Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder associated with a selective loss of the neurons containing dopamine (DA) in the substantia nigra pars compacta. Lines of evidence suggest that oxidative stress is a major factor contributing to the vulnerability of DA cells and that the enzyme NAD(P)H quinone oxidoreductase (NQO1) provides protection in these cells. In the present study, we report the synthesis of a novel compound KMS04014 and show that it induces NQO1 gene expression and protects DAergic neuronal cells in both cell culture and animal models of PD. In vitro, KMS04014 increased both mRNA and protein levels of NQO1 and induced nuclear translocation of Nrf2 in the DAergic neuronal cell line CATH.a. It also protected the cells against oxidative stress generated by tetrahydrobiopterin, 1-methyl-4-phenylpyridinium (MPP+), and H2O2. In vivo, KMS04014 attenuated the loss of tyrosine hydroxylase-immunopositive DAergic neurons in the substantia nigra and reduced degeneration of the nigral neurons and striatal fibers in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice, an animal model of PD. Taken together, KMS04014 may be utilized toward development of neuroprotective therapy for PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease that is accompanied by four cardinal symptoms: tremor, rigidity, bradykinesia, and postural imbalance. In PD patients, dopamine (DA)rgic neurons of the substantia nigra pars compacta undergo selective and progressive degeneration. The current treatment for PD is mainly focused on alleviation of the symptoms using the DA precursor L-DOPA. Unfortunately, chronic treatment with L-DOPA often causes motor and psychiatric side effects. At present, there is no therapy available that can delay or prevent the neurodegeneration itself, and therefore, drugs that modify the course of degeneration are being actively sought.
Evidence suggests a major role of oxidative stress in the pathogenesis of PD. The nigral DAergic neurons are particularly vulnerable to oxidative stress, due to low levels of antioxidant molecules and the presence of reactive oxygen species (ROS)-generating biomolecules such as DA, iron, tyrosine hydroxylase (TH), and monoamine oxidase. DA is oxidized to the highly reactive DA quinone, which, in turn, causes modification of cellular proteins at sulfhydryl groups and also leads to generation of ROS through its redox cycling (Graham et al. 1978; Asanuma et al. 2003). We have previously demonstrated that induction of the enzyme NAD(P)H:quinone oxidoreductase [NAD(P)H-(quinone acceptor) oxidoreductase; EC 1.6.99.2; NQO1], which catalyzes removal of the quinone, leads to protection of DAergic cells in vitro (Choi et al. 2003; Han et al. 2007; Lim et al. 2008). In addition, overexpression of NQO1 protected cells from DA-induced cell death (Zafar et al. 2006a). The substantia nigra has been shown to express NQO1 in the normal brain as well as in PD patients (van Muiswinkel et al. 2004).
Because of the inherent vulnerability of DAergic neurons mentioned above, the issue of cytotoxicity is especially important for compounds to be used for this cell system. Ferulic acid (3-(4-hydroxy-3-methoxyphenyl)-2-propenoic acid) has been shown to induce NQO1 in human umbilical vein endothelial cells (Ma et al. 2010). However, phenolic compounds such as ferulic acid, 4-allyl-2-methoxyphenol (eugenol), and 2-t-butyl-4-methoxyphenol (BHA) are known to have a prooxidant property that can cause adverse effects (Fujisawa et al. 2002; Hirata et al. 2005; Murakami et al. 2006; Maurya and Devasagayam 2010), including loss of cell viability (Inoue et al. 1994; Sergediene et al. 1999). On the other hand, dimerization of eugenol and BHA led to reduced cytotoxicity (Fujisawa et al. 2002, 2004), higher antioxidant activity, and lower prooxidant activity compared to their respective monomers (Fujisawa et al. 2005; Hirata et al. 2005; Murakami et al. 2006).
In the present study, we successfully synthesized a dimeric derivative of ferulic acid and observed that the dimerization lowered ferulic acid’s cytotoxicity to DAergic cells. The compound, KMS04014, retained the NQO1-inducing activity and effectively protected DAergic neurons in both cell culture and animal models of PD, making it a potential compound for the development of PD therapy.
Materials and Methods
Synthesis of KMS04014 (Fig. 1)
Synthesis of 1,2-Di(2-methoxy-4-formyl)phenoxyethane
5 g (32.8 mmol) of 3-methoxy-4-hydroxybenzaldehyde was dissolved in 200 ml of anhydrous dimethylformamide, and then, 1.58 g (39.4 mmol) of 60 % NaH was slowly added at room temperature. The reaction mixture was stirred for 30 min, wherein 5.78 g (15.6 mmol) of ethyleneglycol ditosylate was added. The reaction mixture was stirred at 80 °C for 5 h and then cooled to room temperature after the completion of reaction was confirmed by thin layer chromatography. The reaction mixture was added to 1000 ml of water and stirred vigorously. The solid product was filtered, washed with 1000 ml of water and 500 ml of hexane, and then dried in a vacuum dryer to yield 4.86 g (94.1 %) of 1,2-[2-(para-methoxybenzyloxy)-5-formyl]phenoxyethane as white solids. 1H NMR (300 MHz, DMSO) δ 9.86 (s, 2H), 7.57 (dd, 2H, J = 1.8, 8.2 Hz), 7.41 (d, J = 1.8 Hz, 2H), 7.27 (d, 2H, J = 8.2 Hz), 4.48 (s, 4H), 3.82 (s, 6H); 13C NMR (75 MHz, DMSO) δ 191.92, 153.57, 149.63, 130.40, 126.43, 112.84, 110.19, 67.63, 55.96; Anal. Calcd for C22H22O8: C 65.45, H 5.49; Found: C 65.4, H 5.4.
