Abstract
Lesion-induced cochlear damage can result in synaptic outgrowth in the ventral cochlear nucleus (VCN). Tinnitus may be associated with the synaptic outgrowth and hyperactivity in the VCN. However, it remains unclear how hearing loss triggers structural synaptic modifications in the VCN of rats subjected to salicylate-induced tinnitus. To address this issue, we evaluated tinnitus-like behavior in rats after salicylate treatment and compared the amplitude of the distortion product evoked otoacoustic emission (DPOAE) and auditory brainstem response (ABR) between control and treated rats. Moreover, we observed the changes in the synaptic ultrastructure and in the expression levels of growth-associated protein (GAP-43), brain-derived neurotrophic factor (BDNF), the microglial marker Iba-1 and glial fibrillary acidic protein (GFAP) in the VCN. After salicylate treatment (300 mg/kg/day for 4 and 8 days), analysis of the gap prepulse inhibition of the acoustic startle showed that the rats were experiencing tinnitus. The changes in the DPOAE and ABR amplitude indicated an improvement in cochlear sensitivity and a reduction in auditory input following salicylate treatment. The treated rats displayed more synaptic vesicles and longer postsynaptic density in the VCN than the control rats. We observed that the GAP-43 expression, predominantly from medial olivocochlear (MOC) neurons, was significantly up-regulated, and that BDNF- and Iba-1-immunoreactive cells were persistently decreased after salicylate administration. Furthermore, GFAP-immunoreactive astrocytes, which is associated with synaptic regrowth, was significantly increased in the treated groups. Our study revealed that reduced auditory nerve activity triggers synaptic outgrowth and hyperactivity in the VCN via a MOC neural feedback circuit. Structural synaptic modifications may be a reflexive process that compensates for the reduced auditory input after salicylate administration. However, massive increases in excitatory synapses in the VCN may represent a detrimental process that causes central hyperactivity, leading to tinnitus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subjective tinnitus is the perception of a phantom ringing sound in the absence of external stimulation. Approximately 1–3 % of patients with severe tinnitus exhibit a reduced daily quality of life and various psychological disturbances, such as depression, stress or sleep disturbance [1]. Tinnitus is commonly associated with hyperactivity in the central auditory system, including the ventral cochlear nucleus (VCN) [2–5]. Although tinnitus is generated in the auditory center, there is strong evidence that cochlear damage triggers pronounced neuroplasticity changes and hyperactivity in the central auditory region in various tinnitus models [6–8]. For example, hearing loss due to noise exposure, ototoxic drug administration or cochlear ablation commonly induces structural synaptic modifications in the central auditory system [7–9]. Salicylate can also significantly reduce the auditory input due to cochlear damage [10]. Neural feedback circuits may be involved in structural synaptic modifications and central hyperactivity after the reduction of auditory input [11]. However, the neural mechanism underlying the generation of tinnitus has remained unclear.
The MOC efferent reflex diminishes outer hair cell (OHC) electromotility and reduces cochlear amplification. In response to auditory nerve malfunction, there is a reflexive decrease in MOC efferent reflex strength, leading to the enhancement of distortion product otoacoustic emission (DPOAE) amplitudes [12, 13]. This change may compensate for the loss of auditory input and may keep the balance of excitatory input [14]. Furthermore, MOC neurons send axon collaterals to T-stellate cells in the VCN to form excitatory synapses [15, 16]. After cochlear damage, MOC neurons may contribute to structural synaptic modifications in the VCN [14]. Therefore, we hypothesized that salicylate-induced hearing loss triggers structural synaptic modifications and hyperactivity in the VCN via a neural feedback circuit including MOC neurons.
To determine the neural correlates of these pathological regions we detected changes in the DPOAE and ABR amplitude, the synaptic ultrastructure and the pattern of GAP-43 immunostaining in the VCN of rats subjected to salicylate-induced tinnitus. GAP-43 is a member of the protein kinase C family and is highly expressed in the synaptic growth cone. It plays an important role in synaptic growth and is associated with neurotransmitter regulation and structural synaptic modifications [17, 18]. Additionally, the expression levels of BDNF (a neurotrophic factor), Iba-1 (a microglial marker) and GFAP (an astrocyte marker) in the VCN were analyzed.
Materials and Methods
Subjects
All experimental procedures were approved by the Laboratory Animal Care and Use Committee of Fudan University and adhere to its ethical guidelines. The subjects consisted of 42 adult male Wistar rats (6–8 weeks old, 200–280 g). The rats were divided into the following four groups: [1] control group (n = 10), [2] treatment group with repeated injections of salicylate for 4 days (n = 10; S4), [3] treatment group with repeated injections of salicylate for 8 days (n = 10; S8), and [4] recovery group with 1 week after 8 days salicylate administration (n = 12; S8 + R7).
Design and Salicylate Administration
Sodium salicylate (Sigma-Aldrich, Shanghai, China) was dissolved in 0.9 % sodium chloride (sterile physiological saline) to achieve a concentration of 200 mg/ml. The treatment group was injected intraperitoneally with sodium salicylate (300 mg/kg) once daily at 08:00 for 4 consecutive days (S4) and 8 consecutive days (S8), respectively. The recovery group (S8 + R7) was given intraperitoneal injections for 8 consecutive days followed by a 7-day recovery period after treatment cessation. Rats treated with equal amounts of normal saline served as the control group.
