Abstract
In the present study, we investigated the effect of three different sources of hydrogen sulfide (H2S) on sympathetic neurotransmission from isolated superfused bovine iris-ciliary bodies. The three agents under consideration were: ACS67, a hybrid of latanoprost and a H2S-donating moiety; l-cysteine, a substrate for endogenous production of H2S and GYY 4137, a slow donor of H2S. We also examined the contribution of prostaglandins to the pharmacological actions of the H2S donors on release of [3H]-norepinephrine ([3H]NE) triggered by electrical field stimulation. ACS67, l-cysteine and GYY 4137 caused a concentration-dependent inhibition of electrically-evoked [3H]NE release from isolated bovine iris-ciliary bodies without affecting basal [3H]NE efflux. The cyclooxygenase inhibitor, flurbiprofen enhanced the inhibitory action of ACS67 and l-cysteine on stimulated [3H]NE release. Both aminooxyacetic acid, an inhibitor of cystathionine-β-synthase and glibenclamide, a KATP channel blocker reversed the inhibition of evoked NE release induced by the H2S donors. We conclude that H2S donors can inhibit sympathetic neurotransmission from isolated bovine iris-ciliary bodies, an effect partially dependent on the in situ production of H2S and prostanoids, and is mediated by an action on KATP channels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen sulfide (H2S) is a colorless, flammable and water-soluble gas [1–4]. Although it was known for its toxic nature for centuries, evidence from recent studies support a biological role for H2S as a gaseous neurotransmitter in mammals [1–4]. Evidence from literature supports a pathophysiological role of this gas in several mammalian organ systems such as the cardiovascular and central nervous systems [5–8]. H2S is synthesized endogenously in various tissues from amino acids, l-cysteine and homocysteine in the presence of enzymes of the sulfur metabolic network such as cystathionine-β-synthase (CBS) or cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3MST) along with cysteine aminotransferase [2, 9, 10]. There is evidence confirming the activity and expression of CBS and CSE in the liver, kidney, brain, ileum, uterus and placenta [2]. Furthermore, CBS, CSE and 3MST are both present and are enzymatically active in ocular tissues [11, 12]. In isolated bovine eyes, Kulkarni et al. [12] found that while the highest concentrations of H2S was detected in the cornea and neural retina, no such gas was present in the vitreous humor [12]. Deficiency of CBS has been associated with many eye disorders such as retinal degeneration, retinal detachment, optic atrophy and glaucoma [13, 14], suggesting a significant role for the transsulfuration pathway in ocular pathophysiology.
Evidence from literature supports a physiological and pharmacological role for H2S in numerous mammalian cells and tissues. For instance, H2S modulates long term potentiation in rat hippocampus in the central nervous system [3] and elicits relaxation of the vasculature in the cardiovascular system [2]. H2S has been reported to act as an intracellular antioxidant, conferring cytoprotection to various organ systems, including the heart, central nervous system and digestive system [4]. Presumably, its free radical scavenging activity and ability to elevate glutathione concentrations contribute to its cytoprotective actions in these tissues [4]. In ocular tissues, H2S plays a neuroprotective role in the posterior segment of the eye where it prevents light-induced retinal deterioration in mouse retina [11]. Evidence from our laboratory affirm a pharmacological role for H2S in the anterior and posterior segments of the eye. Both H2S donors, Na2S and NaHS relaxed carbachol-induced tone in isolated porcine irides, an action that was dependent on endogenous production of prostanoids and the biosynthesis of CSE and CBS [15]. Furthermore, Monjok et al. [15] found that while KATP channels were involved in the relaxation caused by the H2S donors, inhibition of nitric oxide biosynthesis had no effect on this response [15]. In another study, both NaHS and Na2S produced a concentration-dependent inhibition of electrically-evoked [3H]NE release from the isolated porcine iris-ciliary bodies, a response that was attenuated by inhibitors of CSE and CBS [16]. These investigators also found that, NaHS caused a concentration-dependent decrease in the concentrations of endogenous norepinephrine and epinephrine without affecting dopamine content in isolated porcine iris ciliary bodies [16]. In 2010, Ohia et al. [17] showed evidence that l-cysteine, a substrate for the production of H2S, can relax isolated porcine irides under conditions of tone induced by the muscarinic receptor agonist, carbachol. The inhibitory action of l-cysteine in porcine isolated irides was dependent upon the endogenous production of H2S by both CSE and CBS, and on activity of KATP channels [17]. In the posterior segment of the eye, both NaHS and Na2S inhibited potassium-evoked [3H]d-aspartate release from isolated superfused bovine and porcine neural retinae, a response that was dependent on the intramural biosynthesis of H2S [18]. In rat retinal pigment epithelial cells, Njie-Mbye and coworkers provided evidence that both l-cysteine and NaHS can increase cyclic AMP production through an action on KATP channels [19]. Chitnis and coworkers found that in isolated bovine posterior ciliary arteries, both fast releasing H2S donors such as NaHS and its slow-releasing counterparts (GYY4137, AP67 and AP72) relaxed tone induced by phenylephrine, an effect that was dependent on endogenous production of prostanoids, H2S and on the activity of KATP channels [20, 21]. Other investigators have also reported pharmacological actions of H2S donors on ocular tissues [4, 12].
