Abstract
Hypoglycemia has emerged as a prominent complication in anti-diabetic drug therapy or negative energy balance of animals, which causes brain damage, cognitive impairment, and even death. Brain injury induced by hypoglycemia is closely related to oxidative stress and the production of reactive oxygen species (ROS). The intracellular accumulation of ROS leads to neuronal damage, even death. Ketone body β-hydroxybutyrate (BHBA) not only serves as alternative energy source for glucose in extrahepatic tissues, but is also involved in cellular signaling transduction. Previous studies showed that BHBA reduces apoptosis by inhibiting the excessive production of ROS and activation of caspase-3. However, the effects of BHBA on apoptosis induced by glucose deprivation and its related molecular mechanisms have been seldom reported. In the present study, PC12 cells and primary cortical neurons were used to establish a low glucose injury model. The effects of BHBA on the survival and apoptosis in a glucose deficient condition and related molecular mechanisms were investigated by using flow cytometry, immunofluorescence, and western blotting. PC12 cells were incubated with 1 mM glucose for 24 h as a low glucose cell model, in which ROS accumulation and cell mortality were significantly increased. After 24 h and 48 h treatment with different concentrations of BHBA (0 mM, 0.05 mM, 0.5 mM, 1 mM, 2 mM), ROS production was significantly inhibited. Moreover, cell apoptosis rate was decreased and survival rate was significantly increased in 1 mM and 2 mM BHBA groups. In primary cortical neurons, at 24 h after treatment with 2 mM BHBA, the injured length and branch of neurites were significantly improved. Meanwhile, the intracellular ROS level, the proportion of c-Fos+ cells, apoptosis rate, and nuclear translocation of NF-κB protein after treatment with BHBA were significantly decreased when compared with that in low glucose cells. Importantly, the expression of p38, p-p38, NF-κB, and caspase-3 were significantly decreased, while the expression of p-ERK was significantly increased in both PC12 cells and primary cortical neurons. Our results demonstrate that BHBA decreased the accumulation of intracellular ROS, and further inhibited cell apoptosis by mediating the p38 MAPK signaling pathway and caspase-3 apoptosis cascade during glucose deprivation. In addition, BHBA inhibited apoptosis by activating ERK phosphorylation and alleviated the damage of low glucose to PC12 cells and primary cortical neurons. These results provide new insight into the anti-apoptotic effect of BHBA in a glucose deficient condition and the related signaling cascade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucose is a key energy substrate of brain and plays a critical role in energy metabolism. It is considered hypoglycemia if the plasma glucose concentration reduces below 3.9 mmol/L (70 mg/dL), regardless of whether the patient is in a fasting state (Rehni and Dave 2018). Hypoglycemia is a common metabolic state in patients treated with anti-diabetic drug as well as in pregnant and lactating animals. It not only impairs the health of many people and animals, but also causes great economic loss. During lactation, cows often suffer from negative energy balance, leading to decreased blood sugar and ketosis, which results in the loss of appetite, decrease of milk production, and even death (Najm et al. 2020). As for humans, it has been reported that it is a common complication of patients with diabetes who receive insulin or insulin secretion promoting therapy (Kittah and Vella 2017). Recent evidence suggests that hypoglycemia is associated with increased risks of neuropathy (Mohseni 2001), cognitive dysfunction (Lacy et al. 2020), and cardiovascular events (AAmiel et al. 2019). In the central nervous system (CNS), hypoglycemia causes partial necrosis of neurons in the cerebral cortex, especially in the dentate gyrus of hippocampus (De Angelis et al. 2021). Degeneration and even loss of granule cells in the dentate gyrus were observed in hypoglycemic animal models (Auer 2004). In addition, different degrees of nonspecific neuronal necrosis were also observed in hippocampus proper (Jackson et al. 2018). One of the important pathological features of low glucose-induced cells damage is the accumulation of ROS and Ca2+ overload. In the early stage of hypoglycemia, neuronal depolarization induces a large influx of calcium ions, which cause mitochondrial Ca2+ overload and further leads to an increase of ROS. The intracellular accumulation of ROS activates PARP-1, which leads to glycolytic inhibition and disrupts neuronal homeostasis (Isaev et al. 2007). Furthermore, decreased glucose uptake or glucose deprivation leads to ATP depletion, oxidative stress, and increased expression of hypoxia-inducible factor-1α, resulting in the activation of apoptotic pathway, which in turn trigger the death cascade (Moley and Mueckler 2000).
