Abstract
Glucose is the major energy substrate in brain, however, during ketogenesis induced by starvation or prolonged hypoglycemia, the ketone bodies (KB), acetoacetate and β-hydroxybutyrate (BHB) can substitute for glucose. KB improve neuronal survival in diverse injury models, but the mechanisms by which KB prevent neuronal damage are still not well understood. In the present study we have investigated whether protection by the D isomer of BHB (D-BHB) against neuronal death induced by glucose deprivation (GD), is related to autophagy. Autophagy is a lysosomal-dependent degradation process activated during nutritional stress, which leads to the digestion of damaged proteins and organelles providing energy for cell survival. Results show that autophagy is activated in cortical cultured neurons during GD, as indicated by the increase in the levels of the lipidated form of the microtubule associated protein light chain 3 (LC3-II), and the number of autophagic vesicles. At early phases of glucose reintroduction (GR), the levels of p62 declined suggesting that the degradation of the autophagolysosomal content takes place at this time. In cultures exposed to GD and GR in the presence of D-BHB, the levels of LC3-II and p62 rapidly declined and remained low during GR, suggesting that the KB stimulates the autophagic flux preventing autophagosome accumulation and improving neuronal survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Correct brain functioning depends on the continuous supply of glucose from blood. Disruption of blood flow during an ischemic episode or a decrease in blood glucose concentration during severe hypoglycemia, leads to brain injury. Other energy sources such as the ketone bodies (KB) acetoacetate and β-hydroxybutyrate (BHB) can be used by brain as alternative substrates to glucose in certain conditions. KB are breakdown products of fatty acid metabolism in the liver, and normally during adulthood their concentration in blood is low (0.1 mM) [1]. However, during the suckling period, KB concentration in blood increases due to the high fat content in maternal milk, representing the major fuel for the immature brain [2, 3]. Nevertheless, the adult brain is capable to transport and oxidize KB whenever their concentration rises due to ketogenesis, during starvation, prolonged hypoglycemia [4] or when KB are supplied by the ketogenic diet or an exogenous infusion [5, 6].
Protection of neuronal death by KB has been demonstrated in several pathological conditions associated with energy depletion, including hypoxia [7] ischemia [8–10] excitotoxicity [11, 12] and severe hypoglycemia [13, 14]. We have recently reported that the D-isomer of BHB (D-BHB) prevents the decline in ATP levels induced by glucose deprivation (GD), improves ATP recovery during glucose reperfusion (GR) and reduces neuronal death in cortical neurons, suggesting it can be used as an energy substrate [15]. In addition, a significant reduction in the number of degenerating neurons is observed in the cerebral cortex of severe hypoglycemic animals rescued with glucose and D-BHB [15]. The mechanism underlying the protective effect of KB is not completely understood, but it has been mainly attributed to the improvement of mitochondrial metabolism as KB incorporate to the tricarboxylic acid cycle [15–17].
To further investigate the actions of KB, in the present study we have explored whether autophagy is involved in the protective effect of D-BHB against GD-induced neuronal damage in cortical cultured neurons. Macroautophagy (here named as autophagy) is an intracellular catabolic process dependent on lysosome hydrolytic activity responsible for the recycling and digestion of damaged or altered proteins and organelles [18, 19]. Autophagy is a highly conserved process occurring in physiological conditions and stimulated under different types of stress including nutritional stress, as a mechanism to provide energy and sustain cell survival [20–22]. However, excessive autophagic digestion can lead to cell death [23, 24]. Autophagy is initiated by the formation of a multiprotein complex containing Beclin 1 and class III PI3K, which are essential for the formation of double membrane vesicles or autophagosomes. During autophagosome formation, the microtubule-associated protein 1 light chain 3 (LC3-I), is conjugated with phosphatidylethanolamine to form LC3-II, which translocates from the cytosol to double membrane vesicles, where damaged proteins and cellular components are engulfed and degraded by lysosomal hydrolytic enzymes in autophagolysosomes, formed by the fusion of autophagosomes with lysosomes [25]. The processes of autophagosome formation and the subsequent degradation of its content in the autophagolysosome, is referred as the autophagic flux. Impairment of the autophagic flux leads to the excessive accumulation of autophagosomes, which can result in neuronal cell death [26, 27].