Structure, synthesis, and effect on cell viability of KMS04014. a The synthesis scheme of KMS04014 using 3-methoxy-4-hydroxybenzaldehyde as a starting material. Details of the synthesis are described in “Methods.” b Structures of KMS04014 and ferulic acid. c CATH.a cells were treated with KMS04014 or ferulic acid at the indicated concentrations. After 24 h, the cell viability was assessed by measuring intracellular ATP. The results are mean ± SEM of three independent experiments. *P < 0.05 and **P < 0.01 vs. untreated control
Synthesis of 1,2-Di[2-methoxy-4-(2-carboxyvinyl)]-phenoxyethane
5.66 g (17.1 mmol) of 1,2-di(2-methoxy-4-formyl)phenoxyethane and 8.92 g (85.6 mmol) of malonic acid were fully dissolved in 70 ml of anhydrous pyridine, and then, 0.5 ml of piperidine was added. The reaction mixture was stirred at 80 °C for 8 h and then cooled to room temperature after the completion of reaction had been confirmed. After filtering, the solid was washed with 500 ml of ethanol and then dried in a vacuum dryer to obtain 6.97 g (98.1 %) of 1,2-di[2-methoxy-4-(2-carboxylvinyl)]phenoxyethane as white crystals. 1H NMR (300 MHz, DMSO) δ 12.1 (bs, 2H), 7.44 (d, 2H, J = 15.8 Hz), 7.24 (s, 2H), 7.12 (d, 2H, J = 8.2 Hz), 6.95 (d, 2H, J = 8.2 Hz), 6.38 (d, 2H, J = 15.8 Hz), 4.24 (s, 4H), 3.70 (s, 6H); 13C NMR (75 MHz, DMSO) δ 168.79, 150.55, 149.83, 144.93, 128.28, 123.44, 117.77, 113.53, 111.38, 67.84, 56.39; IR (KBr) 2954, 1692, 1512, 1260 cm−1; Anal. Calcd for C22H22O8: C 63.76, H 5.35; Found: C 63.4, H 5.4.
Preparation of Disodium Salt of KMS04014
2.2 g (5.3 mmol) of KMS04014 was added to 0.1 M NaOH solution (prepared by dissolving 0.4 g of NaOH in reverse osmosis water) and stirred for 3 h at room temperature. Insoluble materials were removed by filtration, and the filtrate was lyophilized to obtain the salt form.
Cell Culture
CATH.a cells were grown in RPMI 1640 containing 8 % horse serum, 4 % fetal bovine serum, 100 IU/l penicillin, and 10 μg/ml streptomycin (GibcoBRL, Gaithersburg, MD) at 37 °C in 95 % air and 5 % CO2 in humidified atmosphere. For experiments, the cells were plated at densities of 2 × 104 cells/well in 96-well culture plates, 1 × 106 cells/well in 6-well culture plates, or 2 × 106 cells/60 mm plate. After 24 h, the cells were fed with fresh medium and treated.
ATP Assay
Cells were seeded on 96-well culture plates and treated with various concentrations of KMS04014 and ferulic acid for 24 h. To measure cytotoxicity, the level of ATP in live cells was measured using the CellTiter-Glo luminescent cell viability assay kit (Promega Corp., Madison, WI) as previously described (Woo et al. 2014).
Lactate Dehydrogenase (LDH) Assay
As previously described (Hwang et al. 2001), aliquots (50 μl) of cell culture medium were incubated in the presence of 0.26 mM NADH, 2.87 mM sodium pyruvate, and 100 mM potassium phosphate buffer (pH 7.4) in a total volume of 200 μl. The rate of NAD+ formation was measured spectrophotometically at 340 nm.
Western Blot Analysis
As described previously (Kim et al. 2010), equal amounts of protein (20 μg) were separated on 10 % SDS polyacrylamide gel and transferred onto polyvinylidene difluoride-nitrocellulose filters. After blocking for 1 h in 20 mM Tris-HCl containing 137 mM NaCl, 0.05 % Tween 20, and 6 % skim milk at room temperature, the membrane was incubated overnight with primary antibody against NQO1 (1:1000), Nf-E2-related factor 2 (Nrf2; 1:200), or β-actin (1:200) (Santa Cruz Biotechnology, Santa Cruz, CA) at 4 °C followed by horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Protein bands were detected by chemiluminescence (Pierce Chemical, Rockford, IL).