The ketone body β-hydroxybutyrate (BHBA) is an important product of fat metabolism and can replace glucose as the energy substrate for CNS if the organism lacks glucose. In addition to serving as an alternative energy source for glucose in extrahepatic tissues, BHBA is also involved in cellular signaling transduction, such as epigenetic gene regulation, neuronal function, lipid metabolism, and metabolic rate (Newman and Verdin 2017; Dąbek et al. 2020). The most widely proved effects of BHBA are suppression on oxidative stress (Shimazu et al. 2013), alleviation of Alzheimer’s disease (AD) pathology (Shippy et al. 2020), and reduction of ischemia damage (Tajima et al. 2019). The neuroprotective mechanism of BHBA is mainly to reduce glutamate-induced mitochondrial free radical production and enhance mitochondrial aerobic respiratory function (Maalouf et al. 2007). It was reported that microglia overactivation mediated by amyloid β (Aβ) deposition and G protein-coupled receptor 109A (GPR109A) in AD can be attenuated by BHBA (Wu et al. 2020b). The regulation of GPR109A by BHBA is not only involved in the reduction of Aβ precursor protein, but also plays an important role in aging, Parkinson’s disease and other neurodegenerative diseases (Wu et al. 2020b; Fu et al. 2015). In addition, BHBA prevents apoptosis by inhibiting the excessive production of intracellular reactive oxygen species (ROS), increasing the content of GSH, restoring MMP,and reducing the activation of caspase-3 (Cheng et al. 2013). Previous study showed that binding of BHBA to HCAR2 plays an important anti-apoptosis role by activating IP3-dependent intracellular calcium release and inhibiting downstream NF-κB activation (Newman and Verdin 2017). The effects of BHBA on apoptosis induced by glucose deprivation and its related molecular mechanisms have been seldom reported in vitro. In the current study, we characterized low glucose-induced cell injury and demonstrated that the BHBA prevents the cells from apoptosis induced by hypoglycemia. It provides evidence that BHBA has the potential to be a new candidate for the prevention or treatment of hypoglycemia.
Materials and Methods
Materials
High glucose (25 mmol/L, 25 mM), Dulbecco’s modified Eagle’s medium (DMEM), glucose-free DMEM, neurobasal medium, B27 supplement, GlutaMAX supplement, HBSS, fetal bovine serum (FBS), and horse serum were obtained from Gibco (Life Technologies, USA). Methylthiazolyldiphenyl-tetrazolium bromide (MTT), reactive oxygen species assay kit (DCFH-DA), and dimethyl sulfoxide (DMSO) were obtained from Solarbio (Beijing, China). Annexin V-FITC Apoptosis Detection Kit was obtained from Beyotime Biotechnology (Shanghai, China). The antibodies specific for, β-actin (#58,169), caspase-3 (#9662), NF-κB (#8242), ERK (#9102), p-ERK (#4370), p38 (#9212), and p-p38 (#4511), anti-mouse IgG-HRP (#7076), and anti-rabbit IgG-HRP (#7074) were purchased from Cell Signaling Technology (Danvers, MA, USA). Microtubule-associated protein-2 (MAP-2) (ab254143) and c-Fos (ab190289) were purchased from Abcam Biotechnology (Cambridge, UK). Poly-L-Lysine, β-hydroxybutyrate (BHBA), propidium iodide (PI), and penicillin–streptomycin were purchased from Sigma-Aldrich (St. Louis, MO, USA).
ICR mice were obtained from the experimental animal center of Xi’an Jiaotong University, China. The mice were housed with a 12-h light/dark cycle (lights on from 07:00 to 19:00) in constant temperature (22–24 °C) and free access to food and water. All of the care and handling of animals and the experiment activities were conducted according to the Guide for Care and Experimental of Laboratory Animals of Northwest A&F University, and were carried out in accordance with the Animal Care Commission of the College of Veterinary Medicine, Northwest A&F University (certificate no.: SCXK [SHAAN] 2017–003).
