway. Likewise, ELISA assay demonstrated that post-treatment with Troxerutin considerably suppressed LPS-induced acute neuroinflammation, oxidative stress, apoptosis and subsequently memory impairments by targeting SIRT1/SIRT3 signaling pathway. Western blot assay confirmed ELISA results about SIRT1/SIRT3 and NF-κB proteins. These results suggest that Troxerutin can be a suitable candidate to treat neuroinflammation caused by neurodegenerative disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroinflammation is a pathogenic mechanism that plays an essential role in neurodegenerative disorders such as Huntington’s disease, Parkinson’s disease, stroke, and Alzheimer’s disease (AD). Neuroinflammation is characterized by glial cell activation and up-regulation of major inflammatory mediators (Stephenson et al. 2018). Lipopolysaccharides (LPS) are endotoxins from the outer membrane of Gram-negative bacteria that can evoke the immune response. A valid animal model for evaluation of the neuroprotective effects in new therapeutic agents is LPS-induced neuroinflammation (Martins 2018). Generally, activation of the primary and secondary inflammatory in the body following systemic administration of LPS damages vital organs such as the liver and brain. It has been reported that a single intraperitoneal administration of LPS is enough to induce neurodegenerative effects in the mouse brain for 10 months (Qin et al. 2007). Several studies have shown LPS firstly activates microglia and astrocytes in the central nervous system (CNS) through binding to their CD14/toll-like receptor 4 (TLR-4) receptor complex (Okun et al. 2011). Afterward, activated CD14/TLR-4 receptor complex leads to activation of the TLR4/nuclear factor (NF)-κB pathway and subsequently the release of inflammatory mediators such as Tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β) (Badshah et al. 2016). On the other hand, a consequence of TLR and NF-κB activation is excessive production of reactive oxygen species (ROS) which, in turn, leads to overwhelming endogenous antioxidant defense, lipid peroxidation, protein oxidation, DNA damage, and consequently apoptotic cell death (Bromfield and Iacovides 2017).
Likewise, the transactivational activity of NF-ĸB can be inhibited by the silent information regulator transcript-1 (SIRT1) that is a nicotinamide adenine dinucleotide (NAD)-dependent nuclear histone deacetylase and regulates a numerous of physiological processes such as energy metabolism, the maintenance of genomic integrity, DNA repair, healthspan extension and longevity, deacetylation of histones and nonhistone proteins (Yeung et al. 2004). Several previous reports have shown that activation of SIRT1 under pathological condition reduce the extent of the inflammation and oxidative stress factors (Salminen et al. 2011). Moreover, previous studies have shown that pharmacological activation of SIRT3 results in the increase of the mitochondrial oxygen consumption rate and inhibition of ROS production following LPS -induced acute respiratory distress syndrome (Chen et al. 2018). Owing to their multifaceted properties and low side effects, medicinal plants are increasingly used to treat neurodegenerative disorders, neuroinflammation and other diseases (Zhào et al. 2018; Amani et al. 2017). It has been reported that natural products can contribute to the attenuation of oxidative stress through targeting sirtuins (Su et al. 2014; Na et al. 2016). Troxerutin (also known as vitamin P4) is a derivative of the glucosidal natural bioflavonoid that is isolated from Sophora japonica and Dimorphandra gardneriana (Kessler et al. 2002). Troxerutin can act as an anti-inflammatory, antioxidant and anticancer agent (Panat et al. 2016; Zhang et al. 2015). Nevertheless, the effects of Troxerutin on LPS-induced neuroinflammation and LPS-induced oxidative stress have not been explained. This study was designed to investigate the impact of Troxerutin on LPS-induced neuroinflammation, LPS-induced oxidative stress, and LPS-induced neuroinflammatory-memory deficiencies in the hippocampus region of rats.
Methods and materials
Chemicals
Troxerutin and Lipopolysaccharides (LPS) were purchased from Sigma Company (St. Louis, MO, USA). SIRT1 antibody and SIRT3 was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). NFĸB p65 antibody, Anti-rabbit IgG, HRP-linked Antibody, and β-Actin Antibody were purchased from Cell Signalling Technology, Inc. (CST) (Danvers, Massachusetts). Bovine serum albumin (BSA), Immobilon®-FL Polyvinylidene Fluoride (PVDF) membrane and RIPA buffer were purchased from Sigma (Sigma Aldrich Company, USA).