Based on our earlier observations that H2S donors can regulate sympathetic neurotransmission from isolated, superfused porcine iris-ciliary bodies [16], the present study sought to compare the pharmacology of different types of gas donors on electrically-evoked radiolabeled norepinephrine release from bovine iris-ciliary bodies. The aim of the present study was twofold: (1) investigate the differential ability of three H2S donors: ACS67, a hybrid of latanoprost; and a H2S-donating moiety [22]; l-cysteine, a substrate for endogenous production of H2S [2]; and GYY 4137, a slow donor of H2S [23] to regulate electrical field-stimulated norepinephrine release from isolated bovine iris-ciliary bodies, and (2) investigate the mechanisms by which these H2S donors regulate neurotransmitter release. A preliminary account of this study has been communicated in an abstract form [24].
Methods
Chemicals
GYY 4137, ACS67, latanoprost and flurbiprofen were purchased from Cayman Chemicals, Ann Arbor, MI. l-cysteine, AOA and gibenclamide were procured from Sigma Chemical Co., St. Louis, MO. Ecolume® scintillation fluid was obtained from ICN Biomedicals Inc., Costa Mesa, CA and [3H] Norepinephrine was purchased from Amersham Bioscience Piscataway, NJ.
Tissue Preparation
Freshly harvested bovine eyes were obtained from a local slaughter house (Greater Omaha Packing Co., Omaha, Nebraska) within 3 h of enucleation and shipped (on ice) to the laboratory. An incision was made along the equator of each eye and aqueous humor, cornea and lens was removed. The iris-ciliary body was isolated and then incubated in 4 ml of oxygenated (95 % O2:5 % CO2) Krebs buffer solution containing [3H]-norepinephrine ([3H]NE) (2.5 μCi/ml) at 37 °C for 1 h. The Krebs buffer solution had the following composition (mM); NaCl 118; KCl 4.8; CaCl2 1.3; KH2PO4 1.2; NaHCO3 25; MgSO4 2.0; dextrose 10 (pH 7.4). The Krebs buffer also contained ascorbic acid (56.7 mM) and in some experiments, flurbiprofen (FBF 3 µM), a cyclooxygenase (COX) inhibitor was added.
Studies on [3H]Norepinephrine Release
The methodology employed in studies of [3H]NE release is essentially the same as has been described by the authors [25–27]. Following incubation, tissues were rinsed twice with non-radioactive buffer for 5 min, mounted between nylon mesh cloth and placed in thermostatically-controlled Superfusion Chambers (Warner Instrument Corp., CT). Tissues were superfused with oxygenated Krebs solution at 2 ml/min, with effluent collected every 4 min. After an initial 60 min of superfusion to establish a stable baseline of spontaneous tritium efflux, release of [3H]NE was evoked by 300 d.c electrical pulses (5 Hz, 2 ms pulse width, 12 V/cm, 60 s, supramaximal voltage) applied at 13 min (S1) and 45/53 min (S2) after the onset of superfusion. Collected fractions of superfusates were analyzed for radioactivity by liquid scintillation spectrometry. Electrically evoked [3H]NE release were estimated by subtraction of the extrapolated basal tritium efflux from total tritium released in the 28-min period after the onset of stimulation. Basal tritium efflux was assumed to decline linearly between pre-stimulation and post-stimulation fractions. To examine the effect of ACS67, l-cysteine and GYY 4137 on [3H]NE release, each agent was applied at 29 min after the onset of superfusion. For GYY 4137 and ACS67, S2 was applied at 45 min. For l-cysteine, the treatment was relatively longer and S2 was applied at 53 min. To determine the effect of AOA and glibenclamide on the activity of the drugs, these agents were present 20 min prior to S1 and also during S2. Stimulation-evoked [3H] NE release during S1 and S2 was determined graphically, and the ratio (S2/S1) was calculated and compared with untreated controls.