Cell Culture and Treatment
Rat pheochromocytoma (PC12) cells were derived from pheochromocytoma in the adrenal medulla of rattus norvegicus, which have some characteristics of mature dopaminergic neurons and are widely used in neurobiological research (Wiatrak et al. 2020). In the present experiment, PC12 cells were cultured in complete medium containing 25 mM glucose DMEM with 5% FBS, 5% horse serum, and 1% penicillin–streptomycin (10 000 U/mL) at 37 °C and 5% CO2 for 1 day. Then, the medium was replaced with 1 mM low glucose complete medium (LG-CM), 5 mM medium glucose complete medium (MG-CM), or 25 mM high glucose complete medium (HG-CM) respectively for subsequent experiments.
Isolation and culture of primary cortical neurons: All of the pregnant mice at embryonic day 18 (E18) were euthanized and removed uterus into 100-mm dish containing cold HBSS under sterile conditions. The cortical hemispheres of offsprings were separated, and the meninges removed. Then, cerebral cortices were trypsinized for 15 min with 4 ml of 0.25% trypsin at 37 °C. The supernatant was removed, and cold HBSS was added to rinse tissue. Whereafter, trituration with a pipette was performed to separate the tissue blocks. Cells were collected by centrifugation for 5 min at 800 rpm. The cells were resuspended in HG-CM, plated onto 12 mm2 poly-L-lysine-pretreated glass coverslips and placed in a 37 °C, 5% CO2 incubator. After 2 h, the medium was replaced with Neurobasal medium containing 2 mM Glutamine and 2% B27 supplement (Neurobasal/B27). After 24 h, the neurons were divided into control group, low-glucose group, and BHBA-treated group. A total of 25 mM and 1 mM glucose Neurobasal/B27 medium were used in the control group and low-glucose group respectively for 48 h. BHBA-treated group was cultured with 1 mM glucose Neurobasal/B27 medium for 24 h, and then treated with 2 mM BHBA for 24 h.
Measurement of Cell Viability
Detection of Cell Viability of LG-CM Cultured PC12 Cells
Cell viability was detected by the MTT assay. PC12 cells were suspended in 25 mM glucose complete medium and seeded at 1 × 103 cells/well in 96-well plates. After 1 day of adherent cell culture, the medium was replaced with 100 µL LG-CM for the indicated time (0 h, 6 h, 12 h, 24 h, 48 h). Ten replicate culture wells are set at each time points. Whereafter, 10 µL MTT (5 mg/mL) was added and the cells were incubated at 37 °C for 4 h. At the same time, a zero-adjustment hole (complete medium, MTT) was set. After incubation, the formazan was solubilized by 100 µL of DMSO for 20 min and the absorbance at 570 nm was measured by a micro plate reader (Bio-Rad, Hercules, CA, USA). The experiment was repeated three times.
MTT Assay of BHBA Treated PC12 Cells
PC12 cells were suspended in 100 µL LG-CM and seeded at 5 × 103 cells/well in 96-well plates. After 24 h of adherent cell culture, the cells were treated with different concentrations of BHBA (0 mM/BHBA-free, 0.05 mM, 0.5 mM, 1 mM, 2 mM) for the indicated time (6 h, 12 h, 24 h, 48 h). Similarly, the control groups were established which were treated with HG-CM and without BHBA. Ten replicate culture wells were set at each concentration. MTT was added and measured as described in “Detection of Cell Viability of LG-CM Cultured PC12 Cells.” The experiment was repeated three times.
Measurement of Intracellular ROS
Detection of Intracellular ROS of LG-CM Treated PC12 Cells Within 48 h
PC12 cells were treated with LG-CM for 0 h, 6 h, 12 h, 24 h, and 48 h in 24-well plates. Production of intracellular ROS was determined by using a fluorescent probe DCFH-DA according to the manufacturer’s instructions. Cells were incubated with DMEM containing 10 µM DCFH-DA for 20 min. Cells were washed three times with, and then, the images were taken using a fluorescence microscope with excitation at 488 nm and emission at 525 nm wavelengths. Four replicate culture wells were set at each time points. Random fields (n = 20 per group) were selected for images acquisition. The experimenter was blinded to culture condition while acquiring and analyzing images. The resulting data were analyzed by using the Image J.