Ethical statement and animals
A total number of 48 male Wistar rats (200–230 g), were used in this study. The animals were supplied by the Animal Experimental Unit of Iran University of Medical Sciences, Tehran, Iran. The animals were maintained in a controlled environment (22 ± 2 °C) with 60% humidity on a 12 h light/dark schedule with free access to sufficient food and water. All the experiments and protocols were accredited by the Institutional Animal Ethical Committee of Iran University of Medical Sciences.
Experimental design
The animals were randomly assigned into five groups: 1) this group was included normal or healthy rats (Control); 2) normal or healthy rats intraperitoneally received 100 mg/kg of Troxerutin for 6 consecutive days (Control+ Troxerutin); 3) rats intraperitoneally received a single dose (500μg/kg) of LPS to induce neuroinflammation (LPS); 4) the animals intraperitoneally received a single dose (500μg/kg) of LPS and then Troxerutin with 100 mg/kg concentration was intraperitoneally administrated for 5 consecutive days (LPS+ Troxerutin); 5) the animals intraperitoneally received a single dose (500μg/kg) of LPS and then dexamethasone with 100 μg/kg concentration was intraperitoneally administrated for 5 consecutive days (LPS + dexamethasone). LPS + dexamethasone group is a positive control.
Y-maze task
Y-maze apparatus was employed to determine short-term spatial working memory by investigation of spontaneous alternations in behavior. The Y-maze defines as a hippocampal dependent–spatial working memory task that animals using external maze cues to steer the identical internal arms. This apparatus is fabricated from a plastic maze including three arms (30 cm height, 15 cm width and, 40 cm length). As previously reported (Jamali-Raeufy et al. 2014) each rat, naive to the maze, was placed at the end of one arm and was allowed to move through the maze freely for an 8 min session. We visually recorded entries into all arms and counted spontaneous alternations when a rat entered three various arms constantly. When the base of the rat’s tail entirely placed in the arm, entry was considered to be completed. Alternation was defined as successive entries into the three arms on overlapping triplet sets. As for the analysis, the percentage of spontaneous alternations was calculated as: [(actual alternation)/ (maximal alternation −2)] × 100. The Y-maze test was performed by an examiner blinded to experimental design.
Single-trial passive avoidance test
To evaluate memory retention deficit, single-trial passive avoidance test was performed at 3 days after LPS injection according to previous reports (Golechha et al. 2011; Jamali-Raeufy et al. 2015). Passive avoidance instrument is fabricated from light and dark compartments (30 × 20 × 30 cm) with equal sizes. A 40-W lamp was fixed in 30 cm above the floor in the center of the light chamber. A guillotine door separates light and dark compartments. Each rat was habituated with passive avoidance instrument for first and second days (15 min per day). On the third day, an acquisition trial was performed. Rats were put in the light chamber individually and following 5 min habituation, the guillotine door was opened. After entering the rats to the dark chamber, the guillotine door was closed and an electric shock (1 mA for 1 s) was exerted via the stainless-steel grid floor. Time-lapse from putting the rat in the light chamber before it entered the dark and had all four paws inside the chamber is described as Initial latency (IL). Afterward, the animals were returned to their cages. Once again, 24 h after the IL, the latency time was measured as mentioned above in the acquisition trial without electric shock and was described as the retention trial. In retention trial, step-through latency (STL) was defined as the interval between placement of animal in the light chamber and entry into the dark chamber.
Novel object recognition test
An open field test box was used to perform a novel object recognition test. The animals were habituated to test box for 10 min in the absence of any objects in the test arena. At the end of the habituation period, each rat was individually housed into the test box with two identical objects and permitted to search for 5 min. The time spent for exploration of each object by rat was measured. After 4 h of delay, one of the original objects was removed and a new object was replaced. Once again, rats were allowed to explore in the test arena for 5 min. Determination of discrimination ratio (DR) was based on the difference between the exploration time of the novel and familiar objects divided by the total exploration time×100 (D = t[novel]-t [familiar] /t[novel] + t[familiar] ×100).
Tissue homogenization
The brain tissues were rapidly removed from skull bone on ice. After dissecting and weighing of hippocampus tissue, it was homogenized within 1 mL phosphate buffer using a homogenizer at 4 °C for 5 min. After centrifugation at 3000 rpm for 2 min, the supernatant was collected and was used to determine the expression of proteins.