Experimental Design
In the first series of experiments, we compared the pharmacological actions of the slow-releasing H2S donor, GYY 4137 with those elicited by the substrate, l-cysteine and the latanoprost-H2S hybrid molecule, ACS67. To determine the role of prostaglandins in the inhibitory actions of l-cysteine and ACS 67, we omitted the cyclooxygenase inhibitor, flurbiprofen in the buffer solution. In the next series of experiments, we investigated the role of endogenous biosynthesis of H2S and KATP channels in the pharmacological actions of GYY4137, ACS67 and l-cysteine by using the inhibitor, AOA and the channel blocker, glibenclamide. In the last series of experiments, we sought to compare the pharmacological actions of latanoprost with that of its hybrid molecule, ACS67. Table 1 provides a summary of H2S producing compounds and their potential pharmacological targets evaluated in this study.
Data Analysis
Results were expressed as the absolute S2/S1 ratios and/or as percentage inhibition of [3H] NE outflow relative to untreated controls. All three H2S donors were tested on separate iris-cilary body preparations (i.e., each donor was tested on a separate iris-ciliary body). Both control and test experiments for each series of studies were performed on separate tissues. For statistical purposes, tissues from control and test experiments were then pooled and compared with each other. Except where indicated, values given are arithmetic mean ± standard error of the mean (SEM). Significance of differences between control and agent-treated preparations was evaluated using analysis of variance (ANOVA) followed by Tukey’s post-test. Differences with P values <0.05 were accepted as statistically significant.
Results
Effect of H2S Donors on Sympathetic Neurotransmitter Release
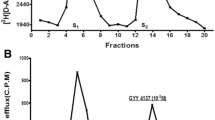
As illustrated in Fig. 1a, stimulation of isolated, superfused bovine iris-ciliary bodies preloaded with [3H]NE using electrical impulses (5 Hz, 2 ms pulse duration, 12 V, 60 s) for two successive periods elicited tritium efflux, yielding S2/S1 ratios of 0.99 ± 0.04 (n = 12). Application of the slow H2S donor, GYY 4137 (30 μM), 12 min prior to second electrical stimulus significantly attenuated electrically-induced [3H]NE overflow [S2/S1 ratios of 0.79 ± 0.03 (n = 4); P < 0.001] without affecting basal tritium overflow (Fig. 1b).
Effect of GYY 4137 on field-stimulated [3H]NE release from isolated superfused bovine iris-ciliary body. Trains of electrical stimulation (5 Hz, 2 ms pulse duration, 12 V, 60 s) were applied at fraction 5 (S1) and fraction 12 (S2). a Control. b GYY 4137(30 µM) was present in the buffer 12 min before and during S2. Fractions of superfusate containing [3H]NE were collected at 4 min intervals and analyzed for radioactivity by liquid scintillation spectrometry
We next examined the effect of different concentrations of H2S donors, GYY 4137 (1–30 µM); ACS67 (10 nM–10 µM) and l-cysteine (10 nM–10 µM) on field-stimulated [3H]NE overflow from isolated bovine irides. All three compounds caused a concentration-dependent inhibition of electrically-evoked [3H]NE release overflow (Fig. 2a–c). At an equimolar concentration of 10 µM, ACS67, l-cysteine and GYY 4137 elicited a 37.6 % (n = 5, P < 0.001), 26.1 % (n = 5, P < 0.05) and 13.7 % (n = 5, P < 0.001) reduction of field-stimulated [3H]NE release.