Detection of Intracellular ROS of BHBA Treated PC12 Cells and Primary Cortical Neurons
PC12 cells were seeded at 5 × 104 cells/well in 24-well plates and cultured in LG-CM for 24 h. Then, the cells were treated with different concentrations of BHBA (0 mM, 0.05 mM, 0.5 mM, 1 mM, 2 mM) for 24 h and 48 h. Meanwhile, the control group was treated with HG-CM and without BHBA. Four replicate culture wells were set at each concentration. Primary cortical neurons were tested in control group, low-glucose group, and BHBA-treated group (2 mM). Intracellular ROS were measured and analyzed as described in “Detection of Intracellular ROS of LG-CM Treated PC12 Cells Within 48 h.”
Flow Cytometry of Apoptosis
Apoptosis was assessed with flow cytometry by AnnexinV (AV) and PI. A total of 1 × 105 PC12 cells were seeded in 6-well plates and treated with different concentrations of BHBA (0 mM, 0.05 mM, 0.5 mM, 1 mM, 2 mM) for 24 h and 48 h, respectively. Similarly, the control group was treated with HG-CM and without BHBA. Cells were collected and stained for AV and PI using the Annexin V-FITC Apoptosis Detection Kit according to the manufacturer’s instructions. A Beckman CytoFLEX flow cytometer was used to acquire the data and for data analysis. Gating was set according to live unstained PC12 cells growing in HG-CM (negative control for apoptosis). Primary cortical neurons were tested in control group, low-glucose group, and BHBA-treated group (2 mM).
Immunofluorescence Staining
Each group of primary neurons were fixed in 4% paraformaldehyde at room temperature for 30 min, and rinsed three times with PBS. The cell coverslips were incubated with primary antibodies diluted in blocking solution (4% BSA, 1% NGS, 0.3% Triton in phosphate buffer) at 4 °C overnight. The following primary antibodies were used: mouse anti-MAP2, rabbit anti-c-Fos, rabbit anti-caspase-3, and rabbit anti-NF-κB. After rinsing 3 times in PBS, all cell coverslips were incubated with second antibodies for 2 h at room temperature in the dark. The following second antibodies were used in this study: Alexa Fluor 488 donkey anti-rabbit IgG and Alexa Fluor 568 donkey anti-mouse IgG. After rinsing 3 times in PBS for 1 h, the cell coverslips were mounted with Fluorescence Mounting Medium (Dako), and observed under a fluorescence microscope (Zeiss observer Z1) with 10 × or 20 × objective. The positive cells were counted using the software ImageJ Fiji. Random fields were selected for images acquisition. The experimenter was blinded to culture condition while acquiring and analyzing images.
Western Blotting Analysis
Western blotting analysis was carried out as described previously (Wang et al. 2020). In brief, cells treated with BHBA were collected and lysed in 360 μL RIPA buffer supplemented with 1× protease and phosphatase inhibitors, then total proteins were extracted and denatured. The protein samples (20 μg) were separated by a 10% SDS-PAGE and electro-transferred onto PVDF membranes. The membranes were blocked with 5% skim milk in Tris-buffered saline (TBS) buffer followed by immunoblotting using specific antibodies (NF-κB, caspase-3, p38, p-p38, ERK, p-ERK, β-actin, 1:1000 dilution) overnight 4 °C. After rinsing with TBS with Tween 20 (TBST), the membranes were incubated with the corresponding secondary antibodies conjugated to horseradish peroxidase (anti-mouse IgG-HRP or anti-rabbit IgG-HRP, 1:2000 dilution). The blots of proteins were detected by chemiluminescence system (Alliance Q9 Advanced, England).
Statistics
The data were analyzed by using GraphPad Prism V8.0 (GraphPad Software Inc., USA). Statistical analysis was performed using one-way ANOVA followed by Tukey’s multiple comparisons test. The error bars indicate the SEM. Differences were considered to be statistically significant at p < 0.05.
Results
Identification of the Glucose Concentration of PC12 Cells in Low Glucose Model Medium
To model a low glucose in vitro, PC12 cells were treated with medium containing varying concentrations of glucose. PC12 cells with HG-CM (25 mM glucose) showed good state with strong proliferation and a large quantity after 48 h adherent culture (Fig. 1A). Although the cell density is lower than that in control group, PC12 cells cultured with MG-CM (5 mM glucose) grew well and fewer apoptotic or dead cell fragments were observed suspended in the medium (Fig. 1B). The density of PC12 cells cultured with LG-CM (1 mM glucose) was greatly reduced with poor growth, and a large number of apoptotic cells or fragments of dead cells were observed (Fig. 1C). MTT assay showed that the OD value of PC12 cells cultured with LG-CM decreased significantly after 6 h culture when compared with that in cells for 0 h treatment with LG-CM, and the difference is more obvious after 24–48 h culture (p < 0.001). As a result, the medium containing 5 mM glucose could reduce cell proliferation but did not cause cell apoptosis or death. Therefore, in the present study, 1 mM glucose was used as low glucose model medium (LG-CM) for PC12 cells.