Immunocytochemistry
Cells were plated at 5 × 104 cells/well in 8-well Lab-Tek II chamber slides (Nalge Nunc International, Naperville, IL). Immunocytochemistry was performed as described previously (Lee et al. 2007). In brief, cells were washed in phosphate-buffered saline (PBS) and fixed in 4 % paraformaldehyde in PBS for 1 h. They were then incubated with blocking solution containing 1 % bovine serum albumin (BSA), 0.2 % Triton X-100, and 0.05 % sodium azide in PBS, washed in 0.5 % BSA in PBS twice, and then incubated with anti-Nrf2 antibody (1:200; Santa Cruz Biotechnology) for 1 h. The cells were washed twice and incubated for 60 min with Fluor Alexa 546-labeled anti-rabbit IgG (1:200; Molecular Probes, Eugene, OR). The samples were coverslipped with fluorescent mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (0.5 μg/ml) and observed under a confocal microscope (TCS-ST2; Leica, Wetzlar, Germany).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
RT reactions were performed using 5 μg of total RNA and the First Strand cDNA Synthesis kit (MBI Fermentas, Ontario, Canada). PCR for NQO1 was performed at 94 °C for 30 s, 55 °C for 40 s, and 72 °C for 1 min for 25 cycles using the following primers: forward, CCATTCTGAAAGGCTGGTTTG; reverse, CTAGCTTTGATCTGGTTGTC. Glyceraldehyde 3-phosphate dehydrogenase (forward, CACCACCATGGAGAAGGCTGG; reverse, TTGTCATGGATGACCTTGGCCAGG) was used as an internal control. Analysis of each PCR product on 1 % agarose gel showed a single band with the expected size.
Animals
All procedures were pre-approved by the Animal Experiment Review Committee of the Asan Institute for Life Science and University of Ulsan College of Medicine. Male C57Bl/6 mice (Orient Corp., Sungnam, Korea) weighing 23–25 g were maintained in a temperature- and humidity-controlled room with a 12 h light-dark cycle and food and water available ad libitum. The animals (n = 8 per group) were given KMS04014 disodium salt suspended in 0.5 % sodium carboxymethylcellulose solution by oral gavage at 30 mg/kg every 24 h for three consecutive days. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; administered four times, 2 h apart at 20 mg/kg, dissolved in saline) was injected intraperitoneally in a single day. The first MPTP injection was made 30 min after the second KMS04014 administration. The animals were sacrificed 7 days after the first MPTP injection. The animals were deeply anesthetized (80 mg/kg ketamine and 20 mg/kg xylazine, intraperitoneally (ip)) and transcardially fixed in 4 % paraformaldehyde as described previously (Hwang et al. 1998). Brains were promptly removed and postfixed in 4 % paraformaldehyde. After cryoprotection, 20-μm sections were made on a vibrating blade microtome (VT 1000S; Leica, Nussloch, Germany).
TH Immunohistochemistry and Counting
The substania nigra pars compacta area was delineated according to the mouse brain atlas (Franklin and Paxinos 1997). A total of eight sections (every fourth section, 80 μm apart) from all animals were taken. They were subjected to immunostaining as described previously (Son et al. 2012), by sequential incubation in rabbit polyclonal anti-TH antibody (1:1000; Protos, New York, NY), biotinylated-conjugated polyclonal anti-rabbit antibody, and horseradish-peroxidase-conjugated avidin/biotin complex (Vector Laboratories, Burlingame, CA). The samples were visualized by incubation in 0.05 % 3,3′-diaminobenzidine and 0.003 % H2O2. All TH-immunopositive cells in each section were counted with the aid of a computer program (Mousotron 3.8.3, Black Sun Software, Turnhout, Belgium).
FluoroJade C and TH Double Fluorescence Staining
A total of eight sections (every fourth section, 80 μm apart) from all animal were incubated in blocking solution containing 1 % BSA, 0.2 % Triton X-100, and 0.05 % sodium azide, rinsed in 0.5 % BSA in PBS twice and then incubated with anti-TH antibody (1:2000; Protos) for 2 h. The sections were washed three times and incubated for 90 min with Fluor Alexa 546-labeled anti-rabbit IgG (1:200; Molecular Probes). After washing twice, they were mounted onto gelatin-coated slides and dried at 50 °C for 30 min. The samples were rehydrated, incubated in 0.06 % potassium permanganate for 6 min, and then rinsed for 2 min in distilled water. The samples were subjected to FluoroJade C (0.0001 % dissolved in 0.1 % acetic acid; Histochem Inc., Jefferson, AR) for 10 min and rinsed. After air drying and clearing in xylene, they were coverslipped with fluorescent mounting medium. FluoroJade C and TH were detected under a confocal microscope (TCS-ST2; Leica, Wetzlar, Germany).
Amino-Cupric Silver Staining
Amino-cupric silver degeneration staining was carried out by the method described before (Kim et al. 2005) on eight nigral and striatal sections, each 80 μm apart, from all animals. For counterstaining, the sections previously stained and mounted on gelatin-coated slides were immersed in 0.5 % neutral red for 15 min, rinsed twice in distilled H2O, dehydrated, cleared in pure xylene, and coverslipped using DPX mounting medium (Sigma-Aldrich). For quantitation, a total of 250 small circles (10 μm in diameter) were randomly selected from microscopic fields of each section, avoiding the myelinated fibers of passage in the striatum, at 100× magnification. Their optical density was measured using Science Lab Image Gauge software (Fuji Photo, Tokyo, Japan).
Data Analyses
Data are expressed as mean ± SEM of independent experiments. Comparisons of three or more groups were analyzed by one-way analysis of variance (ANOVA) and post Dunnett’s multiple comparison tests. Statistical tests were carried out using PRISM (GraphPad Software, San Diego, CA). A value of P < 0.05 was considered statistically significant.