Effect of hybrid of latanoprost and H2S-donating moiety, ACS67 (a); substrate for endogenous H2S production, l-cysteine (b) and the slow H2S donor, GYY 4137 (c) on electrically evoked [3H]NE release in bovine ICB, in vitro. Control or in presence of ACS67 (10 nM–10 µM), l-cysteine (10 nM–10 µM) and GYY 4137 (1–30 µM). Vertical bars represent mean ± SEM. Number of observations is in parenthesis. *P < 0.05; ***P < 0.001 significantly different from control
Role of Endogenous Prostaglandins
There is evidence that products of the arachidonic acid pathway could be involved in the pharmacological actions of H2S in isolated rat cardiomyocytes [28]. Therefore, in a series of experiments, we sought to delineate the role of endogenous PGs in the inhibitory action of H2S donors on sympathetic neurotransmission in the bovine anterior uvea by excluding the COX enzyme inhibitor, FBF from the Krebs buffer solution. Interestingly, exclusion of FBF (3 µM) partially reversed the inhibitory action of both ACS67 and l-cysteine (0.1 and 10 µM) on [3H]NE release (Table 2).
Effect of a CBS Inhibitor and a KATP Channel Blocker
We next investigated the effect of an inhibitor of biosynthetic enzymes of the H2S pathway (AOA) and a blocker of KATP channels on the inhibitory action of the H2S donors. As illustrated in Fig. 3a–c, AOA (3 mM) reversed the inhibitory responses induced by ACS67, l-cysteine and GYY 4137, on field-stimulated [3H]NE overflow. Likewise, glibenclamide (300 µM) reversed the inhibition of evoked [3H]NE release caused by ACS67 (Fig. 4a) and l-cysteine (Fig. 4b) and GYY 4137 (Fig. 4c). It is pertinent to note that on their own, both AOA and glibenclamide had no significant effect on evoked NE release (Figs. 3, 4).
Effect of the CBS/CSE inhibitor, aminooxyacetic acid (AOA; 3 mM) on ACS67 (a), l-cysteine (b) and GYY 4137 (c)-mediated inhibition of electrically evoked [3H]NE release in bovine ICB, in vitro: control or ACS67 (0.1–10 µM), l-cysteine (10 µM) and GYY 4137 (10–30 µM) in absence and presence of AOA. Vertical bars represent mean ± SEM. Number of observations is in parenthesis. *P < 0.05; ***P < 0.001 significantly different from control
Effect of the KATP inhibitor, glibenclamide (Glb; 300 μM) on ACS67 (a), l-cysteine (b) and GYY 4137 (c)-mediated inhibition of electrically evoked [3H]NE release in bovine ICB, in vitro: control or ACS67 (0.1–10 µM), l-cysteine (10 µM) and GYY 4137 (10–30 µM) in absence and presence of Glb. Vertical bars represent mean ± SEM. Number of observations is in parenthesis. *P < 0.05; ***P < 0.001 significantly different from control
Role of Latanoprost in the Effect of ACS67 on Sympathetic Neurotransmitter Release
ACS67 is a hybrid of latanoprost (the prostanoid FP-receptor agonist that is used for glaucoma therapy) and a H2S-donor moiety. To delineate the role of latanoprost in the inhibitory action elicited by ACS67, we compared the effect of ACS67 to that of latanoprost on field-stimulated [3H]NE release. As shown in Fig. 5, ACS67 displayed a higher potency (IC30 of 2 µM) than that of latanoprost (IC30 of 10 µM) on the neurotransmitter release. Whereas an inhibitor of CBS enzyme, AOA (3 mM) reversed the inhibitory action of ACS67 (0.1–10 µM), the antagonist had no effect on the effect elicited by latanoprost (10 µM; Fig. 6).