Behavioral Detection of Tinnitus
The rats were assessed for tinnitus-like behavior using the gap-prepulse inhibition of the acoustic startle (GPIAS) paradigm as described in previous studies [19]. In a soundproof room, each rat was placed in an organic glass cage containing holes drilled on the roof for sound passage, which limited excessive animal movement. This cage was mounted on an acrylic base attached to a sensitive piezoelectric transducer on the cage floor that detected the startle responses of the rats. Using Kinder Scientific Startle Monitor (Kinder Scientific Co., CA, USA), the maximum peak-to-peak amplitude of the startle response was recorded over a 100-ms interval following the onset of the startle stimulus. Sound stimuli and background noise were generated by a computer using Multi-Instrument v3.2 (Virtins Technology, Singapore), were amplified by a power amplifier and were presented using a high frequency dome speaker located 15 cm above the animal’s head. The startle-eliciting stimulus consisted of a broadband noise burst presented at 110 dB SPL (white noise, 20 ms duration). Background sound consisted of 65 dB SPL level continuous narrow band noise (1000-Hz bandwidth) centered at 8, 12, 16 and 20 kHz. Baseline noise levels for the frequency range were controlled below 20 dB SPL in the test chamber.
GPIAS testing began 4 h after the final injection for each group. The peak-to-peak amplitude of the acoustic startle response was measured in the presence of continuous background noise with “no gap”(STng) or with a 50-ms silent “gap”(STg). The gap in the background noise preceded the onset of the startle sound by 100 ms. For each testing frequency, the GPIAS sessions contained 30 “no-gap” and 30 “gap” trials in a randomized order. The interval between each session was randomly set from 30 to 35 s. The significant difference in GPIAS between the gap and no-gap conditions indicated that the animal was able to detect the silent gap. In contrast, in rats with tinnitus, the startle amplitude under the “gap” condition was significantly increased, approaching that of the “no gap” condition. The percentage of the GPIAS was calculated using the following formula: [(STng-STg)/STng × 100 %] [20].
Auditory Brainstem Response (ABR) Measurement
The ABR amplitude was examined before, after 8-day salicylate treatment, and 1 week after 8-day treatment in six recovery rats. The animals were anesthetized via intraperitoneal injection of a mixture of ketamine (Ketaset; 80 mg/kg) and xylazine (Rompun; 4 mg/kg). The test temperature was maintained at 25 °C in a sound-proof room. Alternating phase tone bursts (5-ms duration with a 1-ms rise/fall time) at 8, 16, 24, and 32 kHz were generated using BioSigRP Stimulate System (Tucker-Davis Technologies, Gainesville, FL, USA) and were presented at a rate of 21 bursts/s. The stimuli were delivered to the left or right ear using a high-frequency transducer. Needle electrodes were placed at the vertex (ground), apex nasi (reference), and the mastoid (active). The responses were amplified 5000 times by an amplifier. Then the response was averaged 500 times and collected by BioSigRP Record System (Tucker-Davis Technologies, Gainesville, FL, USA). The vertical distance between N3 and P3 was measured as ABR amplitude [21].
Distortion Product Evoked Otoacoustic Emission (DPOAE) Measurement
The DPOAE amplitude was tested before, after 4 and 8-day salicylate treatment, and 1 week after 8-day treatment in the recovery rats (n = 6). The DPOAE testing was conducted in a sound-proof room. The awake animals were fixed on a rat holder (Fudan Apparatus) for the procedure. TDT SigGen software (Tucker-Davis Technologies, Gainesville, FL, USA) created a two-tone stimulation which was delivered to the ear canal using flexible tubes connected to the earpiece through two high frequency transducers. The primary tone F 2 was set at 8, 12, 16, 20, 24, 28, 32 kHz. The frequency ratio F 2/F 1 was set at 1.2. Both F 2 and F 1 were given an intensity of 65 dB SPL. TDT BioSig software (Tucker-Davis Technologies, Gainesville, FL, USA) recorded the DPOAE amplitudes in the ear canal using an Intel microprocessor-based computer. At all frequencies (F 2), the input of the Smart DPOAE system received, digitized and evaluated the output of the microphone using the system software. The acoustic signal was recorded over a period of 204 ms (sampling rate 40 kHz) and averaged 32 times. The noise floor was measured in a 24 Hz band surrounding 2F 2 − F 1.
Transmission Electron Microscopy (TEM)
The anesthetized rats were perfused with 50 ml of normal saline followed by a mixture of 4 % paraformaldehyde and 1.0 % glutaraldehyde in 0.1 M phosphate buffer (pH7.2) for 15 min. Then, the tissue samples were immersed in 2.5 % glutaraldehyde for 24 h at 4 °C and dissected into 200-μm slices using a vibratome (LEICA VT1200S, Microtome Leica, Wetzlar, Germany). In the present study, we selected the medial portion of VCN approaching the root of the cochlear nerve in the VCN for synaptic comparison. Subsequently, the tissue was sliced into 1 × 1 mm sections that were routinely dehydrated using a graded ethanol series. After replacing the ethanol with propylene oxide, the tissues were embedded in Epon. A diamond knife was used to prepare a series of consecutive 80-nm ultra-thin sections. Finally, the sections were stained with lead citrate and observed under a JEM-1230 TEM (JEOL, JAPAN). The image J program (Bethesda, MA, USA) for quantitative analysis. The number of synaptic vesicles, the thickness and length of postsynaptic density (PSD) and the width of synaptic cleft were measured.