The activation of autophagy under different conditions of cellular stress is well known in the nervous system, and its role in either neuronal survival [28–30] or neuronal death [31, 32] has been suggested. The role of autophagy in hypoglycemia- and GD-induced neuronal damage has not been well characterized, but a recent study suggests that the disruption of the autophagic flux during glucose reperfusion is involved in the death of neurons exposed to glucose starvation [33].
In the present study we have investigated the effect of D-BHB on autophagy induced by GD in cultured neurons. We have evaluated the changes in the levels of three key autophagic proteins: Beclin 1, a protein that interacts with class III PI3K and is part of the complex necessary for the initiation of autophagosome formation [34], the transformation of LC3-I to LC3-II, which is essential for the formation of double membrane vesicles [25]; and SQSTM1/p62, a protein that interacts with LC3, recruits ubiquitinated proteins to the autophagosome and is finally degraded into the autophagolysosome [35]. Results show a rapid conversion of LC3-I to LC3-II and autophagosome formation during glucose withdrawal, followed by the degradation of autophagosome content when glucose is replenished. In the presence of D-BHB the transformation of LC3-I to LC3-II and the formation of autophagosomes decreases significantly and the rate of degradation of p62 occurs more rapidly, suggesting that D-BHB stimulates the autophagic flux preventing the accumulation of autophagosomes.
Materials and Methods
Materials
Neurobasal medium, B27, gentamicin and Dulbecco’s Modified Eagle Medium (DMEM) were obtained from Gibco life technologies (Grand Island, USA); MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide, l-Glutamine, poly-l-lysine, NADH, pyruvate, Hoechst, 3-Methyl adenine (3-MA) and chloroquine (CQ) from Sigma-Aldrich (St. Louis, MO, USA); D-BHB was from Fluka (Sigma-Aldrich). Calcein-AM/ethidium homodimer (live/death kit, Molecular Probe, Eugene, Oregon, USA); protease inhibitor cocktail (Roche complete, 11626200, Indianapolis, IN, USA); anti-LC3 antibody (MBL international, PD014); anti-Beclin 1 antibody (Sigma-Aldrich, PRS3613); anti-SQSTM1/p62 antibody (Cell signaling technology, 51146); anti-actin antibody (Chemicon, Merck, Millipore, MAB1501); goat anti-mouse (Jackson Immunoresearch Laboratories, 115035-062); goat anti-rabbit (Jackson Immunoresearch Laboratories, 115035-003) and goat anti-rabbit (Zymed, 62-6111) secondary antibodies; Chemiluminescent HRP substrate (Millipore Corporation, P90720); Fluoromount-G™ (Electron Microscopy Sciences 17984); Cyto-ID (autophagy detection kit, Enzo Life Sciences, 51031-K200).
Cell Culturing
Cortical primary cultures were prepared from Wistar rat embryos of 17–18 days of gestation obtained from the animal house of the Instituto de Fsiología Celular (Universidad Nacional Autónoma de México, UNAM) as previously described [15]. Animals were handled following the rules of the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23 revised 1996) with the approval of the Animal Care committee (CICUAL) of the Instituto de Fisiología Celular. Briefly, cells were suspended in Neurobasal medium supplemented with 1 % B27 + 1 % B27 Minus AO, 0.5 mM l-glutamine and 20 μg/ml gentamicin. Cells were plated at a density of 2.2 × 105/cm2 in 12-well plates precoated with poly-l-lysine (5 μg/ml). Cells were cultured for 8 days in vitro (DIV) at 37º C in a humidified 5 % CO2/95 % air atmosphere. At 4 DIV, cytosine arabinoside (0.8 μM) and 400 μl of fresh Neurobasal medium (containing 2 % B27 Minus AO) was added.
Cell Treatment
At 8 DIV Neurobasal medium was withdrawn and cells were exposed to glucose free (GD) medium (DMEM) for 1 and 2 h in the presence or the absence of 10 mM D-BHB. Afterwards, GD medium was changed for the Neurobasal glucose-containing medium previously withdrawn (GR) containing or not 5 mM D-BHB. We have previously determined that at these doses and following this protocol of administration, D-BHB efficiently prevents GD-induced neuronal death [15]. Cultures were also treated with the class-III PI3K inhibitor 3-MA (10 mM) or chloroquine (CQ) (20 μM), to inhibit autophagosome formation and the autophagic flux, respectively. Trehalose (150 mM) was used as an autophagy inducer and it was incubated during 4 h in Neurobasal medium.