Inhibitory effect of ACS67 and latanoprost (LP) on electrically evoked release of [3H]norepinephrine ([3H]NE) from isolated, superfused bovine ICB in presence of aminooxyacetic acid (AOA; 3 mM). Vertical bars represent mean ± SEM; each data point represents 4–8 observations. ***P < 0.001 significantly different from control
Discussion
In the past decade, H2S has been identified as an endogenously-produced gasotransmitter in several mammalian tissues and organs [1, 2, 4]. Indeed, its role as a neuromodulator, neuroprotectant, cardioprotectant, anti-apoptotic and as an antioxidant agent in various biological systems is well established [4]. In addition to studies that have described the pharmacological actions of H2S on mammalian tissues and organs, its physiological and pharmacological role in ocular tissues has been reported by us and several investigators [11, 12, 15–21, 29]. Based on evidence from our laboratory that H2S donors (NaHS and Na2S) inhibited sympathetic neurotransmission from isolated porcine iris-ciliary bodies [16], the present study was focused on comparing the pharmacology of three different types of H2S donors on electrically-evoked radiolabeled [3H]NE release from bovine iris-ciliary bodies, in vitro. While NaHS and Na2S (sulfide salts) have been used as tools to simulate the biological actions of the endogenous H2S gas, these agents release large amounts of gas within a short duration of time. Since the release of endogenous H2S from cells may occur in lesser amounts and at a much slower rate, the release of the gas from sulfide salts may be different from that of endogenously generated H2S. In the present study, the following compounds were used: ACS67, a hybrid of latanoprost and a H2S-donating moiety [22]; l-cysteine, a substrate for endogenous production of H2S [2]; and GYY4137, a slow donor of H2S [23]. We employed ACS67 in these studies because latanoprost, an analog of prostaglandin F2α is currently used in the therapy of glaucoma [30]. It was, therefore, of interest to determine whether the new hybrid compound with a H2S moiety can alter sympathetic neurotransmission in bovine anterior uvea. Furthermore, these studies would allow us to compare the pharmacological actions of ACS67 with that of the latanoprost, the parent compound.
We found that all three compounds that can produce H2S, GYY4137, ACS67 and l-cysteine elicited a concentration-dependent inhibition of field-stimulated [3H]NE release from isolated, superfused bovine iris-ciliary bodies without affecting basal tritium efflux. The rank order of inhibitory activities caused by these compounds are as follows: ACS67 > l-cysteine > GYY4137. The relatively higher potency of ACS67 when compared to l-cysteine and GYY4137 could be due to the fact that this compound is a hybrid of a prostanoid FP-receptor agonist with a H2S moiety. Be that as it may, the present observation that all three compounds inhibited sympathetic neurotransmission suggests that H2S may interfere with the pathway leading to NE release from the bovine iris-ciliary bodies. A similar inhibitory action was displayed by H2S donors, NaHS and Na2S on electrically-evoked [3H]NE release from isolated porcine iris-ciliary bodies [16].
Products of the COX pathway have been implicated in the cardioprotection induced by H2S in isolated rat cardiomyocytes [28] and in the relaxation of vascular smooth muscles to H2S donors [31, 32]. We have evidence that products of the COX pathway are involved in the relaxations elicited by H2S donors in the isolated bovine and porcine iris-ciliary bodies [15, 17, 20]. In the present study, removal of FBF from the perfusion buffer caused a reversal of the inhibitory action of both ACS 67 and l-cysteine on field stimulated [3H]NE release from the bovine anterior uvea. Taken together, these observations suggest that arachidonic acid metabolites mediate the inhibitory effects of ACS 67 and l-cysteine on sympathetic neurotransmission in the isolated bovine iris-ciliary body.
Recently, AOA has been reported to inhibit both CBS and CSE, enzymes responsible for the endogenous synthesis of H2S in biological tissues [33]. In the present study, we examined the effect of AOA on basal and electrically-evoked [3H]NE release from isolated bovine iris-ciliary bodies. In these experiments, we only examined the effect of AOA on concentrations of ACS67, GYY4137 and l-cysteine that elicited a significant inhibition of evoked neurotransmitter release. Although AOA had no effect on [3H]NE release, it blocked the inhibitory actions of ACS67, l-cysteine and GYY4137 on neurotransmitter release suggesting that effects caused by all three compounds are dependent upon endogenous synthesis of H2S. A similar antagonistic action of AOA has been reported on the inhibitory action induced by H2S donors, NaHS and Na2S on field stimulated [3H]NE release from isolated porcine iris-ciliary bodies [16]. It appears endogenously produced H2S is involved in the pharmacological actions of chemical donors of this gas and in the actions of its substrate, l-cysteine. The exact mechanism whereby exogenous gas donors can interact with the endogenous pathway leading to additional H2S production is unknown. It is tempting to speculate that these donors could elicit an indirect action on the pathway leading to endogenous H2S production via an effect on other mediators involved in the release of this gas.