Immunohistochemistry
The rats were sacrificed at 8:00 on the day following the final injection. Under deep anesthetization, the rats were transcardially perfused with normal saline for 5 min followed by 4 % paraformaldehyde in 0.1 M phosphate buffer (pH 7.2) for 30 min at room temperature. The brainstem was dissected coronally and post-fixed in 4 % paraformaldehyde fixative for 24 h. Then, the tissues were embedded in paraffin blocks and sectioned at 4-µm thickness. The medial AVCN approaching the root of the cochlear nerve was selected for investigation. This region is the major target innervated by MOC collateral fibers. Moreover, a previous study demonstrated that the medial AVCN is a reliable site for analyzing structural synaptic modifications in the VCN [6]. These sections were deparaffinized, rapidly rehydrated and blocked with normal goat serum. Then, the sections were incubated in primary antibody overnight at 4 °C. For immunohistochemistry, the following antibodies were used: 1:200 GAP-43 (BOSTER, BA0878), 1:200 rabbit BDNF antibody (Abcam, AB6201), 1:1000 goat Iba-1 antibody (Abcam, AB107159) and 1:50,000 rabbit GFAP antibody (Abcam, AB7260). After treatment with the primary antibody, the sections were incubated in secondary antibodies for 1 h. Immunoreactivity was visualized using 3,3′-diaminobenzidine tetrahydrochloride (DAB) as a chromogen. To evaluate the expression of GAP-43, BDNF, Iba-1 and GFAP, measurements of relative optical density (ROD) were performed in the selected region of the VCN according to the previous method [22]. The OD of immunoreactive structures in every section was obtained using Image Pro Plus 6.0. After the background density was subtracted, a ratio of the OD of every section was calibrated as % (ROD). The average value from 5 sections per rat was calculated by a blinded investigator.
Western Blot
The VCN regions of the rats were rapidly dissected and stored in a freezer at −80 °C as described by Friedland et al. [23]. After the cells were disrupted, their protein content was determined using an ultraviolet spectrophotometer (752-P, XianKe, China). The GAP-43, BDNF, Iba-1 and GFAP proteins were separated via 8 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5 % skim milk powder for 1 h at room temperature, and then incubated in primary antibodies at 4 °C overnight. Secondary antibodies were applied to the membranes for 1 h at room temperature. Finally, the peroxidase bands were visualized using the SuperSignal Chemiluminescent Substrate system (Pierce). After scanning, the pixel densities of the immunoreactive bands were quantified using AlphaEaseFC software (Alpha Innotech), and the band intensities corresponding to GAP-43, BDNF, Iba-1 and GFAP were normalized to Actin levels. The following antibodies were used: 1:200 rabbit GAP-43 antibody (Boster, BA0878), 1:200 rabbit BDNF antibody (Abcam, AB6201), 1:1000 goat Iba-1 antibody (Abcam, AB107159) and 1:50,000 rabbit GFAP antibody (Abcam, AB7260).
Statistical Analysis
All data are presented as the mean ± standard error of mean (SEM). Based on the data distribution and the homogeneity tests, paired and unpaired, two-sided Student’s t tests and one-way analysis of variance (ANOVA) was selectively performed for group comparisons. A level of P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 14.0 (Chicago, IL, USA).
Results
Salicylate-Induced Tinnitus-Like Behavior in Rats
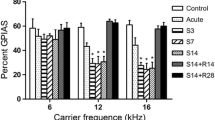
For the mean percentage of the GPIAS, an effect of salicylate treatment was observed at 12 kHz (F3,20 = 42.638, P < 0.05) and 16 kHz (F3,20 = 24.657, P < 0.05), but not at 8 kHz (F3,20 = 0.429, P = 0.734) and 20 kHz (F3,20 = 2.905, P = 0.060). In the control group, the mean percentage of the GPIAS was 48.0 ± 3.5 % for 8 kHz, 45.6 ± 10.9 % for 12 kHz, 50.5 ± 7.4 % for 16 kHz and 51.7 ± 14.1 % for 20 kHz. After 4 days of salicylate treatment, the rats showed a significant reduction in the mean percentage of the GPIAS at 16 kHz (P < 0.05, vs. the control group), which indicated that tinnitus-like behavior appeared after 4-day salicylate injection. Moreover, the difference in the mean percentage of the GPIAS between the control and S8 groups was significant at 12 kHz (P < 0.05) and 16 kHz (P < 0.05), which indicated that these rats presented with persistent tinnitus during salicylate injection. However, the recovery group showed no difference in the mean percentage of the GPIAS compared with the control group (P > 0.05), which indicated that tinnitus-like behavior disappeared 1 week after salicylate treatment (see Fig. 1).