Cell Survival
Cell survival was monitored 22 h after glucose reintroduction by the determination of lactic acid dehydrogenase (LDH) activity present in the medium, MTT reduction and the calcein-AM/ethidium homodimer method (live/death kit) as previously described [15]. After 22 h of GR cells were incubated with MTT (150 μM) for 1 h at 37 °C in 5 % CO2/95 % air atmosphere; the medium was withdrawn and acidic isopropanol was added to solubilize the precipitated formazan salts. Formazan absorbance was measured spectrophotometrically at 570 nm. Cell viability is expressed as percentage of MTT reduction relative to control. LDH activity was determined in the culture medium by measuring the decrease in the optical density resulting from the oxidation of NADH at 340 nm adding pyruvate as a substrate. Culture medium was collected and added to potassium phosphate buffer (0.05 M, pH 7.5) with NADH (9.4 mM). Pyruvate (20 mM) was added to the mixture, and the change in optical density was monitored after 5 min in a spectrophotometer. Data are expressed as percent LDH activity relative to control. LDH activity in control cultures not exposed to GD was normalized to 100 %. To corroborate cell survival the fluorescent markers for live and dead cells, calcein-AM and ethidium homodimer, respectively, were used. These markers (calcein 2 μM and ethidium homodimer 1 μM) were added to culture wells 22 h after GR during 30 min, cells were washed with Lockey medium and images were obtained using confocal microscopy (FV 1000; Olympus) motorized FV10ASW 2.1, with Ar 488 laser (for FITC) and Ar 596 nm (for ethidium) and images from the different treatments were captured.
Immunoblotting
Cells cultured in 35 mm dishes were exposed to GD for 1 or 2 h or 2 h of GD + 3, 6 and 20 h of GR. After the different treatments cells were washed in ice-cold PBS 0.1 M and lysed with a buffer containing: Tris–HCl pH 8.0 50 mM, NaCl 150 mM, Triton X-100 1 %, sodium deoxycholate 0.5 % and SDS 1 % and 2 mg/ml of protease inhibitor cocktail, and were centrifuged at 10 000 rpm at 4 °C for 5 min. Protein concentration was determined by the Lowry method and 30 μg was separated in 10 % (Beclin 1 and p62) or 20 % (LC3) SDS-PAGE and subsequently transferred to PVDF membranes. Membranes were immunoblotted with specific antibodies against the different autophagic markers: LC3 antibody that recognizes both LC3-I and LC3-II (1:1000), Beclin 1 (1:1000) and SQSTM1/p62 (1:500). Stripped blots were incubated with antibody against actin (1:7000) used as a loading control. The reactions of primary antibodies were detected using the respective horseradish peroxidase, goat anti-mouse or goat anti-rabbit secondary antibody and immunoreactivity was detected by chemiluminescent HRP substrate.
Immunocytochemistry
Cells were cultured on cover slips and exposed to 2 h of GD and to 2 h of GD + 3 h of GR. They were washed with ice-cold PBS 0.1 M and fixed with methanol for 20 min on ice. They were blocked with PBS-albumin 5 %, horse serum 5 %, Triton X-100 0.1 % for 1 h at room temperature. Primary antibody anti-LC3 (1:500) was incubated overnight at 4 °C and detected using FITC-coupled secondary anti-rabbit antibody (1:500) incubated at room temperature for 2 h. Cells nuclei were stained with Hoechst 0.001 % in PBS immediately after immunostaining and cells covered with Fluoromount-G™. Images were obtained by confocal microscopy (Leica TCS SP5) using a 63X objective with UV-405 nm laser for Hoechst and Arg-488 nm for LC3 immunoreactivity.