Results
KMS04014 Induces NQO1 Gene Expression in DAergic Cells
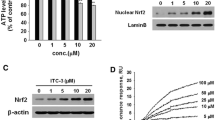
KMS04014 was synthesized using 3-methoxy-4-hydroxybenzaldehyde as a starting material (the structure and synthesis scheme shown in Fig. 1a). The compound contains two ferulic acid moieties linked together through the hydroxyl group (Fig. 1b). We first assessed the cytotoxicity of this compound in CATH.a cells, a DAergic neuronal cell line extensively used to study DAergic cell death (Choi et al. 2000, 2003, 2005, 2008). As shown in Fig. 1c, while ferulic acid showed significant cytotoxicity from 100 μM, KMS04014 had no apparent effect, showing that we have successfully lowered ferulic acid’s cytotoxic property by dimerization.
We tested whether KMS04014 might still retain the NQO1-inducing activity. RT-PCR against NQO1 in cells treated with KMS04014 showed a dose-dependent increase. Significant induction was observed at 1 μM (3.1 ± 0.1-fold), and the degree of induction reached 5.4 ± 0.3-fold at 50 μM (Fig. 2a). Western blot analysis (Fig. 2b) also revealed a dose-dependent elevation of the NQO1 protein level, reaching a 4.1 ± 0.6-fold increase at 50 μM.
KMS04014 induces NQO1 expression and Nrf-2 nuclear translocation. CATH.a cells were treated with KMS04014 at various concentrations. a After 6 h, total RNA was isolated and subjected to RT-PCR for NQO1. b After 24 h, cell lysate subjected to Western blot analysis against NQO1 (mean ± SEM of three independent experiments. *P < 0.05 and **P < 0.01 vs. untreated control for a and b). c After a 1 h exposure to 50 μM KMS04014, the cells were subjected to immunocytochemistry against Nrf2 (red); the nuclei were stained with DAPI (blue), and the samples were viewed under confocal microscopy. Scale bar = 20 μm
Because NQO1 gene expression is regulated by the transcription factor Nrf2 (Venugopal and Jaiswal 1996), it was possible that KMS04014 may influence Nrf2. Normally located in the cytosol, Nrf2 translocates to the nucleus upon activation (Itoh et al. 1999). As shown in Fig. 2c, within 1 h, the Nrf2 immunoreactivity in the nucleus was elevated and overlapped with the nucleus-specific DAPI staining. In addition, the immunoreactivity in the cytosol was higher than the untreated control, as expected for activated Nrf2, as it would no longer be targeted for degradation (Kobayashi et al. 2004). We also compared the NQO1-inducing activity of KMS04014 with that of ferulic acid. As shown in Fig. 3a, b, ferulic acid (50 μM) elevated the NQO1 mRNA and protein levels by 3.9 ± 0.4 and 2.7 ± 0.1-folds, respectively, which were approximately half of the effects of KMS04014 (5.4 ± 0.3 and 4.1 ± 0.6-folds, respectively). This was also observed with the total Nrf2 level (Fig. 3c), showing 2.5 ± 0.1 and 4.5 ± 0.1-fold increases by ferulic acid and KMS04014, respectively. The finding that the effect of KMS04014 was consistently about 2-fold higher suggested that the active moiety in ferulic acid most likely remained uninterrupted by the linking.
Comparison of KMS04014 with ferulic acid. CATH.a cells were treated with KMS04014 (50 μM) or ferulic acid (50 μM). a After 6 h, total RNA was isolated and subjected to RT-PCR for NQO1; after 24 h, cell lysate subjected to Western blot analysis against b NQO1 and c Nrf2. The results are mean ± SEM of three independent experiments. **P < 0.01 vs. untreated control
KMS04014 Provides Protection to DAergic Cells In Vitro
Whether KMS04014 might be neuroprotective was tested in vitro. For this, tetrahydrobiopterin (BH4)-treated CATH.a cells, a cellular model previously used to study DAergic cytotoxicity (Choi et al. 2000, 2003; Lee et al. 2007), was used. CATH.a cells were exposed to 200 μM BH4 for 24 h in the presence of various concentrations of KMS04014. As shown in Fig. 4a, KMS04014 was able to completely protect the cells from BH4-induced death at a concentration as low as 1 μM (P > 0.05 vs. untreated control). Cell deaths caused by hydrogen peroxide or the DAergic toxin 1-methyl-4-phenylpyridinium (MPP+) were also blocked by 1 μM KMS04014 (Fig. 4b) (P > 0.05 vs. untreated control).