The change in the gap prepulse inhibition of acoustic startle (GPIAS) values in all groups after salicylate treatment. The S4 group showed a significant reduction in the mean percentage of the GPIAS compared with the control group at 16 kHz (*P < 0.05). The S8 group showed a significant reduction in the percentage of the GPIAS at 12 kHz (*P < 0.05) and 16 kHz (*P < 0.05), but not at 8 kHz or 20 kHz (P > 0.05)
ABR
The present study showed that salicylate injection at 300 mg/kg/day for 8 days caused a reduction in the ABR amplitude. Figure 2 shows the ABR amplitudes as a function of frequency at 70 dB SPL at baseline, after 8-day treatment and 1 week recovery after 8 days of salicylate administration. The baseline ABR amplitude shifted from more than 2 µV at 8 kHz to approximately 0.58 µV at 32 kHz. The ABR amplitudes following salicylate treatment were significantly smaller than those at baseline at every frequency analyzed (P < 0.05 by t test); this amplitude reduction was greater at 8 and 16 kHz than at 24 and 32 kHz. Expectedly, 1 week after the 8-day treatment, they recovered to the baseline values.
The effects of salicylate treatment on the ABR amplitudes in the rates. The ABR amplitudes as a function of frequency at 70 dB SPL were measured at baseline, after 8-day salicylate treatment and 1 week after 8-day treatment. The S8 group showed a significant reduction in the ABR amplitude, compared with the control rats at every frequency analyzed (P < 0.05). One week after 8-day treatment, they recovered to the baseline values
DPOAE
In the present study, the DPOAE amplitudes were measured before salicylate treatment, after 4, 8-day treatment and 1 week recovery after 8-day treatment in awake rats to determine if consecutive salicylate administration had an effect on the DPOAE amplitudes. Figure 3 presents the DPOAE amplitudes at a stimulation level of 65 dB SPL (L 1 and L 2) at different F 2 frequencies. After 4-day salicylate treatment, DPOAE amplitudes increased slightly (1.8–3.7 dB SPL) compared with the baseline values but no significant differences. The 8-day salicylate treatment induced a significant elevation of DPOAE amplitudes at 16, 20, 24 kHz compared with the baseline values (P < 0.05 by t test), except for at 8, 12, 28 and 32 kHz (P > 0.05 by t test). However, 1 week after the 8-day treatment, the DPOAE amplitude recovered or dropped below pre-treatment baseline values.
The effects of a consecutive salicylate treatment on DPOAE in awake rats. DPOAE amplitudes at a stimulation level of 65 dB SPL (L1 = L2) as a function of Frequency (F2) in a group of rats (n = 6). DPOAE recording: before, after 4 and 8-day treatment, and 1 week recovery after 8-day treatment. The salicylate treatment induced a significant elevation at 16, 20, 24 kHz after 8-day treatment (P < 0.05), except for at 8, 12, 28 and 32 kHz (P > 0.05). One week after the 8-day treatment, they recovered or dropped below pre-treatment baseline values
Synaptic Changes in the VCN
The synaptic ultrastructure of the VCN was observed via TEM after salicylate administration for 4 or 8 consecutive days. Representative images are shown in Fig. 4. Analysis revealed a main effect of salicylate treatment on synaptic vesicles (F2,33 = 48.871, P < 0.05). Compared with the VCN neurons from the control rats, those from the salicylate-treated rats displayed a significantly increased number of synaptic vesicles (P < 0.05). Furthermore, for the length of the postsynaptic density (PSD), a main effect of salicylate treatment was observed (F2,33 = 4.735, P < 0.05). The length of the postsynaptic density was significantly increased from 0.23 ± 0.11 nm in the control group to 0.38 ± 0.18 nm in the S4 group (P < 0.05) and 0.37 ± 0.11 nm in the S8 group (P < 0.05). However, the width of the synaptic cleft and the thickness of the PSD in the treated groups were not significantly altered compared to the control group (P > 0.05) (Table 1).
Expression of GAP-43 in the VCN
The expression of GAP-43 in the VCN was evaluated via immunohistochemistry (IHC) and Western blot. Figure 5 shows the relative immunoreactive and protein expression levels of GAP-43 in the different groups. For GAP-43 Immunohistochemistry expression, analysis revealed a main effect of salicylate treatment (F2,12 = 144.470, P < 0.05). In the control group, GAP-43 expressed at a low level in the medial AVCN in every section. With salicylate administration, GAP-43 immunoreactivity in the medial AVCN were significantly increased in the treated groups (P < 0.05) (Fig. 5a, b). Moreover, for GAP-43 protein levels, we observed a main effect of salicylate treatement (F3,16 = 224.749, P < 0.05). Post-hoc analysis indicated that the GAP-43 protein levels in the VCN of the treated groups (S4 and S8) were also significantly higher than in the control group (P < 0.05) (Fig. 5c). Thus, salicylate-induced synaptic outgrowth was very prominent in the VCN.