ATP-sensitive potassium channels (KATP) have been reported to mediate the pharmacological actions of H2S in the cardiovascular [20, 21] and central nervous systems [34, 35]. In the present study, we investigated the effect of the KATP channel inhibitor, glibenclamide on basal and field-stimulated [3H]NE overflow from isolated bovine iris-cilary bodies. In these experiments, we only examined the effect of glibenclamide on concentrations of ACS67, GYY4137 and l-cysteine that elicited a significant inhibition of evoked neurotransmitter release. In the present study, the KATP channel inhibitor, glibenclamide reversed the inhibitory actions of GYY4137, ACS67 and l-cysteine on electrically-induced [3H]NE overflow in the isolated bovine iris-ciliary body. These results support a role for KATP channels in the responses caused by the two H2S donors and its substrate, l-cysteine on NE release.
One of the H2S donors employed in the present study, ACS67, is a hybrid of latanoprost (a prostanoid FP-receptor agonist) with a H2S donor moiety [36]. It was, therefore, of interest to delineate the role of latanoprost in the observed inhibitory responses to ACS67 on electrically-evoked [3H]NE overflow. Data obtained in the present study shows that ACS67 was more potent than latanoprost in inhibiting field stimulated [3H]NE release in the isolated bovine iris-ciliary bodies. These results indicate that the H2S moiety can cause an additional effect on the inhibitory responses elicited by the FP-receptor agonist, lantanoprost. Indeed, there is evidence that FP-receptor agonists can inhibit sympathetic neurotransmission in the isolated rabbit iris-ciliary body [37] and dopaminergic neurotransmission in the isolated rabbit [38] and bovine [39] neural retina. The observation that ACS67 is more effective than latanoprost in the present study affirms a role for the released H2S in eliciting an inhibitory action on sympathetic neurotransmission. Interestingly, ACS67 has been reported to exhibit a more potent ocular hypotensive effect than latanoprost in glaucomatous pigmented rabbits [40]. It is pertinent to note that latanoprost is among the first-line agents used in management of open angle glaucoma [30]. Thus, the ability of H2S to potentiate the regulatory effect of latanoprost on the sympathetic neurotransmission could, in part, explain enhanced ocular hypotensive potency of ACS67 over latanoprost and could have significant therapeutic implications in management of glaucoma.
We conclude that the H2S donors (GYY4137 and ACS67) and its substrate, l-cysteine can inhibit sympathetic neurotransmission for isolated bovine iris-ciliary bodies. The effect caused by H2S donors and its substrate are partially dependent upon endogenously produced H2S and on intramural prostanoid biosynthesis. Furthermore, KATP channels appear to mediate the responses elicited by all three compounds on sympathetic neurotransmission.
References
Wagner F, Asfar P, Calzia E, Radermacher P, Szabó C (2009) Bench-to-bedside review: hydrogen sulfide—the third gaseous transmitter: applications for critical care. Crit Care 13(3):213. doi:10.1186/cc7700. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2717401/pdf/cc7700.pdf
Kimura H (2011) Hydrogen sulfide: its production, release and functions. Amino Acids 41(1):113–121
Zhou CF, Tang XQ (2011) Hydrogen sulfide and nervous system regulation. Chin Med J 124(21):3576–3582
Predmore BL, Lefer DF, Gojon G (2012) Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal 17(1):119–140. doi:10.1089/ars.2012.4612
Wallace JL, Wang R (2015) Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov 5:329–345. doi:10.1038/nrd4433
Olas B (2015) Hydrogen sulfide in signaling pathways. Clin Chim Acta 15(439):212–218. doi:10.1016/j.cca.2014.10.037