KMS04014 protects DAergic cells in vitro. CATH.a cells were treated with various concentrations of KMS04014 and 200 μM BH4 (a) and H2O2 (50 nM) or MPP+ (1 mM) (b). After 24 h, degrees of cell death were assessed by LDH activity in the medium. The results are mean ± SEM of three independent experiments. **P < 0.01 vs. untreated control; ## P < 0.01 vs. BH4, H2O2, or MPP+ alone-treated
KMS04014 Protects the Nigral DAergic Neurons in Animal Model of PD
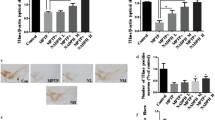
We tested whether KMS04014 could protect the in vivo nigral DAergic neurons in an animal model of PD. KMS04014 was given three times, 24 h and 30 min before MPTP, and 24 h after the first MPTP injection, a regimen we have previously used to successfully test and develop candidate compounds for PD therapy (Cho et al. 2009; Woo et al. 2014; Lee et al. 2014). A dose of 30 mg/kg was chosen because ferulic acid had been reported to be effective at doses between 50 and 100 mg/kg (Yeh et al. 2009; Thyagaraju and Muralidhara 2008), and KMS04014 had a 2-fold effect compared to ferulic acid on NQO1 expression in vitro. Immunohistochemistry against TH, the marker enzyme for DAergic cells (Fig. 5a), revealed that the loss of the nigral TH-positive neurons by the MPTP exposure was attenuated by KMS04014. Quantitative analysis showed that the number of TH-positive nigral neurons in the MPTP alone and MPTP/KMS04014 animals were 58 ± 2 and 79 ± 4 % of vehicle-treated control, respectively. KMS04014 alone had no effect.
KMS04014 attenuates the degeneration of the nigrostriatal system in animal model of PD. Mice were administered with KMS04014 (orally at 30 mg/kg/day × 3 days) and/or MPTP (intraperitoneally at 20 mg/kg × 4) and sacrificed after 7 days as described in “Methods.” a Typical TH-immunostained nigral sections to detect DAergic neurons (top; scale bar = 200 μm) and quantitation of the TH-positive neurons (bottom; n = 8 per group), expressed as mean ± SEM in percent of control. b Typical photographs of confocal microscopy showing nigral sections subjected to FluoroJade C staining and TH immunostaining. Scale bar = 20 μm. Typical amino-cupric silver-stained nigral (c) and striatal (d) sections (top; scale bars = 200 μm) and respective quantification of silver staining (bottom; n = 8 per group). **P < 0.01 vs. vehicle-treated control and ## P < 0.01 vs. MPTP treated
In order to confirm that this apparent neuroprotection was not due to changes in TH immunoreactivity, we subjected the adjacent nigral tissue sections to FluoroJade C staining, which allows for labeling of degenerating neurons (Schmued et al. 2005). As shown in Fig. 5b, FluoroJade C-positive staining was evident in the MPTP-treated animals and overlapped with the TH-positive DAergic neurons, confirming that the DAergic neurons are undergoing degeneration. In comparison, in the MPTP/KMS04014 samples, no apparent FluoroJade C staining and a higher number of TH-positive cells were observed. KMS04014 alone had no effect on the FluoroJade C staining pattern.
For quantitation of the degeneration, we also performed amino-cupric silver staining. As shown in Fig. 5c, d, the MPTP administration caused increased silver staining in the substantia nigra and the striatum, to which the nigral neurons send their fibers, and this was attenuated by the KMS04014 cotreatment. Quantitative analyses revealed that the optical density was increased to 158 ± 3 % in the nigra and 209 ± 3 % in the striatum by the MPTP treatment but that this was suppressed by KMS04014 to 118 ± 5 and 129 ± 5 %, respectively.
Discussion
In the present study, we demonstrate that the novel compound KMS04014 protects DAergic neuronal cells from various DAergic neurotoxins in vitro and from MPTP-induced degeneration in vivo. The compound also increased NQO1 gene expression and protein levels as well as nuclear translocation of Nrf2.
Oxidative metabolism of DA and the consequent cellular events contribute to the susceptibility of the nigral DAergic neurons. Oxidation of DA in the cytosol results in production of the highly reactive DA quinone (Hastings and Zigmond 1997), which then inactivates essential cellular macromolecules (Graham et al. 1978; Asanuma et al. 2003) whose dysfunction has been associated with the pathogenesis of PD. For example, DA quinone modification of Parkin renders the protein insoluble and causes inactivation of its E2 ubiquitin ligase activity (LaVoie et al. 2005). The quinone promotes the conversion of α-synuclein to the cytotoxic protofibril form (Conway et al. 2001), and DA quinone-modified α-synuclein is not readily degraded and impedes with the normal degradation of other proteins (Martinez-Vicente et al. 2008). It also covalently modifies DJ-1 and ubiquitin C-terminal hydrolase L1, the enzymes whose gene mutation leads to genetic forms of PD (Van Laar et al. 2009). DA quinone has also been reported to lead to proteasome inhibition (Zafar et al. 2006b; Zhou and Lim 2009). In addition, it modifies the sulfhydryl moiety of the reduced form of glutathione, the major cellular antioxidant (Zhou and Lim 2010; Bisaglia et al. 2010), as well as superoxide dismutase 2 (Belluzzi et al. 2012). Accumulation of DA quinine-protein adducts and DA quinone-DNA adducts has been associated with etiology of PD (Zahid et al. 2011; Wang et al. 2011).