Assessment of brain acetyl-cholinesterase activity
Brain acetyl-cholinesterase activity was measured based on the method of Ellman et al. (Ellman et al. 1961). Briefly, 0.4 ml supernatant and 100 μl of Ellman’s reagent (0.5 mM, 19.8 mg DTNB and 0.1 M sodium phosphate, pH 7.2) were added to a cuvette, containing 2.6 ml of sodium phosphate buffer (0.1 M, pH 7.2). Subsequently, we measured absorbance utilizing spectrophotometer at 412 nm until the increasing absorbance become stable. Later on, after shifting of stable absorbance toward zero and mixing with 20 μl of acetylthiocholine iodide (substrate), variations in absorbance per minute were recorded for 10 min. The rate was calculated based on the following formula: R = 5.74 (10–4) × A / Co. Brain acetyl-cholinesterase activity was presented as μM/l/min/g tissue. In the case of the mentioned formula, R is rate; A is alterations in absorbance per minutes; Co is the original concentration of tissue (mg/ml).
Assessment of lipid peroxidation
MDA level is one of the end products of lipid peroxidation and usually is determined the method of Esterbauer and Cheeseman (Esterbauer and Cheeseman 1990). This method is based on thiobarbituric acid-reactive substance (TBARS) formation. Briefly, thiobarbituric acid (2 ml, 0.67%) and trichloroacetic acid (1 ml, 20%) were mixed with tissue homogenates, including 1 mg protein concentration. Then, the resulting substance was incubated at 100 °C for 60 min. After centrifugation and removing of the precipitate, the rate of the absorbance change of reaction mixtures was measured at γ = 532 nm against a blank including all ingredients except for brain tissue homogenates.
Evaluation of ROS
To measure ROS, 2, 7-dichlorofluorescein diacetate fluorescent probe (DCFH-DA, Molecular Probes, Eugene, OR) was used. The Interaction between intracellular ROS and DCFH-DA result in fluorescent dichlorofluorescein formation. Briefly, 10 μl of DCFH-DA (10 μM) was mixed with 150 μl tissue homogenates and incubated at 37 °C for 40 min. Quantification of the DCF fluorescence intensity at an excitation of 488 nm and an emission of 525 nm was performed using a fluorescence microplate reader.
Assessment of NF-κ B, TNFα, SIRT1, SIRT3, and P53
The expressions of NF-κ B, TNFα, SIRT1, SIRT3, and P53 were measured using commercially available kits inconsistent with the manufacturer’s instructions.
Western blot assay
Western blot assay was performed according to a previous study (Amani et al. 2019). The hippocampus tissues were homogenized in RIPA buffer. Afterward, the lysate tissues were centrifuged at 13300 g at 4 °C for 20 min. In the next step, the supernatant was collected and the total protein concentrations were determined using the Nanodrop equipment. Soluble proteins (50 μg) were separated into the electrophoresis chamber in 4–20% gradient SDS/PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and then transferred to PVDF membranes. PVDF membranes were incubated with a blocking solution (3% solution of BSA and 0.1% Tween-20 (TBST)) to black nonspecific reaction sites. Then, the membranes were incubated with the primary antibodies including anti-SIRT1 antibody, SIRT3 antibody and NFĸB p65 antibody at 4 °C overnight. After washing, the membranes were exposed to anti-rabbit IgG peroxidase-conjugated secondary antibody for 1 h at room temperature. Finally, the membranes were exposed to chemiluminescent HRP Substrate (Millipore) as a detector of immunoreactivity onto Kodak X-OMAT films for 5 min. Alpha Ease ® FC Imaging System was used to determine the mean values of proteins based on their optical densities. The β-actin was used as a loading control and for normalization.
Data analysis
All the experimental data are expressed as mean ± standard deviation (SD). One-way analysis of variance (one-way ANOVA) followed by Tukey test as post hoc analysis was used to analyze statistical differences between different groups. P < 0.05 was accepted to be statistically significant.