di Masi A, Ascenzi P (2013) H2S: a “double face” molecule in health and disease. BioFactors 39(2):186–196. doi:10.1002/biof.1061
Wang R (2012) Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 92(2):791–896. doi:10.1152/physrev.00017.2011
Chen X, Jhee KH, Kruger WD (2004) Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J Biol Chem 279(50):52082–52086
Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H (2009) 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11(4):703–714. doi:10.1089/ARS.2008.2253
Mikami Y, Shibuya N, Kimura Y, Nagahara N, Yamada M, Kimura H (2011) Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem 286(45):39379–39386. doi:10.1074/jbc.M111.298208
Kulkarni M, Njie-Mbye YF, Okpobiri I, Zhao M, Opere CA, Ohia SE (2011) Endogenous production of hydrogen sulfide in isolated bovine eye. Neurochem Res 36(8):1540–1545. doi:10.1007/s11064-011-0482-6
Kaliaperumal S, K Praveen Kumar B (2014) Varied phenotypic presentations of Homocystinuria in two siblings. Indian J Ophthalmol 62(1):93–94. doi:10.4103/0301-4738.126190.PMCID:PMC3955077
Kraus JP, Kozich V (2001) Cystathionine β-synthase and its deficiency. In: Carmel R, Jacobsen DW (eds) Homocysteine in health and disease. Cambridge University Press, Cambridge, pp 223–243
Monjok EM, Kulkarni KH, Kouamou G, McKoy M, Opere CA, Bongmba ON, Njie YF, Ohia SE (2008) Inhibitory action of hydrogen sulfide on muscarinic receptor-induced contraction of isolated porcine irides. Exp Eye Res 87(6):612–616. doi:10.1016/j.exer.2008.09.011
Kulkarni KH, Monjok EM, Zeyssiq R, Kouamou G, Bonqmba ON, Opere CA, Nije YF (2009) Effect of hydrogen sulfide on sympathetic neurotransmission and catecholamine levels in isolated porcine iris-ciliary body. Neurochem Res 34(3):400–406. doi:10.1007/s11064-008-9793-7
Ohia SE, Opere CA, Monjok EM, Kouamou G, LeDay AM, Njie-Mbye YF (2010) Role of hydrogen sulfide production in inhibitory action of l-cysteine on isolated porcine irides. Curr Eye Res 35(5):402–407. doi:10.3109/02713680903576716
Opere CA, Monjok EM, Kulkarni KH, Njie YF, Ohia SE (2009) Regulation of [3H]d-aspartate release from mammalian isolated retinae by hydrogen sulfide. Neurochem Res 34(11):1962–1968. doi:10.1007/s11064-009-9984-x
Njie-Mbye YF, Kulkarni M, Opere CA, Ohia SE (2012) Mechanism of action of hydrogen sulfide on cyclic AMP formation in rat retinal pigment epithelial cells. Exp Eye Res 98:16–22. doi:10.1016/j.exer.2012.03.001
Chitnis MK, Njie-Mbye YF, Opere CA, Wood ME, Whiteman M, Ohia SE (2013) Pharmacological actions of the slow release hydrogen sulfide donor GYY4137 on phenylephrine-induced tone in isolated bovine ciliary artery. Exp Eye Res 116:350–354. doi:10.1016/j.exer.2013.10.004
Kulkarni-Chitnis M, Njie-Mbye YF, Mitchell L, Robinson J, Whiteman M, Wood ME, Opere CA, Ohia SE (2015) Inhibitory action of novel hydrogen sulfide donors on bovine isolated posterior ciliary arteries. Exp Eye Res 134:73–79. doi:10.1016/j.exer.2015.04.001
Osborne NN, Ji D, Abdul Majid AS, Fawcett RJ, Sparatore A, Del Soldato P (2010) ACS67, a hydrogen sulfide-releasing derivative of latanoprost acid, attenuates retinal ischemia and oxidative stress to RGC-5 cells in culture. Invest Ophthalmol Vis Sci 51(1):284–294. doi:10.1167/iovs.09-3999
Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, Deng LW (2011) The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One 6(6):e21077. doi:10.1371/journal.pone.0021077
Opere CA, Salvi A, Bankhele P, Jamil J, Njie-Mbye YF, Kulkarni K, Ohia SE (2013) Inhibitory action of hydrogen sulfide donor, ACS 67 on sympathetic neurotransmitter release in isolated bovine iris-ciliary bodies. Presented at the: ISER symposium, abstract #15, Sarasota, Fl, Sept 29–Oct 2, 2013