The toxic accumulation of the DA quinone can be prevented by the action of the enzyme NQO1, which catalyzes two-electron reduction of quinone to the redox-stable hydroquinone (Joseph et al. 2000; Cavelier and Amzel 2001). NQO1 is expressed in the substantia nigra and is markedly increased in the Parkinsonian substantia nigra (van Muiswinkel et al. 2004). In addition, NQO1 is known to maintain both α-tocopherol and coenzyme Q10 in their reduced, antioxidant state (Beyer et al. 1997; Siegel et al. 1997) and directly scavenge superoxide (Siegel et al. 2004). An epidemiological study showed that a polymorphism (C609T) of NQO1 that results in a decrease or total loss of its expression is associated with PD (Harada et al. 2001; Siegel et al. 2001). Taken together, means to induce NQO1 activity in DAergic neurons should lead to protection. In concert with previous observations that compounds known to induce NQO1 protect DAergic cells from cytotoxic insults made by us (Choi et al. 2003; Han et al. 2007; Lim et al. 2008; Woo et al. 2014) and others (Duffy et al. 1998; Hara et al. 2003; Miyazaki et al. 2006; Jia et al. 2008, 2009; Siebert et al. 2009), we introduce a novel synthetic compound that induces NQO1 expression and provides protection of DAergic neuronal cells.
We observed that KMS04014 leads to an increase in the nuclear Nrf2. Since NQO1 expression is known to be governed by Nrf2 (Venugopal and Jaiswal 1996), it is likely that the mechanism by which KMS04014 leads to NQO1 induction at least in part involves Nrf2 activation. Once in the nucleus, Nrf-2 can also induce the transcription of a number of other genes, whose products are involved in antioxidant defense mechanisms (Jazwa et al. 2011). It is possible that the concurrent expression of these enzyme genes, along with NQO1, may take a part in the neuroprotective effect of KMS04014. On the other hand, we have previously noted that pharmacological inhibition of NQO1 enzyme activity led to almost complete reversal of the DAergic neuroprotection that was rendered by Nrf2 activation (Han et al. 2007), which suggested that NQO1 may play a major role in this cell system.
KMS04014 appears to enter the brain and to be metabolically stable. Our pharmacokinetic study showed that the brain to plasma ratio was 23.6 %, and the T max for the brain tissue was the same as plasma (15 min), suggesting that the compound can rapidly enter the brain (data not shown). The peak plasma concentration was reached at 15 min showing rapid absorption, and the terminal half-life was determined to be 184 min, indicating sufficient stability. KMS04014 incubated with liver microsomal enzyme preparations also showed stability against catabolism, with no significant changes in the amount of KMS04014 in 240 min (data not shown). These observations indicate the utility of this compound as a drug targeted for the brain.
In conclusion, our novel compound KMS04014 has NQO1-inducing and neuroprotective properties on DAergic neurons both in vivo and in vitro and may be useful toward development of disease-modifying drugs for PD.
Abbreviations
- BH4:
-
Tetrahydrobiopterin
- BSA:
-
Bovine serum albumin
- DA:
-
Dopamine
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- LDH:
-
Lactate dehydrogenase
- MPP+ :
-
1-Methyl-4-phenylpyridinium
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NQO1:
-
NAD(P)H quinone oxidoreductase
- Nrf2:
-
Nf-E2-related factor 2
- PD:
-
Parkinson’s disease
- PBS:
-
Phosphate-buffered saline
- ROS:
-
Reactive oxygen species
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- TH:
-
Tyrosine hydroxylase
References
Asanuma M, Miyazaki I, Ogawa N (2003) Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson's disease. Neurotox Res 5:165–176
Belluzzi E, Bisaglia M, Lazzarini E, Tabares LC, Beltramini M, Bubacco L (2012) Human SOD2 modification by dopamine quinones affects enzymatic activity by promoting its aggregation: possible implications for Parkinson's disease. PLoS One 7:e38026
Beyer RE, Segura-Aguilar J, di Bernardo S et al (1997) The two-electron quinone reductase DT-diaphorase generates and maintains the antioxidant (reduced) form of coenzyme Q in membranes. Mol Asp Med 18(Suppl):S15–S23
Bisaglia M, Soriano ME, Arduini I, Mammi S, Bubacco L (2010) Molecular characterization of dopamine-derived quinones reactivity toward NADH and glutathione: implications for mitochondrial dysfunction in Parkinson disease. Biochim Biophys Acta 1802:699–706
Cavelier G, Amzel LM (2001) Mechanism of NAD(P)H:quinone reductase: Ab initio studies of reduced flavin. Proteins 43:420–432
Cho Y, Son HJ, Kim EM et al (2009) Doxycycline is neuroprotective against nigral dopaminergic degeneration by a dual mechanism involving MMP-3. Neurotox Res 16:361–371
Choi HJ, Jang YJ, Kim HJ, Hwang O (2000) Tetrahydrobiopterin is released from and causes preferential death of catecholaminergic cells by oxidative stress. Mol Pharmacol 58:633–640