Live Imaging of Autophagosome Formation
Cyto-ID Autophagy detection kit was used to label autophagosomes in living cells [43]. Cells cultured in 35 mm dishes were exposed for 30, 60 and 90 min to GD. Before the onset of GD, Cyto-ID was incubated for 20 min in culture medium. Neurobasal medium was washed using a reperfusion chamber and progressively substituted with DMEM free-glucose medium. Confocal images (Leica TCS SP5 using 63X water immersion objective with UV-405 nm laser for Hoechst and Arg-488 nm for Cyto-ID) were taken at the onset of GD and at different times after GD. Hoechst was used as a nuclear counterstain. The number of Cyto-ID-positive vesicles was counted in the different experimental conditions from confocal images taken from 3 independent experiments using the Fiji image analysis software (Image J) [36]. We used the maximum projection and resorted parameters at the “analyze particles” plugin and an area of 0.3–1.5 μm2 and a circularity from 0.2 to 1.0 were fixed as parameters for the identification of posivite particles. The average size of the particles was 0.52 μm2.
Statistics
All data are expressed as Mean ± SEM and were analyzed by One-way ANOVA followed by a Fishers’ post hoc multiple comparison test.
Results
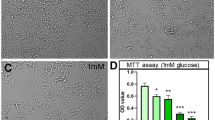
The effect of D-BHB on neuronal death induced after 2 h GD and 22 h GR is shown in Fig. 1. LDH activity in the medium of cultures exposed to GD/GR increased by 100 % relative to non-treated cultures, while a 60 % increase in LDH activity was observed in the medium of cells exposed to GD/GR in the presence of D-BHB (10 and 5 mM, respectively) (Fig. 1a). Similarly, MTT reduction decreased to 40 % of control values in cultures exposed to GD/GR in the absence of D-BHB, while treatment with the KB restored MTT reduction to 70 % of the control (Fig. 1b). These results are in agreement with our previous observations showing efficient protection against GD-induced neuronal death by D-BHB [15]. The effect of the KB on neuronal viability was corroborated by the fluorescent markers, calcein-AM (green) and ethidium homodimer (red), for live and dead neurons, respectively. As observed in Fig. 1c, many green cells with a normal morphology are present in the control condition. In cells exposed to 2 h GD and 22 h GR the number of green cells is substantially reduced while numerous red cells appear. In contrast, cultures treated with D-BHB show more green and well-preserved cells and few red cells as compared to cultures not treated with D-BHB, demonstrating the improvement of cell survival by D-BHB. Neuronal viability was monitored at different times after GR. No significant increase in the number of cells positive to ethidium homodimer was observed before 8 h of GR and this number increased from 8 to 16 h (data not shown). Only a small elevation in the number of death cells occurred from 16 to 22 h, suggesting that most of the cells die between 8 and 16 h.
Protective effect of D-BHB against GD-induced neuronal death in cortical cultures. Cultures were exposed to 2 h of GD and 22 h of GR in the presence of D-BHB (10 and 5 mM, respectively) and cell survival was assessed by LDH activity (a) and MTT reduction (b). Bars represent mean ± SEM (n = 6). Data were analyzed by One-way ANOVA followed a Fisher’s post hoc test *p < 0.005 versus control and & p < 0.005 versus GD. Representative images from cells stained with calcein (green) and ethidium homodimer (red) to monitor live and dead cells, respectively after the different treatments, are shown in c
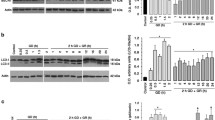
We then aimed to evaluate the changes in the levels of Beclin 1 and SQSTM1/p62, and in the LC3-II/LC3-I ratio. Figure 2a shows a significant decrease in Beclin 1 levels relative to control 3 h after GR, while no significant change was observed during GD. The autophagy inductor trehalose showed no effect on Beclin 1 levels after 4 h incubation, as shown in the representative immunoblot (Fig. 2a). In cultures treated with D-BHB, Beclin 1 content significantly decreased during 1 and 2 h of GD and at 3 h after GR Beclin 1 levels were very low. The transformation of LC3-I to LC3-II increases substantially when glucose is withdrawn and remains elevated at 3 h after glucose replenishment, suggesting an increase in autophagosome formation (Fig. 2b). The levels of LC3-II also increased in cultures exposed to trehalose during 4 h consistent with its action as an autophagy inducer (Fig. 2b). When D-BHB is added to the culture media during GD, the transformation of LC3-I to LC3-II significantly increases after 1 h of GD relative to control cultures but decreases at 2 h GD and 3 h after GR LC3-II content reaches control values (Fig. 2b).