The proliferation of PC12 cells cultured with LG-CM was decreased. PC12 cells were incubated with medium containing different conditions of glucose for 48 h. A HG-CM (25 mM glucose). B MG-CM (5 mM glucose). C LG-CM (1 mM glucose). D OD values detected by MTT in cells incubated with LG-CM at different time points (n = 10 per group). *p < 0.05, **p < 0.01, and ***p < 0.001 relative to 0 h group, scale bar: 100 μm
Identification of the Incubation Time of PC12 Cells with LG-CM
Although 1 mM glucose was identified as the concentration for low glucose model, the optimum time of induction is still crucial. In order to obtain the most suitable low glucose model, ROS detection and PI staining were performed to detect the damage of cells caused by LG-CM over time. The level of ROS in PC12 cells treated with LG-CM gradually increased with the prolonging of time. At 12 h after treatment with LG-CM, the intracellular ROS level was significantly increased when compared with that in control cells (p = 0.0049). The increase lasted up to 48 h and showed time-dependent (p < 0.0001) (Fig. 2A, B). In addition, the PI-positive cells (apoptotic or dead cells) were detected. We found that at 24 h (p = 0.0021) and 48 h (p < 0.0001) after treatment with LG-CM, the proportions of PI positive cells were significantly increased, while the density of PI-negative living cells was significantly reduced compared to that in control condition (Fig. 2C, D). Since the cell death rate at 6–12 h was not significantly different from that in control condition, while the cell death rate at 48 h was 31.5% which was so hard for drug rescue to reverse the effect, LG-CM incubation for 24 h was chosen as the time point for the model of low glucose-injured cells in the following studies.
PC12 cells incubated with LG-CM showed higher ROS level and mortality. A PC12 cells were cultured with LG-CM and ROS was examined at different time points. B Quantitative analysis of ROS (n = 15–20 random fields per group). C PC12 cells were cultured with LG-CM and were stained with PI to visualize the dead cells at different time points. D Quantitative analysis of PI-positive cells (n = 10 random fields per group). **p < 0.01 and ***p < 0.001 relative to 0 h group
BHBA Reduced ROS Production in Low Glucose–Injured Cells
Our data found that the viability of PC12 cells cultured with 1 mM glucose was greatly reduced with poor growth. To investigate whether BHBA treatment can increase low glucose-injured cells viability, the cells in LG-CM conditions were incubated with containing different concentrations of BHBA (0 mM, 0.05 mM, 0.5 mM, 1 mM, 2 mM) for 6–48 h. According to MTT assay, we found that the OD value of the BHBA-free (BHBA 0 mM) group gradually decreased with the extension of time. At 6–12 h treatment with different concentrations of BHBA, the OD values did not show significant difference (Fig. 3A). However, at 24 h treatment with BHBA, the OD values in BHBA 0.5–2 mM groups were significantly higher than that in BHBA-free group (p < 0.01), but no difference when compared with that in control group (Fig. 3B). At 48 h treatment with BHBA, the OD value in BHBA 0.5–2 mM groups was lower than that at 24 h (Fig. 3A), but still significantly higher than that in BHBA-free group (p < 0.001) and lower than that in control group (Fig. 3C). According to these results, the time points of 24 h and 48 h treated with BHBA were conducted in the following experiments.