Results
The effects of Troxerutin on cognitive and behavioral evaluations
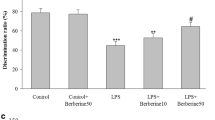
Y-maze test revealed that the rate of alternations was markedly decreased in the LPS group compared with control and control+ Troxerutin (Fig. 1a). Post-treatment with Troxerutin considerably reversed the percentage of alternation while dexamethasone failed to exert a significant effect. As shown in Fig. 1b, a significant decrease in STL was observed in the LPS group compared with control and control+ Troxerutin. Both Troxerutin and dexamethasone significantly increased STL compared to the LPS group. Moreover, the novel object recognition test revealed that LPS group had a significant decrease in discrimination ratio between the novel and familiar objects relative to control and control+ Troxerutin. Intraperitoneal administration of Troxerutin significantly increased the DR compared to the LPS group whereas increased DR by dexamethasone did not reach a significant level (Fig. 1c).
Post-treatment with Troxerutin significantly reduced LPS-induced memory impairments (a) Effect of Troxerutin on Y-maze spatial memory (*P < 0. 05, **P < 0. 01 vs. controls; #P < 0.05 vs. LPS group). b The effects of Troxerutin on step-through latency (*P < 0. 05, and ***P < 0. 001 vs. controls; #P < 0.05 vs. LPS group). c Effect of Troxerutin on novel object recognition test (*P < 0. 05, and **P < 0. 01 vs. controls; #P < 0.05 vs. LPS group)
The effects of Troxerutin on expression levels of inflammatory mediators (ELISA assay)
As depicted in Fig. 2a, b, ELISA assay confirmed that levels of NF-κ B and TNFα significantly elevated in the LPS group in comparison with control and control+ Troxerutin. Subsequently, the levels of NF-κ B and TNFα in the LPS rats which treated with Troxerutin and dexamethasone were reduced.
The effects of Troxerutin on the expression levels of SIRT1, SIRT3, and P53 (ELISA assay)
Our results revealed that SIRT1 and SIRT3 expression were reduced in contrast to control group upon LPS administration for 6 days. As shown in Fig. 3a, b, Troxerutin administration significantly promoted SIRT1 and SIRT3 expression levels as compared with the LPS group while dexamethasone failed to exert a significant effect. Likewise, a significantly increased level of P53 was observed in the LPS-induced adult rat hippocampus compared with control and control+ Troxerutin. Both Troxerutin and dexamethasone markedly decreased the expression level of P53 relative to the LPS group (Fig. 3c).
Troxerutin administration affects the expression of the SIRT1/SIRT3 signaling pathway, p53, as well as brain acetyl-cholinesterase activity. The effects of Troxerutin on expression levels of (a) SIRT1 (*P < 0. 05, and **P < 0. 01 vs. controls; #P < 0.05 vs. LPS group), (b) SIRT3 (**P < 0. 01 vs. controls; #P < 0.05 vs. LPS group), C) p53 (**P < 0. 01 vs. controls; #P < 0.05 vs. LPS group) as well as D) brain acetyl-cholinesterase activity (*P < 0. 05, and **P < 0. 01 vs. controls; #P < 0.05 vs. LPS group)
Brain acetyl-cholinesterase activity
Systemic administration of LPS increased the activity of brain acetyl-cholinesterase compared to control and control+ Troxerutin. Troxerutin injection markedly reduced the activity of this enzyme whereas dexamethasone failed to decrease its activity significantly (Fig. 3d).
The effects of Troxerutin on the expression levels of NF-κB, SIRT1, and SIRT3 (Western blot assay)
To confirm the above-mentioned result in targeting the SIRT1/SIRT3 signaling pathway and inhibition of inflammation by Troxerutin, we also determined the expression levels of NF-κ B, SIRT1, and SIRT3 using Western blot assay. In keeping with ELISA assay, the expressions of SIRT1 and SIRT3 were significantly reduced in LPS rats that reversed by Troxerutin (Fig. 4a, b). Likewise, a significant increased expression level of NF-κ B was found in LPS rats that reversed by Troxerutin (Fig. 4c).
Western blot assay confirmed that Troxerutin administration affects the expression of the SIRT1/SIRT3 signaling pathway and NF-κ B signaling pathway. The effects of Troxerutin on expression levels of (a) SIRT1 (*P < 0. 05 vs. control; ##P < 0.01 vs. LPS group), (b) SIRT3 (*P < 0. 05 vs. control; ##P < 0.01 vs. LPS group), and (c) NF-κ B (**P < 0. 01 vs. control; #P < 0.05 vs. LPS group)
Oxidative stress
ROS production in the LPS-induced adult rat hippocampus was considerably increased and suppressed by Troxerutin treatment. There is no significant difference between LPS + dexamethasone and LPS group (Fig. 5a). As shown in Fig. 5b, a significant increment in MDA level was found upon LPS administration contrast to control and control+ Troxerutin groups. Post-treatment with Troxerutin markedly reduced MDA level compared with the LPS group. There is no significant difference between LPS + dexamethasone and LPS group.