Changes in Beclin 1, LC3-II/LC3-I ratio and p62 in cultures exposed to GD/GR in the presence or the absence of D-BHB. Representative western-blots and quantification of the changes in Beclin 1/actin levels (a), LC3-II/LC3-I ratio (b) and p62/actin levels (c) are shown. Bars represent mean ± SEM (n = 3–6). Data were analyzed by One-way ANOVA followed by a Fisher’s post hoc test *p < 0.05 versus control and & p < 0.05 versus D-BHB. OD optical density
To monitor autophagic degradation, the levels of p62, a protein hydrolyzed within the autophagolysosome, were measured. As can be observed in Fig. 2c, p62 levels did not change during 1 and 2 h of GD whereas they decreased significantly 3 h after glucose replenishment suggesting it is degraded at this time. When cultures were treated with D-BHB, p62 levels significantly diminished below control levels after 1 and 2 h of GD and remained low at 3 h GR (Fig. 2c).
To corroborate that the transformation of LC3-I to LC3-II corresponds to the formation of autophagosomes, we performed immunocytochemistry using an antibody that recognizes both LC3-I and LC3-II, after the exposure of cortical cultures to 2 h GD and to 2 h GD followed by 3 h GR. As observed in Fig. 3, LC3 immunoreactivity increases 2 h after GD as compared to control and at 3 h after GR, LC3 puncta are visible in many cells. In contrast, in D-BHB-treated cultures immunoreactivity is more diffuse and basically no cells containing LC3 puncta are observed after GR. To confirm these data we followed autophagosome formation by time-lapse live confocal microscopy using the fluorescent marker Cyto-ID, which labels autophagic vesicles [37]. As observed in Fig. 4 (upper panel), 30 min after GD the number of Cyto-ID-positive green vesicles increased in many cells as compared to the control condition (time 0), and were still present after 60 min. After 90 min GD the number of autophagosomes diminished in many cells although in some others Cyto-ID-positive vesicles remained. These observations were confirmed by the quantification of Cyto-ID-positive vesicles, as shown in Fig. 4b, and suggest that the formation of autophagic vesicles increases when glucose is withdrawn and progressively decreases during the last phases of GD. In cells exposed to GD in the presence of D-BHB, the number of Cyto-ID-positive vesicles increases at 30 min, but decreased substantially at 60 and 90 min of GD remaining only few cells containing green particles (Fig. 4a). These observations were confirmed by the quantification of the number of Cyto-ID-positive-puncta (Fig. 4b). Overall, these results suggest that a lower number of autophagosomes is accumulated in the presence of D-BHB, possibly due to the stimulation of the autophagic flux.
Time-lapse of in vivo autophagosome formation in cortical cultures exposed to GD in the presence or the absence of D-BHB. Representative images showing autophagosome formation using the Cyto-ID detection kit (green) and Hoechst counterstaining (blue), during GD (from 0.5 to 1.5 h). The graph below shows the number of Cyto-ID puncta in cells exposed to GD in the presence or the absence of D-BHB. *p < 0.05 versus GD (time 0), # p < 0.05 versus GD (0.5 h with or without D-BHB, respectively),& p < 0.05 versus GD (0.5 h without D-BHB)
To investigate the time-course of the changes in autophagy proteins and its correlation to neuronal death, we monitored LC3-II and p62 levels at longer times after GR, in the presence or the absence of D-BHB. As indicated in Fig. 5a, the increased transformation of LC3-I to LC3-II is sustained at 6 and 20 h after GR, while in cells treated with D-BHB, LC3-II levels remain below those of non-treated cells. These observations suggest that the KB stimulates the rate of autophagosome degradation. To test this hypothesis we used CQ to inhibit lysosome acidification and thus the autophagic flux. As observed (Fig. 5a) LC3-II levels significantly increased when CQ was added to cultures non-treated with D-BHB, and the effect of D-BHB was completely abated by CQ. This result is consistent with immunocytochemistry observations showing that more cells containing LC3 puncta were evident when CQ was added to D-BHB-treated cultures (Fig. 3). These observations support the hypothesis that the autophagic flux is accelerated in the presence of D-BHB. To further confirm these findings the changes in p62 content were also analyzed at longer times after GR in the presence or absence of D-BHB with and without CQ. As observed in Fig. 5b, p62 levels returned to control values at 6 and 20 h after GR. In the presence of D-BHB, p62 also increased after 6 and 20 h relative to the levels observed at 3 h, but remained below control values (Fig. 5b). When cells were exposed to D-BHB in the presence of CQ, p62 levels increased in agreement with the hypothesis that D-BHB stimulates the autophagic flux. Overall, the above-described results suggests that the accumulation of autophagosomes along the GR period precedes neuronal death and that protection by D-BHB is related to an accelerated rate of autophagic degradation.