The expression of GAP-43 was evaluated via IHC staining (×400) and Western bolt a the expression of GAP-43 immunoreactivity in the control and treatment groups (S4, S8). b Relative optical density (ROD) as % of GAP-43 immunoreactive structures of the medal VCN (*P < 0.05), c the expression levels of GAP-43 protein in all groups (*P < 0.05)
Expression of BDNF, Iba-1 and GFAP in the VCN
The expression of BDNF, Iba-1 and GFAP in the VCN was evaluated using IHC and Western blot. For BDNF Immunohistochemistry expression, we observed a main effect of salicylate treatment (F3,16 = 541.821, P < 0.05). In the treated groups, BDNF-positive cells were distinctively decreased in number and showed lower immunoreactivity than that in the control group (P < 0.05) (Fig. 6a, b). Moreover, we observed a main effect of salicylate treatment on Iba-1 Immunohistochemistry expression (F3,16 = 292.907, P < 0.05). Post-hoc analysis indicated that Iba-1-immunoreactive microglia were significantly decreased following salicylate treatment (P < 0.05) (Fig. 6a, c). In addition to the decrease in the number of immunostained cells, the expression level of each of the proteins was significantly reduced in the VCN of the treated groups (P < 0.05, vs. the control group) (Fig. 6c, d). ANOVA analysis revealed a main effect of salicylate treatment on the protein expression of BDNF (F3,16 = 202.592, P < 0.05) and Iba-1 (F3,16 = 283.947, P < 0.05). However, in the recovery group, the protein expression of BDNF and Iba-1 returned to the level in the control group. ANOVA analysis revealed a main effect of salicylate treatment on both GFAP Immunohistochemistry (F3,16 = 332.852, P < 0.05) and protein expression (F3,16 = 252.728, P < 0.05). In the treated group, GFAP-immunoreactive astrocytes were distinctively increased in number and showed stronger immunoreactivity than that in the control group (P < 0.05) (Fig. 6a, b). GFAP protein expression in the VCN of the treated groups (S4 and S8) was also significantly higher than that in the control group (P < 0.05) (Fig. 6e).
The expression of BDNF, lba-1 and GFAP was evaluated via IHC staining (×400) and Western bolt a the immunoreactive expression of BDNF, lba-1 and GFAP in the control treatment groups (S4, S8). b ROD as % of GFAP, lba-1 and BDNF immunoreactive structures of the medical VCN after salicylate treatment (*P < 0.05). c, d, e the expression levels of GFAP, lba-1 and BDNF protein. There was a significant difference between the control treatment groups (S4, S8) (*P < 0.05)
Discussion
Our study confirmed that consecutive salicylate administration resulted in a reduction in the ABR amplitudes which may result from a reversible outer hair cell (OHC) and spiral ganglion neurons (SGN) dysfunction. In vitro, salicylate competitively binds to prestin to affect interactions of chloride with prestin or changes the conformation of prestin to decrease chloride permeability, resulting in a reversible inhibition of OHC electromotility and temporary loss of cochlear amplification [24, 25]. Moreover, a previous study demonstrated that salicylate administration caused a significant loss of SGN in the cochlea, leading to auditory nerve dysfunction [26].
Despite the reduction of the ABR amplitudes, our study showed that the DPOAE amplitude was paradoxically increased following consecutive salicylate administration. Previously, Huang et al. [27]. reported that long-term salicylate treatment caused temporary elevation of DPOAE amplitudes in awake guinea pigs. Chen et al. [26]. showed that discontinuing our high-dose salicylate treatment enhanced DPOAE amplitudes by 3–5 dB for up to 8 weeks post-treatment. Similarly, the chronic salicylate treatment caused a reduction in ABR amplitude during the time when cochlear sensitivity was improved. Therefore, long-term salicylate treatment can produce hearing changes, characterized by a reduction in the ABR amplitude and an opposing increase in the DPOAE amplitudes. The treated rats in our study also exhibited these characteristics, suggesting that they must be related to salicylate-induced tinnitus.
The enhancement of the DPOAE amplitudes was possibly due to inhibited MOC efferent reflex and increased cochlear sensitivity [28]. Salicylate-induced hearing loss may inhibit MOC efferent reflex, thereby increasing cochlear sensitivity, which may partially compensate for the reduced auditory input at the cochlear level. Clinical studies reported that tinnitus patients often exhibit a reduction in MOC efferent activity [29]. Furthermore, stimulation of the MOC efferent reduced spontaneous hyperactivity in the auditory center [30]. These studies suggested that tinnitus might be associated with effects on the MOC efferent at the OHC level. However, the functional relationship between MOC efferent activity and hyperactivity in the auditory center remains unclear.
In the present study, the salicylate-treated rats showed a significant up-regulation of GAP-43 in the VCN despite auditory nerve dysfunction. Furthermore, the expression of GAP-43 immunoreactivity showed a big difference between S4 and S8 group, but the same pattern was not observed in Western blot. Thus, there is a discrepancy between GAP-43 immunohistochemistry and western blot results, which can be explained that immunohistochemistry method is not as precise as western blot in GAP-43 protein quantification because immunohistochemistry result is liable to be affected by various factors, such as secondary antibody and negative control, although it has an advantage in antigen location of the VCN. The GAP-43 expression level returned to normal 7 days after the cessation of this treatment. GAP-43 expression is an important marker for synaptic plasticity, which may be involved in tinnitus generation. Furthermore, the rats subjected to salicylate administration displayed a greater number of synaptic vesicles and longer PSDs in the VCN. Several models of hearing loss or tinnitus through cochlear ablation, noise trauma or ototoxicity also produced GAP-43 up-regulation in the VCN region [7, 9, 31]. Synaptic outgrowth may represent a detrimental process to cause central hyperactivity and tinnitus. Although GAP-43 is also transported by the auditory nerve, the increased GAP-43 expression in the VCN did not arise from malfunctioning auditory nerve. The auditory nerve is not the only source that regulates GAP-43 expression in the VCN. In addition to the auditory nerve, the MOC neuron extends axon collaterals that innervate the VCN. Some studies have demonstrated that MOC axon collaterals are the predominant source of GAP-43 expression in the VCN after cochlear damage [32].