Choi HJ, Kim SW, Lee SY, Hwang O (2003) Dopamine-dependent cytotoxicity of tetrahydrobiopterin: a possible mechanism for selective neurodegeneration in Parkinson's disease. J Neurochem 86:143–152
Choi HJ, Lee SY, Cho Y, Hwang O (2005) Inhibition of vesicular monoamine transporter enhances vulnerability of dopaminergic cells: relevance to Parkinson's disease. Neurochem Int 46:329–335
Choi DH, Kim EM, Son HJ et al (2008) A novel intracellular role of matrix metalloproteinase-3 during apoptosis of dopaminergic cells. J Neurochem 106:405–415
Conway KA, Rochet JC, Bieganski RM, Lansbury PT Jr (2001) Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science 294:1346–1349
Duffy S, So A, Murphy TH (1998) Activation of endogenous antioxidant defenses in neuronal cells prevents free radical-mediated damage. J Neurochem 71:69–77
Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic Press, San Diego, pp 59–67
Fujisawa S, Atsumi T, Kadoma Y, Sakagami H (2002) Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity. Toxicology 177:39–54
Fujisawa S, Atsumi T, Kadoma Y, Ishihara M, Ito S, Yokoe I (2004) Kinetic radical scavenging activity and cytotoxicity of 2-methoxy- and 2-t-butyl-substituted phenols and their dimers. Anticancer Res 24:3019–3026
Fujisawa S, Atsumi T, Murakami Y, Kadoma Y (2005) Dimerization, ROS formation, and biological activity of o-methoxyphenols. Arch Immunol Ther Exp (Warsz) 53:28–38
Graham DG, Tiffany SM, Bell WR Jr, Gutknecht WF (1978) Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol Pharmacol 14:644–653
Han JM, Lee YJ, Lee SY et al (2007) Protective effect of sulforaphane against dopaminergic cell death. J Pharmacol Exp Ther 321:249–256
Hara H, Ohta M, Ohta K, Kuno S, Adachi T (2003) Increase of antioxidative potential by tert-butylhydroquinone protects against cell death associated with 6-hydroxydopamine-induced oxidative stress in neuroblastoma SH-SY5Y cells. Brain Res Mol Brain Res 119:125–131
Harada S, Fujii C, Hayashi A, Ohkoshi N (2001) An association between idiopathic Parkinson's disease and polymorphisms of phase II detoxification enzymes: glutathione S-transferase M1 and quinone oxidoreductase 1 and 2. Biochem Biophys Res Commun 288:887–892
Hastings TG, Zigmond MJ (1997) Loss of dopaminergic neurons in parkinsonism: possible role of reactive dopamine metabolites. J Neural Transm Suppl 49:103–110
Hirata A, Murakami Y, Atsumi T et al (2005) Ferulic acid dimer inhibits lipopolysaccharide-stimulated cyclooxygenase-2 expression in macrophages. In Vivo 19:849–853
Hwang O, Baker H, Gross S, Joh TH (1998) Localization of GTP cyclohydrolase in monoaminergic but not nitric oxide-producing cells. Synapse 28:140–153
Hwang O, Kim G, Jang YJ et al (2001) Synthetic phytoceramides induce apoptosis with higher potency than ceramides. Mol Pharmacol 59:1249–1255
Inoue M, Suzuki R, Koide T, Sakaguchi N, Ogihara Y, Yabu Y (1994) Antioxidant, gallic acid, induces apoptosis in HL-60RG cells. Biochem Biophys Res Commun 204:898–904
Itoh K, Wakabayashi N, Katoh Y et al (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13:76–86
Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernandez-Ruiz J, Cuadrado A (2011) Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal 14:2347–2360
Jia Z, Zhu H, Misra HP, Li Y (2008) Potent induction of total cellular GSH and NQO1 as well as mitochondrial GSH by 3H-1,2-dithiole-3-thione in SH-SY5Y neuroblastoma cells and primary human neurons: protection against neurocytotoxicity elicited by dopamine, 6-hydroxydopamine, 4-hydroxy-2-nonenal, or hydrogen peroxide. Brain Res 1197:159–169
Jia Z, Zhu H, Li Y, Misra HP (2009) Cruciferous nutraceutical 3H-1,2-dithiole-3-thione protects human primary astrocytes against neurocytotoxicity elicited by MPTP, MPP(+), 6-OHDA, HNE and acrolein. Neurochem Res 34:1924–1934
Joseph P, Long DJ 2nd, Klein-Szanto AJ, Jaiswal AK (2000) Role of NAD(P)H:quinone oxidoreductase 1 (DT diaphorase) in protection against quinone toxicity. Biochem Pharmacol 60:207–214
Kim ST, Choi JH, Chang JW, Kim SW, Hwang O (2005) Immobilization stress causes increases in tetrahydrobiopterin, dopamine, and neuromelanin and oxidative damage in the nigrostriatal system. J Neurochem 95:89–98
Kim EM, Shin EJ, Choi JH et al (2010) Matrix metalloproteinase-3 is increased and participates in neuronal apoptotic signaling downstream of caspase-12 during endoplasmic reticulum stress. J Biol Chem 285:16444–16452
Kobayashi A, Kang MI, Okawa H et al (2004) Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24:7130–7139
LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ (2005) Dopamine covalently modifies and functionally inactivates parkin. Nat Med 11:1214–1221
Lee SY, Moon Y, Choi DH, Choi HJ, Hwang O (2007) Particular vulnerability of rat mesencephalic dopaminergic neurons to tetrahydrobiopterin: relevance to Parkinson's disease. Neurobiol Dis 25:112–120
Lee JA, Kim JH, Woo SY et al (2014) A novel compound VSC2 has anti-inflammatory and antioxidant properties in microglia and in Parkinson's disease animal model. Br J Pharmacol. doi:10.1111/bph.12973