Effect of Chloroquine (CQ) on LC3-II/LC3-I and p62 levels in cultures exposed to 2 GD and 3, 6 and 20 h GR in the presence or the absence of D-BHB. Representative western-blots and quantification of LC3-II/LC3-I and p62 levels are shown. Bars represent mean ± SEM (n = 3–6). Data were analyzed by One-way ANOVA followed by Fisher’s post hoc test *p < 0.05 versus control, & p < 0.05 versus GD without D-BHB, # p < 0.05 versus cells exposed to GD/GR without CQ
Finally, we tested the effect of the autophagy inhibitor, 3-MA, which blocks the activity of class III PI3K, on neuronal viability. As shown in Fig. 6a, 3-MA significantly improved cell survival as LDH activity in the medium was significantly reduced in cells exposed to GD in the presence of 3-MA. Similarly, MTT reduction was restored to 72 % of control values as compared to cells not treated with 3-MA, which showed a 60 % decrease in MTT reduction relative to controls (Fig. 6b). The effect of 3-MA on autophagosome formation was corroborated by LC3-II immunoblotting. As shown in Fig. 6c, the transformation of LC3-I to LC3-II decreased substantially in cultures treated with 3-MA, suggesting that inhibition of autophagy prevents neuronal death induced by glucose starvation.
Protective effect of 3-MA against GD-induced neuronal death in cortical cultures. Cultures were exposed to 2 h of GD in the presence of 3-MA (10 mM) and 22 h of GR and cell survival was assessed by LDH activity (a) and MTT reduction (b). Bars represent mean ± SEM (n = 6). Data were analyzed by One-way ANOVA followed by Fisher’s post hoc test *p < 0.005 versus control and & p < 0.05 versus GD. Representative western-blot showing the inhibitory effect of 3-MA on the transformation of LC3-I to LC3-II is shown in c
Discussion
It is well-known that autophagy is up-regulated after the ischemic episode and its role in ischemic injury has been suggested [38, 39], either due to the excessive degradation of cellular components [31], or to impaired autophagy leading to the accumulation of damaged proteins and organelles within autophagosomes [27, 28, 40]. The role of autophagy in GD- or low glucose-induced neuronal damage has been poorly explored, but recent in vitro studies suggest that the impairment of autophagy during these conditions contributes to neuronal death [41, 33]. The present results show that cultures exposed to glucose starvation activate autophagy, possibly as a response to nutritional stress and a mechanism to gain energy for cell survival. However, blockade of class III PI3K activity reduce neuronal death, suggesting a contribution of autophagy to neuronal damage in the present conditions.
We and others have shown that KB preserve the energy status of neurons, sustain synaptic activity and prevent cell death in different in vitro models of energy depletion [14, 15, 42]. Furthermore, KB prevent neuronal damage induced by ischemia and hypoglycemia in vivo [8, 9, 13–15]. We now show that D-BHB stimulates the autophagic flux and that this effect is involved in its protective action. According to the results, the levels of LC3-II and p62 rapidly decline at 3 h after GR, suggesting that the fusion of autophagosomes with lysosomes and the autophagic degradation take place soon after GR. This hypothesis is further supported by the results showing that the decline in these proteins is abated by CQ, which is commonly used to block the autophagic flux [43]. At later times after GR (6 and 20 h) the levels of LC3-II remained elevated and p62 returned to control values. In contrast, when GD occurs in the presence of D-BHB, LC3-II and p62 levels decrease to control values or below during GD and remain low during the entire GR period. The effect of D-BHB is completely abated by CQ suggesting that the autophagic flux is reestablished in the presence of the KB.