MOC neurons are located in the medial region of the superior olivary complex (SPO) and preferentially form synaptic connections with the OHC [33]. In addition to inhibitory synapses at the level of the cochlea, MOC neurons send collateral projections to the VCN that postsynaptically target glutamatergic T-stellate cells, implying that these projections represent an excitatory synaptic input into the VCN [15, 34]. These T-stellate cells integrate singals from the cochlea and MOC collateral fibers and send ascending fibers directly to the contralateral inferior colliculus (IC), which is a major source of input from the CN to the contralateral IC [35, 36]. T-stellate cells also participate in neuronal feedback circuits that provide feedback inhibition to the cochlea and feed-forward inhibition to the IC via the ventral nuclei of the trapezoid body (VNTB) [37]. The activity of these T-stellate cells is modulated by the positive feedback circuit. Reduced auditory input may activate MOC collateral fibers and may enhance excitation of T-stellate cells of the VCN via the positive feedback circuit. Activated T-stellate cells may be responsible for synaptic outgrowth and hyperactivity in the VCN that could compensate for the reduced activity of the auditory fibers [34]. Furthermore, reduced auditory input inhibits the MOC efferent reflex and enhances OHC electromotility via the negative feedback circuit to improve cochlear sensitivity and increase signal gain in the cochlear, which may explain the increase in DPOAE amplitudes after auditory nerve dysfunction. Additionally, when MOC neurons lose their postsynaptic targets in the cochlea, the synaptic connections of the MOC collateral fibers in the VCN are strengthened or increased in number [32, 38]. Collectively, enhanced synaptic excitability and synaptic outgrowth in the VCN counteract the decreased auditory nerve activity and compensate for the lost signal from the damaged cochlea. However, excessive compensation for the excitatory signal may result in central hyperactivity, which may be relevant to hyperacusis or tinnitus.
BDNF is a member of the neurotrophic factor family and secreted by neurons and microglia [39]. It is involved in regulating the construction and functions of neuronal circuits and plays an important role in the development and maintain of neurons [40, 41]. Microglia is able to modulate the electrical activity within neuronal circuits and protect neurons from various injuries. After peripheral nerve injury, microglia releases neurotrophic factor, such as BDNF, to enhance neural excitability [42, 43]. In this study, both the BDNF expression and the microglial number in the VCN were significantly decreased following salicylate administration, suggesting that the VCN had degenerated and lost their BDNF, which may be induced by the reduction in auditory input signal. In addition to releasing neurotrophic factors, microglia plays important roles in neuroinflammatory responses, a previous study reported that neurogenesis of adult neural progenitor cells is induced by IL-4-activated microglia [44]. However, our study suggest that the change in Iba-1 immunoreactivity and protein expression does not reflect the neuroinflammatory response after salicylate administration. Conversely, these results suggest that this loss of iba-1-positive microglia contributed to the VCN degeneration after salicylate administration. Given that tinnitus originates from a decrease in the spontaneous activity of the auditory nerve, it is conceivable that VCN degeneration results in further loss of auditory afferent activity, promoting synaptic outgrowth and hyperactivity in the VCN through the MOC neural feedback circuit. Previous studies have also showed that cochlear damage causes VCN degeneration and that new synapses regenerated in the VCN regions that underwent neural degeneration [7, 31]. Thus, auditory input reduction and VCN degeneration may play a synergistic role in triggering the development of tinnitus.
Additionally, a dramatic increase in the number of GFAP-immunoreactive astrocytes was accompanied by neural degeneration and followed by synaptic outgrowth in the VCN after salicylate administration. GFAP-immunoreactive cells could be postmitotic astrocytes or neuronal stem cells that participate in neurogenesis in various manners. Astrocytes can secret fibroblast growth factors (FGFs) that are upregulated in response to the neural degeneration and potentially play a role in the synaptic regrowth in the CN [45]. At the stage of VCN degeneration, the GFAP-positive cells may release neurotrophic factor-3 (NT-3), which is useful in promoting axonal sprouting after neural injury [46, 47]. Synapses regenerate when they contact astrocytes and receive sufficient trophic supply from postsynaptic sources. Furthermore, astrocytes play an important role in the neuroinflammatory response because they can produce the proinflammatory cytokine TNF-α in response to numerous extrinsic and intrinsic stimuli [48]. TNF-α up-regulation may enhance overall synaptic activity in the CN and may be linked to salicylate-induced tinnitus [49, 50]. Therefore, GFAP-positive astrocytes are increased in response to cochlear damage, which may be a compensatory mechanism to promote synaptic outgrowth in the VCN.