Lim JH, Kim KM, Kim SW, Hwang O, Choi HJ (2008) Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: novel cytoprotective mechanism against oxidative damage. Pharmacol Res 57:325–331
Ma ZC, Hong Q, Wang YG et al (2010) Ferulic acid protects human umbilical vein endothelial cells from radiation induced oxidative stress by phosphatidylinositol 3-kinase and extracellular signal-regulated kinase pathways. Biol Pharm Bull 33:29–34
Martinez-Vicente M, Talloczy Z, Kaushik S et al (2008) Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest 118:777–788
Maurya DK, Devasagayam TP (2010) Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem Toxicol 48:3369–3373
Miyazaki I, Asanuma M, Diaz-Corrales FJ et al (2006) Methamphetamine-induced dopaminergic neurotoxicity is regulated by quinone-formation-related molecules. FASEB J 20:571–573
Murakami Y, Shoji M, Ogiwara T, Tanaka S, Yokoe I, Fujisawa S (2006) Preventive effect of ortho dimer of butylated hydroxyanisole on activator protein-1 activation and cyclooxygenase-2 expression in macrophages stimulated by fimbriae of Porphyromonas gingivalis, an oral anaerobe. Anticancer Res 26:2915–2920
Schmued LC, Stowers CC, Scallet AC, Xu L (2005) Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res 1035:24–31
Sergediene E, Jonsson K, Szymusiak H, Tyrakowska B, Rietjens IM, Cenas N (1999) Prooxidant toxicity of polyphenolic antioxidants to HL-60 cells: description of quantitative structure-activity relationships. FEBS Lett 462:392–396
Siebert A, Desai V, Chandrasekaran K, Fiskum G, Jafri MS (2009) Nrf2 activators provide neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal cocultures. J Neurosci Res 87:1659–1669
Siegel D, Bolton EM, Burr JA, Liebler DC, Ross D (1997) The reduction of alpha-tocopherolquinone by human NAD(P)H: quinone oxidoreductase: the role of alpha-tocopherolhydroquinone as a cellular antioxidant. Mol Pharmacol 52:300–305
Siegel D, Anwar A, Winski SL, Kepa JK, Zolman KL, Ross D (2001) Rapid polyubiquitination and proteasomal degradation of a mutant form of NAD(P)H:quinone oxidoreductase 1. Mol Pharmacol 59:263–268
Siegel D, Gustafson DL, Dehn DL et al (2004) NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol 65:1238–1247
Son HJ, Lee JA, Shin N et al (2012) A novel compound PTIQ protects the nigral dopaminergic neurones in an animal model of Parkinson's disease induced by MPTP. Br J Pharmacol 165:2213–2227
Thyagaraju BM, Muralidhara (2008) Ferulic acid supplements abrogate oxidative impairments in liver and testis in the streptozotocin-diabetic rat. Zool Sci 25:854–860
Van Laar VS, Mishizen AJ, Cascio M, Hastings TG (2009) Proteomic identification of dopamine-conjugated proteins from isolated rat brain mitochondria and SH-SY5Y cells. Neurobiol Dis 34:487–500
van Muiswinkel FL, de Vos RA, Bol JG et al (2004) Expression of NAD(P)H:quinone oxidoreductase in the normal and Parkinsonian substantia nigra. Neurobiol Aging 25:1253–1262
Venugopal R, Jaiswal AK (1996) Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A 93:14960–14965
Wang N, Wang Y, Yu G, Yuan C, Ma J (2011) Quinoprotein adducts accumulate in the substantia nigra of aged rats and correlate with dopamine-induced toxicity in SH-SY5Y cells. Neurochem Res 36:2169–2175
Woo SY, Kim JH, Moon MK et al (2014) Discovery of vinyl sulfones as a novel class of neuroprotective agents toward Parkinson's disease therapy. J Med Chem 57:1473–1487
Yeh CT, Ching LC, Yen GC (2009) Inducing gene expression of cardiac antioxidant enzymes by dietary phenolic acids in rats. J Nutr Biochem 20:163–171
Zafar KS, Inayat-Hussain SH, Siegel D, Bao A, Shieh B, Ross D (2006a) Overexpression of NQO1 protects human SK-N-MC neuroblastoma cells against dopamine-induced cell death. Toxicol Lett 166:261–267
Zafar KS, Siegel D, Ross D (2006b) A potential role for cyclized quinones derived from dopamine, DOPA, and 3,4-dihydroxyphenylacetic acid in proteasomal inhibition. Mol Pharmacol 70:1079–1086
Zahid M, Saeed M, Yang L, Beseler C, Rogan E, Cavalieri EL (2011) Formation of dopamine quinone-DNA adducts and their potential role in the etiology of Parkinson's disease. IUBMB Life 63:1087–1093
Zhou ZD, Lim TM (2009) Dopamine (DA) induced irreversible proteasome inhibition via DA derived quinones. Free Radic Res 43:417–430
Zhou ZD, Lim TM (2010) Glutathione conjugates with dopamine-derived quinones to form reactive or non-reactive glutathione-conjugates. Neurochem Res 35:1805–1818
Acknowledgments
Hyo Jin Son and Ji Hyun Choi made equal contributions. This work was supported by the National Agenda Project from Korea Research Council of Fundamental Science and Technology (OH) and the National Research Foundation of Korea (NRF-2009-0081675, OH; NRF-2013-R1A1A22059669, HJS).
Conflict of Interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Son, H.J., Choi, J.H., Lee, J.A. et al. Induction of NQO1 and Neuroprotection by a Novel Compound KMS04014 in Parkinson’s Disease Models. J Mol Neurosci 56, 263–272 (2015). https://doi.org/10.1007/s12031-015-0516-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0516-7