Discussion
The particular knowledge about targeting neuroinflammation opens up potential avenues for discovery of efficient approaches to treat neurodegenerative diseases such as Parkinson’s and Alzheimer’s diseases (Amani et al. 2018). Many previous studies have shown that bioactive phytoconstituents of herbal medicines could give rise to suppression of LPS induced memory impairment though targeting neuroinflammation and oxidative stress (Esterbauer and Cheeseman 1990; Song et al. 2013). In a previous study by our group, we observed that Troxerutin confers neuroprotection in a rat model of Parkinson’s disease by targeting some cellular signaling pathways (Baluchnejadmojarad et al. 2017a). Herein, we found the neuroprotective capability of Troxerutin in coping with LPS induced cognitive deficiency via targeting LPS-induced neuroinflammation and oxidative stress in the hippocampus region of rats. The fundamental effector components of the immune system in the CNS which play a pivotal role in host defense mechanisms are neuroglial cells like astrocytes and microglia. They also possess paramount roles in memory, learning and synaptic remodeling (Geinisman 2000). It has been reported that LPS can be bonded to TLR4 receptors that are expressed by astrocytes and microglia cell and initiates acute inflammatory responses. Moreover, CD14 receptors amplify LPS responses to trigger its detrimental effects on vital body organs (Park and Lee 2013; Zanoni et al. 2011). Likewise, it has recognized that LPS-TLR4 signaling leads to phosphorylation and nuclear translocation of the transcription factor NFκ B and subsequent release of pro-inflammatory cytokines such as TNFα and IL1β in the microglial cells (Gorina et al. 2011). In fact, activated microglia and astrocytes are critical factors for exacerbation of neuroinflammation because these cells are pools for secretion and release of pro-inflammatory cytokines (Block et al. 2007). According to previous reports, elevated levels of NFκB and TNFα upon LPS administration for the 6 days were found in this study and were reversed by Troxerutin. It is well documented that sirtuins paramount key role in healthspan extension and longevity as a result of their ability to suppress inflammation and oxidative stress (Salminen et al. 2008). It has been reported that LPS can act as a competitive inhibitor to SRIT1 with glucose and cholesterol toxicity to various cells and tissues (Ian 2017). Activation of SRIT1 by therapeutic agents and nutritional diets during pathological condition results in deacetylation of NF- κB and consequently inhibition of its transactivational activity (Yeung et al. 2004). Also, a previous report showed that activation of the Sirt3/ AMP-activated protein kinase (AMPK) signaling axis by bioactive phytoconstituents led to protection against LPS-induced acute respiratory distress syndrome (Chen et al. 2018). Sirt3 ameliorates microglia activation-induced oxidative stress injury via modulation of the mitochondrial pathway (Jiang et al. 2017). This study also revealed that the expressions of SRIT1 and SIRT3 decreased upon LPS administration for the 6 days and Troxerutin post-treatment increased the expression level of them. In keeping with our findings, a previous report showed that activation of the SIRT1/ nuclear factor erythroid 2-related factor (Nrf2) signaling pathway by pharmacological agents resulted in suppression of LPS-induced oxidative stress in postnatal rat brain (Shah et al. 2017). On the other hand, some studies have shown that LPS as a toxic agent; confers excessive ROS production. Then, LPS-generated ROS phosphorylates NFκ B that, in turn, results in the initiation of a pathological cascade and subsequent acute neuroinflammation (Zhao et al. 2008). In keeping with these studies, excessive ROS production, elevated MDA and apoptosis were found upon LPS administration for the 6 days and reversed by post-treatment with Troxerutin. Additionally, previous studies have shown that neuroinflammation and oxidative stress caused by LPS administration result in LPS-induced memory impairments in rats. This study also confirmed cognitive deficit following injection of a single dose of LPS. Troxerutin showed better improvement of LPS-induced memory impairments compared to dexamethasone. In this study, we also found an increased activity of acetylcholinesterase that was reversed by Troxerutin. It is known that high activity of acetylcholinesterase along with excessive ROS production under pathological condition gives rise to initiation of acute inflammatory responses (Baluchnejadmojarad et al. 2017b).