In conclusion, the present study suggests that GAP-43-mediated structural synaptic modifications in the VCN may be associated with neural hyperactivity and tinnitus in salicylate-treated rats. Salicylate-induced auditory nerve dysfunction, concomitant with VCN degeneration, results in the inhibition of MOC efferent reflex and astrocyte proliferation. The MOC neural feedback circuit and astrocytes promote synaptic outgrowth in the VCN, which, in turn, causes hyperactivity in the auditory center and plays a pivotal role in salicylate-induced tinnitus.
References
Bauer CA (2004) Mechanisms of tinnitus generation. Curr Opin Otolaryngol Head Neck Surg 12:413–417
Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM (2000) GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res 147:251–260
Robertson D, Bester C, Vogler D, Mulders WH (2013) Spontaneous hyperactivity in the auditory midbrain: relationship to afferent input. Hear Res 295:124–129
Vogler DP, Robertson D, Mulders WH (2011) Hyperactivity in the ventral cochlear nucleus after cochlear trauma. J Neurosci 31:6639–6645
Wallhausser-Franke E, Mahlke C, Oliva R, Braun S, Wenz G, Langner G (2003) Expression of c-fos in auditory and non-auditory brain regions of the gerbil after manipulations that induce tinnitus. Exp Brain Res 153:649–654
Kraus KS, Ding D, Jiang H, Lobarinas E, Sun W, Salvi RJ (2011) Relationship between noise-induced hearing-loss, persistent tinnitus and growth-associated protein-43 expression in the rat cochlear nucleus: does synaptic plasticity in ventral cochlear nucleus suppress tinnitus. Neuroscience 194:309–325
Kraus KS, Ding D, Zhou Y, Salvi RJ (2009) Central auditory plasticity after carboplatin-induced unilateral inner ear damage in the chinchilla: up-regulation of GAP-43 in the ventral cochlear nucleus. Hear Res 255:33–43
Muly SM, Gross JS, Morest DK, Potashner SJ (2002) Synaptophysin in the cochlear nucleus following acoustic trauma. Exp Neurol 177:202–221
Illing RB, Horvath M, Laszig R (1997) Plasticity of the auditory brainstem: effects of cochlear ablation on GAP-43 immunoreactivity in the rat. J Comp Neurol 382:116–138
Muller M, Klinke R, Arnold W, Oestreicher E (2003) Auditory nerve fibre responses to salicylate revisited. Hear Res 183:37–43
Eggermont JJ, Roberts LE (2004) The neuroscience of tinnitus. Trends Neurosci 27:676–682
Peng JH, Tao ZZ, Huang ZW (2007) Long-term sound conditioning increases distortion product otoacoustic emission amplitudes and decreases olivocochlear efferent reflex strength. NeuroReport 18:1167–1170
Yang K, Huang ZW, Liu ZQ, Xiao BK, Peng JH (2009) Long-term administration of salicylate enhances prestin expression in ratcochlea. Int J Audiol 48:18–23
Kraus KS, Illing RB (2004) Superior olivary contributions to auditory system plasticity: medial but not lateral olivocochlear neurons are the source of cochleotomy-induced GAP-43 expression in the ventral cochlear nucleus. J Comp Neurol 475:374–390
Benson TE, Berglund AM, Brown MC (1996) Synaptic input to cochlear nucleus dendrites that receive medial olivocochlear synapses. J Comp Neurol 365:27–41
Fujino K, Oertel D (2001) Cholinergic modulation of stellate cells in the mammalian ventral cochlear nucleus. J Neurosci 21:7372–7383
Hanaya R, Boehm N, Nehlig A (2007) Dissociation of the immunoreactivity of synaptophysin and GAP-43 during the acute and latent phases of the lithium-pilocarpine model in the immature and adult rat. Exp Neurol 204:720–732
Kim SH, Kim MK, Yu HS, Kim HS, Park IS et al (2010) Electroconvulsive seizure increases phosphorylation of PKC substrates, including GAP-43, MARCKS, and neurogranin, in rat brain. Prog Neuropsychopharmacol Biol Psychiatry 34:115–121
Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K et al (2006) Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci 120:188–195
Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D et al (2007) Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res 226:244–253
Brandt CT, Caye-Thomasen P, Lund SP, Worsoe L, Ostergaard C et al (2006) Hearing loss and cochlear damage in experimental pneumococcal meningitis, with special reference to the role of neutrophil granulocytes. Neurobiol Dis 23:300–311
Park CW, Lee JC, Ahn JH, Lee DH, Cho GS et al (2013) Neuronal damage using fluoro-Jade B histofluorescence and gliosis in the gerbils eptum submitted to various durations of cerebral ischemia. Cell Mol Neurobiol 33:991–1001
Friedland DR, Popper P, Eernisse R, Cioffi JA (2006) Differentially expressed genes in the rat cochlear nucleus. Neuroscience 142:753–768
McLaughlin S (1973) Salicylates and phospholipid bilayer membranes. Nature 243:234–236
Oliver D, He DZ, Klocker N, Ludwig J, Schulte U et al (2001) Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science 292:2340–2343