These present observations lead us to conclude that D-BHB stimulates the autophagic flux under energy-deficient conditions, possibly because the ATP levels of the cells are better preserved, preventing the overload of autophagosomes and improving neuronal survival. These results add new knowledge about the actions of KB and the mechanisms by which they can prevent neuronal death induced by energy failure. To our knowledge, this is the first study to suggest that protection by D-BHB against GD-induced neuronal death is mediated, at least in part, by the stimulation of the autophagic flux. The molecular mechanisms by which D-BHB modulates autophagy are currently studied.
Abbreviations
- GD:
-
Glucose deprivation
- GR:
-
Glucose reperfusion
- LC3:
-
Microtubule associated protein light chain 3
- LC3-II:
-
Lipidated form of the microtubule associated protein light chain 3
- BHB:
-
β-Hydoxybutyrate
- D-BHB:
-
D isomer of β-hydoxybutyrate
- 3-MA:
-
3-Methyl adenine
- KB:
-
Ketone bodies
References
Robinson AM, Williamsom DH (1980) Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev 60:143–187
Hawkins RA, Williamson DH, Krebs HA (1971) Ketone body utilization by adult and suckling rat brain in vivo. Biochem J 122:13–18
Nehlig A, Pereira de Vasconcelos A (1993) Glucose and ketone body utilization by the brain of neonatal rats. Prog Neurobiol 40:163–221
Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF Jr (1967) Brain metabolism during fasting. J Clin Invest 46:1589–1595
Pan JW, de Graaf R, Petersen KF, Shulman G, Herrington HP, Rothman DL (2002) [2,4-13C2]-beta-hydroxybutyrate metabolism in human brain. J Cereb Blood Flow Metab 22:890–898
Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I (2001) Ketogenic diet, amino acid metabolism and seizure control. J Neurosci Res 66:931–940
Masuda R, Monahan JW, Kashiwaya Y (2005) D-beta-hydroxybutyrate is neuroprotective against hypoxia in serum-free hippocampal primary cultures. J Neurosci Res 80:501–509
Suzuki M, Suzuki M, Kitamura Y, Mori S, Sato K, Dohi S et al (2002) β-Hydroxybutyrate, a cerebral function improving agent, protects rat brain against ischemic damage caused by permanent and transient focal cerebral ischemia. Jpn J Pharmacol 89:36–43
Puchowicz MA, Zechel JL, Valerio J, Emancipator DS, Xu K, Pundik S et al (2008) Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J Cereb Blood Flow Metab 28:1907–1916
Tai KK, Nguyen N, Pham L, Truong DD (2008) Ketogenic diet prevents cardiac arrest-induced cerebral ischemic neurodegeneration. J Neural Transm 115:1011–1017
Massieu L, Haces ML, Montiel T, Hernández-Fonseca K (2003) Acetoacetate protects hippocampal neurons against glutamate-mediated neuronal damage during glycolysis inhibition. Neuroscience 120:365–378
Noh HS, Hah YS, Nilufar R, Han J, Bong JH, Kang SS et al (2006) Acetoacetate protects neuronal cells from oxidative glutamate toxicity. J Neurosci Res 83:702–709
Yamada KA, Rensing N, Thio LL (2005) Ketogenic diet reduces hypoglycemia-induced neuronal death in young rats. Neurosci Lett 385:210–214
Haces ML, Hernández-Fonseca K, Medina-Campos ON, Montiel T, Pedraza-Chaverri J, Massieu L (2008) Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp Neurol 211:85–96
Julio-Amilpas A, Montiel T, Soto-Tinoco E, Gerónimo-Olvera C, Massieu L (2015) Protection of hypoglycemia-induced neuronal death by β-hydroxybutyrate involves the preservation of energy levels and decreased production of reactive oxygen species. J Cereb Blood Flow Metab 35:851–860
Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM (2007) Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience 145:256–264
Zhang J, Cao Q, Li S, Lu X, Zhao Y, Guan JS et al (2013) 3-Hydroxybutyrate methyl ester as a potential drug against Alzheimer’s disease via mitochondria protection mechanism. Biomaterials 34:7552–7562
Mizushima N, Levine B, Cuervo AM, Klionsky D (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Tanida I (2011) Autophagy basics. Microbiol Immunol 55:1–11
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S et al (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26:9220–9231
Kroemer G, Mariño G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40:280–293