Conclusion
Collectively, the present study shows the anti-neuroinflammatory capacity of Troxerutin against LPS-induced memory impairments by targeting SIRT1/SIRT3 signaling pathway, neuroinflammation and oxidative stress in a rat model. Furthermore, our results suggest that Troxerutin can be a suitable candidate to treat inflammatory and neurodegenerative disorders by targeting the SIRT1/SIRT3 signaling pathway.
References
Amani H, Ajami M, Nasseri Maleki S, Pazoki-Toroudi H, Daglia M, Tsetegho Sokeng AJ, Di Lorenzo A, Nabavi SF, Devi KP, Nabavi SM (2017) Targeting signal transducers and activators of transcription (STAT) in human cancer by dietary polyphenolic antioxidants. Biochimie 142:63–79
Amani, Hamed, Ebrahim Mostafavi, Hamidreza Arzaghi, Soodabeh Davaran, Abolfazl Akbarzadeh, Omid Akhavan, Hamidreza Pazoki-Toroudi, and Thomas J Webster. 2018. 'Three-dimensional graphene foams: synthesis, properties, biocompatibility, biodegradability, and applications in tissue engineering'. ACS Biomaterials Science & Engineering, 5: 193–214
Amani H, Habibey R, Shokri F, Hajmiresmail SJ, Akhavan O, Mashaghi A, Pazoki-Toroudi H (2019) Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci Rep 9:6044
Badshah H, Ali T, Myeong OK (2016) Osmotin attenuates LPS-induced neuroinflammation and memory impairments via the TLR4/NFκB signaling pathway. J Scientific Reports 6:24493
Baluchnejadmojarad T, Jamali-Raeufy N, Zabihnejad S, Rabiee N, Roghani M (2017a) Troxerutin exerts neuroprotection in 6-hydroxydopamine lesion rat model of Parkinson’s disease: Possible involvement of PI3K/ERβ signaling. J European J Pharmacology 801:72–78
Baluchnejadmojarad T, Kiasalari Z, Afshin-Majd S, Ghasemi Z, Roghani M (2017b) S-allyl cysteine ameliorates cognitive deficits in streptozotocin-diabetic rats via suppression of oxidative stress, inflammation, and acetylcholinesterase. J European J Pharmacology 794:69–76
Block ML, Zecca L, Hong J-S (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. J Nature Reviews Neuroscience:8–57
Bromfield JJ, Iacovides SM (2017) Evaluating lipopolysaccharide-induced oxidative stress in bovine granulosa cells. J Assist Reprod Genet 34:1619–1626
Chen L, Li W, Qi D, Lu L, Zhang Z, Wang D (2018) Honokiol protects pulmonary microvascular endothelial barrier against lipopolysaccharide-induced ARDS partially via the Sirt3/AMPK signaling axis. J Life Sciences 210:86–95
Ellman GL, Diane Courtney K, Jr VA, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. J Biochemical Pharmacology 7:88–95
Esterbauer, Hermann, and Kevin H Cheeseman. (1990). [42] Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. in Methods in enzymology (Elsevier)
Geinisman Y (2000) Structural synaptic modifications associated with hippocampal LTP and behavioral learning. J Cerebral Cortex 10:952–962
Golechha M, Chaudhry U, Bhatia J, Saluja D, Singh D (2011) Naringin protects against kainic acid-induced status epilepticus in rats: evidence for an antioxidant, anti-inflammatory and neuroprotective intervention. J Biological Arya Pharmaceutical Bulletin 34:360–365
Gorina R, Font-Nieves M, Márquez-Kisinousky L, Santalucia T, Planas AM (2011) Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. J Glia 59:242–255
Ian M (2017) The future of genomic medicine involves the maintenance of sirtuin 1 in global populations. Mol Biol 2:00013
Jamali-Raeufy N, Roghani M, Ramazi S, Mansouri M (2014) Administration of Salvianolic Acid B Attenuates Learning and Memory Deficits in Diabetic Rats: Involvement of Oxidative Stress. J Basic and Clinical Pathophysiology 2:43–50
Jamali-Raeufy N, Roghani M, Nikbakht F, Ramazi S, Zavvary F (2015) Salvianolic acid improves status epilepticus and learning and memory deficiency in rat model of temporal lobe epilepsy. J Basic and Clinical Pathophysiology 3:39–46