Opere CA, Ohia SE (1998) Role of intracellular calcium in peroxide-induced potentiation of sympathetic neurotransmission in bovine irides. Gen Pharmacol 31(5):793–798
Opere CA, Opere D, Ohia SE (1998) Mechanism of peroxide-induced potentiation of sympathetic neurotransmission in bovine irides: role of extracellular calcium. Free Radic Res 28(3):283–292
Opere CA, Ohia SE (1998) Role of prejunctional α2-adrenoceptors in peroxide-induced potentiation of norepinephrine release from the bovine iris. Neurochem Res 23(8):1093–1098
Hu LF, Pan TT, Neo KL, Yong QC, Bian JS (2008) Cyclooxygenase-2 mediates the delayed cardioprotection induced by hydrogen sulfide preconditioning in isolated rat cardiomyocytes. Pflugers Arch 455(6):971–978
Biermann J, Lagrèze WA, Schallner N, Schwer CI, Goebel U (2011) Inhalative preconditioning with hydrogen sulfide attenuated apoptosis after retinal ischemia/reperfusion injury. Mol Vis 17:1275–1286. http://www.molvis.org/molvis/v17/a143
American Academy of Ophthalmology Glaucoma Panel (2010) Preferred practice pattern® guidelines. Primary open-angle glaucome. American Academy of Ophthalmology, San Francisco, CA. www.aao.org/ppp
Koenitzer JR, Isbell TS, Patel HD, Benavides GA, Dickinson DA, Patel RP, Darley-Usmar VM, Lancaster JR Jr, Doeller JE, Kraus DW (2007) Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am J Physiol Heart Circ Physiol 292(4):H1953–H1960
Abe S, Watabe H, Takaseki S, Aihara M, Yoshitomi T (2013) The effects of prostaglandin analogues on intracellular Ca2+ in ciliary arteries of wild-type and prostanoid receptor-deficient mice. J Ocul Pharmacol Ther 29(1):55–60. doi:10.1089/jop.2011.0197
Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A (2013) Selectivity of commonly used pharmacological inhibitors for cystathionine beta synthase (CBS) and cystathionine gamma lyase (CSE). Br J Pharmacol 169(4):922–932. doi:10.1111/bph.12171
Tay AS, Hu LF, Lu M, Wong PT, Bian JS (2010) Hydrogen sulfide protects neurons against hypoxic injury via stimulation of ATP-sensitive potassium channel/protein kinase C/extracellular signal-regulated kinase/heat shock protein 90 pathway. Neuroscience 167(2):277–286. doi:10.1016/j.neuroscience.2010.02.006
Guo Q, Jin S, Wang XL, Wang R, Xiao L, He RR, Wu YM (2011) Hydrogen sulfide in the rostral ventrolateral medulla inhibits sympathetic vasomotor tone through ATP-sensitive K+ channels. J Pharmacol Exp Ther 338(2):458–465. doi:10.1124/jpet.111.180711
Tang G, Zhang L, Yang G, Wu L, Wang R (2013) Hydrogen sulfide-induced inhibition of L-type Ca2+ channels and insulin secretion in mouse pancreatic beta cells. Diabetologia 56(3):533–541. doi:10.1007/s00125-012-2806-8
Ohia SE, Jumblatt JE (1990) Prejunctional inhibitory effects of prostanoids on sympathetic neurotransmission in the rabbit iris-ciliary body. J Pharmacol Exp Ther 255(1):11–16
Al-Zadjali KH, Imler MP, Ohia SE (1994) Inhibitory effect of prostaglandins on dopamine release from the retina. Gen Pharmacol 25(2):289–296
Liu H, Zhao M, Opere CA (2008) Prejunctional inhibitory effects of isoprostanes on dopaminergic neurotransmission in bovine retinae, in vitro. Neurochem Res 33(1):37–42
Perrino E, Uliva C, Lanzi C, Soldato PD, Masini E, Sparatore A (2009) New prostaglandin derivative for glaucoma treatment. Bioorg Med Chem Lett 19(6):1639–1642
Acknowledgments
National Institute of Health, National Eye Institute, 1R15EY022215-01.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salvi, A., Bankhele, P., Jamil, J.M. et al. Pharmacological Actions of Hydrogen Sulfide Donors on Sympathetic Neurotransmission in the Bovine Anterior Uvea, In Vitro. Neurochem Res 41, 1020–1028 (2016). https://doi.org/10.1007/s11064-015-1784-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1784-x