Alirezaei M, Kembal CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB (2010) Short-term fasting induces profound neuronal autophagy. Autophagy 6:702–710
Tsujimoto Y, Shimizu S (2005) Another way to die: autophagic programed cell death. Cell Death Diff 12:1528–1534
Clarke PGH, Puyal J (2012) Autophagic cell death exists. Autophagy 8:867–869
Sou Y-S, Waguri S, Iwata J, Ueno T, Fujimura T, Hara T et al (2008) The Atg8 conjugation system is indispensable for proper development of autophagic isolation membranes in mice. Mol Biol Cell 19:4762–4775
Kulbe JR, Levy JMM, Coultrap SJ, Thorburn A, Baye KU (2014) Excitotoxic glutamate insults block autophagic flux in hippocampal neurons. Brain Res 1542:12–19
Sarkar C, Zhao Z, Aungst S, Sabirzhanov B, Faden AI, Lipinski MM (2014) Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy 10:2208–2222
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I et al (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441:880–884
Carloni S, Buonocore G, Balduini W (2008) Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis 32:329–339
Bukcley KM, Hess DL, Sazonova IY, Periyasamy-Thandavan S, Barrett JR, Kirks R et al (2014) Rapamycin up-regulation of autophagy reduces infarct size and improves outcomes in both permanent MCAL, and embolic MCAO, murine models of stroke. Exp Trans Stroke Med 6:8
Shi R, Weng J, Zhao L, Li X-M, Gao T-M, Kong J (2012) Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosc Ther 18:25–260
Higgins GC, Devenish RJ, Beart PM, Nagley P (2011) Autophagic activity in cortical neurons under acute oxidative stress directly contributes to cell death. Cell Mol Life Sci 68:3725–3740
Jang BG, Choi BY, Kim JH, Kim MJ, Sohn M, Suh SW (2013) Impairment of autophagic flux promotes glucose reperfusion-induced neuro2A cell death after glucose deprivation. PLoS ONE 8:e76466
Yue Z, Zhong Y (2010) From global view to focused examination: understanding cellular function of lipid kinase VPS34-Beclin 1 complex in autophagy. J Mol Cell Biol 2:305–307
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H et al (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Chan LL, Shen D, Wilkinson AR, Patton W, Lai N, Chan E, et al (2012) A novel image-based cytometry method for autophagy detection in living cells. Autophagy 8:1371–1382
Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD et al (2008) Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy 4:762–769
Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S et al (2008) Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol 172:454–469
Liu C, Gao Y, Barrett J, Hu B (2010) Autophagy and protein aggregation after brain ischemia. J Neurochem 115:68–78
Balmer D, Emery M, Andreux P, Auwerx J, Ginet V, Puyal J et al (2013) Autophagy defect is associated with low glucose-induced apoptosis in 66 W photoreceptor cells. PLoS ONE 8:e74162
Izumi Y, Benz AM, Katsuki H, Zorumski CF (1997) Endogenous monocarboxylates sustain hippocampal synaptic function and morphological integrity during energy deprivation. J Neurosci 17:9448–9457
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140:313–326
Acknowledgments
This study was performed in partial fulfillment of the requirements for the Ph.D. degree in Ciencias Biomédicas of L. Camberos-Luna at the Universidad Nacional Autónoma de México. This work was supported by Programa de Apoyo a Proyectos de Investigación e Inovación Tecnológica (PAPIIT) grant IN204213 and Consejo Nacional de Ciencia y Tecnología (CONACYT) Grant CB-239607 to LM and CONACYT scholarship to L. Camberos-Luna. Authors thank Augusto César Poot-Hernández for his help in vesicle counting.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Special Issue: In Honor of Philip Beart.
Lucy Camberos-Luna and Cristian Gerónimo-Olvera have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Camberos-Luna, L., Gerónimo-Olvera, C., Montiel, T. et al. The Ketone Body, β-Hydroxybutyrate Stimulates the Autophagic Flux and Prevents Neuronal Death Induced by Glucose Deprivation in Cortical Cultured Neurons. Neurochem Res 41, 600–609 (2016). https://doi.org/10.1007/s11064-015-1700-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1700-4