Jiang D-Q, Wang Y, Li M-X, Ma Y-J, Wang Y (2017) SIRT3 in neural stem cells attenuates microglia activation-induced oxidative stress injury through mitochondrial pathway. J Frontiers in Cellular Neuroscience 11:7
Kessler, M, G Ubeaud, T Walter, F Sturm, and L Jung. (2002). Free radical scavenging and skin penetration of troxerutin and vitamin derivatives, J Dermatological Treatment 13: 133–41
Martins IJ (2018) Bacterial lipopolysaccharides and neuron toxicity in neurodegenerative diseases. Neurol Neurosurg 1:1–3
Na J-Y, Song K, Kim S, Kwon J (2016) Rutin protects rat articular chondrocytes against oxidative stress induced by hydrogen peroxide through SIRT1 activation. Biochem Biophys Res Commun 473:1301–1308
Okun E, Griffioen KJ, Mattson MP (2011) Toll-like receptor signaling in neural plasticity and disease. J Trends Neurosciences 34:269–281
Panat NA, Singh BG, Maurya DK, Sandur SK, Ghaskadbi SS (2016) Troxerutin, a natural flavonoid binds to DNA minor groove and enhances cancer cell killing in response to radiation. J Chemico-Biological Interactions 251:34–44
Park BS, Lee J-O (2013) Recognition of lipopolysaccharide pattern by TLR4 complexes. J Experimental Molecular Medicine 45:e66
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong J-S, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. J Glia 55:453–462
Salminen A, Kauppinen A, Suuronen T, Kaarniranta K (2008) SIRT1 longevity factor suppresses NF-κB-driven immune responses: regulation of aging via NF-κB acetylation? J Bioessays 30:939–942
Salminen A, Hyttinen JMT, Kaarniranta K (2011) AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med 89:667–676
Shah SA, Khan M, Jo M-H, Jo MG, Amin FU, Kim MO (2017) Melatonin stimulates the SIRT 1/Nrf2 signaling pathway counteracting lipopolysaccharide (LPS)-induced oxidative stress to rescue postnatal rat brain. J CNS Neuroscience Therapeutics 23:33–44
Song J-H, Lee J-W, Shim B, Lee C-Y, Choi S, Kang C, Sohn N-W, Shin J-W (2013) Glycyrrhizin alleviates neuroinflammation and memory deficit induced by systemic lipopolysaccharide treatment in mice. J Molecules 18:15788–15803
Stephenson J, Nutma E, van der Valk P, Amor S (2018) Inflammation in CNS neurodegenerative diseases. J Immunol 154:204–219
Su K-Y, Yu CY, Chen Y-W, Huang Y-T, Chen C-T, Wu H-F, Chen Y-LS (2014) Rutin, a flavonoid and principal component of Saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model. Int J Med Sci 11:528–537
Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW (2004) Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. J The EMBO J 23:2369–2380
Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC (2011) CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. J Cell 147:868–880
Zhang Z-F, Zhang Y-q, Fan S-H, Zhuang J, Zheng Y-L, Lu J, Wu D-M, Shan Q, Hu B (2015) Troxerutin protects against 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47)-induced liver inflammation by attenuating oxidative stress-mediated NAD+-depletion. J Hazardous Materials 283:98–109
Zhao L, Chen Y-H, Wang H, Ji Y-L, Ning H, Wang S-F, Cheng Z, Lu J-W, Duan Z-H, Xu D-X (2008) Reactive oxygen species contribute to lipopolysaccharide-induced teratogenesis in mice. J Toxicol Sci 103:149–157
Zhào, Hóngyi, Yu Liu, Jing Zeng, Dandan Li, Weiwei Zhang, Yonghua Huang. (2018). Troxerutin and Cerebroprotein Hydrolysate Injection Protects Neurovascular Units from Oxygen-Glucose Deprivation and Reoxygenation-Induced Injury In Vitro, J Evidence-Based Complementary and Alternative Medicine 2018
Acknowledgments
The present study was financially supported by research affairs of Iran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jamali-Raeufy, N., Kardgar, S., Baluchnejadmojarad, T. et al. Troxerutin exerts neuroprotection against lipopolysaccharide (LPS) induced oxidative stress and neuroinflammation through targeting SIRT1/SIRT3 signaling pathway. Metab Brain Dis 34, 1505–1513 (2019). https://doi.org/10.1007/s11011-019-00454-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-019-00454-9