Chen GD, Kermany MH, D’Elia A, Ralli M, Tanaka C et al (2010) Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res 265:63–69
Huang ZW, Luo Y, Wu Z, Tao Z, Jones RO, Zhao HB (2005) Paradoxical enhancement of active cochlear mechanics in long-term administration of salicylate. J Neurophysiol 93:2053–2061
Peng JH, Tao ZZ, Huang ZW (2007) Long-term sound conditioning increases distortion product otoacoustic emission amplitudes and decreases olivocochlear efferent reflex strength. NeuroReport 18:1167–1170
Favero ML, Sanchez TG, Bento RF, Nascimento AF (2006) Contralateral suppression of otoacoustic emission in patients with tinnitus. Braz J Otorhinolaryngol 72:223–226
Mulders WH, Seluakumaran K, Robertson D (2010) Efferent pathways modulate hyperactivity in inferior colliculus. J Neurosci 30:9578–9587
Illing RB, Kraus KS, Meidinger MA (2005) Reconnecting neuronal networks in the auditory brainstem following unilateral deafening. Hear Res 206:185–199
Kraus KS, Illing RB (2004) Superior olivary contributions to auditory system plasticity: medial but not lateral olivocochlear neurons are the source of cochleotomy-induced GAP-43 expression in the ventral cochlear nucleus. J Comp Neurol 475:374–390
Varghese GI, Zhu X, Frisina RD (2005) Age-related declines in distortion product otoacoustic emissions utilizing puretone contralateral stimulation in CBA/CaJ mice. Hear Res 209:60–67
Fujino K, Oertel D (2001) Cholinergic modulation of stellate cells in the mammalian ventral cochlear nucleus. J Neurosci 21:7372–7383
Cant NB, Benson CG (2003) Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull 60:457–474
Oertel D, Wright S, Cao XJ, Ferragamo M, Bal R (2011) The multiple functions of T stellate/multipolar/chopper cells in the ventral cochlear nucleus. Hear Res 276:61–69
Fujino K, Oertel D (2001) Cholinergic modulation of stellate cells in the mammalian ventral cochlear nucleus. J Neurosci 21:7372–7383
Kraus KS, Ding D, Jiang H, Kermany MH, Mitra S, Salvi RJ (2013) Up-regulation of GAP-43 in the chinchilla ventral cochlear nucleus after carboplatin-induced hearing loss: correlations with inner hair cell loss and outer hair cell loss. Hear Res 302:74–82
Mehrpouya S, Nahavandi A, Khojasteh F, Soleimani M, Ahmadi M, Barati M (2014) Iron administration prevents BDNF decrease and depressive-like behavior followingchronic stress. LID Brain Res S0006-8993(14)01479-6. doi:10.1016/j.brainres.2014.10.057
Mattson MP, Scheff SW (1994) Endogenous neuroprotection factors and traumatic brain injury: mechanisms of action and implications for therapy. J Neurotrauma 11:3–33
Pardon MC (2010) Role of neurotrophic factors in behavioral processes: implications for the treatment of psychiatric and neurodegenerative disorders. Vitam Horm 82:185–200
Parpura V, Zorec R (2010) Gliotransmission: exocytotic release from astrocytes. Brain Res Rev 63:83–92
Trang T, Beggs S, Salter MW (2011) Brain-derived neurotrophic factor from microglia: a molecular substrate for neuropathic pain. Neuron Glia Biol 7:99–108
Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE et al (2006) Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci 31:149–160
Smith L, Gross J, Morest DK (2002) Fibroblast growth factors (FGFs) in the cochlear nucleus of the adult mouse following acoustic over stimulation. Hear Res 169:1–12
Feng J, Bendiske J, Morest DK (2012) Degeneration in the ventral cochlear nucleus after severe noise damage in mice. J Neurosci Res 90:831–841
Wang T, Wang SW, Zhang Y, Wu XF, Peng Y et al (2014) Scorpion venom heat-resistant peptide (SVHRP) enhances neurogenesis and neurite outgrowth of immature neurons in adult mice by up-regulating brain-derived neurotrophic factor (BDNF). PLoS ONE 9:e109977
Park KM, Bowers WJ (2010) Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal 22:977–983
Hu SS, Mei L, Chen JY, Huang ZW, Wu H (2014) Effects of salicylate on the inflammatory genes expression and synaptic ultrastructure in the cochlear nucleus of rats. Inflammation 37:365–373
Hwang JH, Chen JC, Yang SY, Wang MF, Chan YC (2011) Expression of tumor necrosis factor-alpha and interleukin-1 beta genes in the cochlea and inferior colliculus in salicylate-induced tinnitus. J Neuroinflammation 8:30
Acknowledgments
This study was financed by the National Natural Science Fund Projects (Serial number 81070786), the Zhejiang Natural Science Fund Projects (Serial number LY16H130001), the Shanghai Health System Talents Training Program (XBR2011068) and the Shanghai Science and Technology Committee (12XD1401700).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fang, L., Fu, Y. & Zhang, Ty. Salicylate-Induced Hearing Loss Trigger Structural Synaptic Modifications in the Ventral Cochlear Nucleus of Rats via Medial Olivocochlear (MOC) Feedback Circuit. Neurochem Res 41, 1343–1353 (2016). https://doi.org/10.1007/s11064-016-1836-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1836-x