BHBA reduced low glucose-induced cytotoxicity and ROS production in PC12 cells. A OD values for MTT test in low glucose-injured cells cultured with different concentrations of BHBA for 6–48 h respectively (n = 10 per group). B Quantitative analysis of OD value in low glucose-injured cells with different concentrations of BHBA and in control group cultured for 24 h (n = 10 per group). C Quantitative analysis of OD value in low glucose-injured cells with different concentrations of BHBA and in control group cultured for 48 h (n = 10 per group). D Fluorescence microscope photography for ROS detection of low glucose-injured cells cultured with different concentrations of BHBA for 24 h. E Quantitative analysis of ROS intensity in low glucose-injured cells with different BHBA concentration and in control group cultured for 24 h (n = 12 random fields per group). F Fluorescence microscope photography for ROS detection of low glucose–injured cells cultured with different concentrations of BHBA for 48 h. G Quantitative analysis of ROS intensity in low glucose-injured cells with different BHBA concentrations and in control group cultured for 48 h (n = 20 random fields per group). **p < 0.01 and ***p < 0.001 relative to 0 mM group; ###p < 0.001 relative to control group
The intracellular accumulation of ROS leads to apoptosis and cell death (Wu et al. 2020a). The current results showed that low glucose contributes to intracellular ROS accumulation and increased cell mortality. BHBA treatment for 24–48 h can reduce cell damage caused by low glucose. However, it is unclear if BHBA reverses ROS accumulation caused by low glucose. Intracellular ROS was determined by using a fluorescent probe DCFH-DA. After treatment with BHBA for 24 h, the intracellular ROS levels were significantly lower in the 0.5 mM, 1 mM, and 2 mM groups compared with that in BHBA-free group (p < 0.01), while no difference compared with that in control group (Fig. 3D, E). At 48 h treatment with BHBA, no significant difference was found in the ROS level in the 0.05 mM, 0.5 mM, and 1 mM groups when compared with that in BHBA-free group, while in 2 mM group showed a significant decrease (p < 0.001) (Fig. 3F, G). These data suggest that the increased viability of the low glucose-injured cells in BHBA-treated groups resulted from a reduction of ROS.
BHBA Increased the Cell Survival in Low Glucose Condition
The above results indicate that BHBA treatment increased viability of the low glucose-injured cells. In order to further verify the effect of BHBA treatment on cell survival, the survival rate and apoptotic rate were examined by flow cytometry. The results showed that after treatment with different concentrations of BHBA (0 mM, 0.05 mM, 0.5 mM, 1 mM, 2 mM) for 24 h, the survival rates of low glucose-injured cells in groups treated with 0.5 mM, 1 mM, and 2 mM BHBA were significantly increased compared to that in BHBA-free group (p < 0.001). Compared with that in control group, the survival rates of the cells treated with 0 mM, 0.05 mM, and 0.5 mM BHBA were significantly lower (p < 0.001), while no statistical significance in the 1 mM and 2 mM groups was found (Fig. 4A, C). Correspondingly, the apoptotic rates of cells in groups treated with 0 mM, 0.05 mM, and 0.5 mM BHBA were significantly increased as compared to that in the control group (p < 0.001). The apoptotic rates of cells in groups treated with 1 mM and 2 mM BHBA were significantly decreased as compared to that in BHBA-free group, whereas no significant differences compared to that in control group (p < 0.001) (Fig. 4A, D). Following 48 h treatment with BHBA, the survival rates of low glucose-injured cells with all concentrations of BHBA were significantly increased and the apoptotic rates were significantly decreased as compared to that of BHBA-free group (p < 0.05). Meanwhile, the survival rate and apoptotic rate in all the BHBA treated groups were significantly different from the control group (p < 0.05) (Fig. 4B, E–F). These results demonstrate convincingly that BHBA effectively blocks low glucose-induced apoptosis of PC12 cells.
BHBA increased the survival rate and decreased the death rate of low glucose-injured cells. Flow cytometry detection of low glucose-injured PC12 cells after treatment with different concentrations of BHBA and control group for 24 h A and 48 h B. Quantitative analysis of survival rate C and apoptotic rate D of low glucose-injured cells treated with different concentrations of BHBA for 24 h. Quantitative analysis of survival rate E and apoptotic rate F of low glucose-injured cells treated with different concentrations of BHBA for 48 h (n = 4 per group). *p < 0.05, **p < 0.01, and ***p < 0.001 relative to 0 mM group; #p < 0.05, ##p < 0.01, and ###p < 0.001 relative to control group
BHBA Inhibited p38 MAPK and Activated ERK in Low Glucose–Injured Cells
The above results indicate that low glucose led to intracellular ROS accumulation and apoptosis. Previous study demonstrate that the accumulation of intracellular ROS activates p38 MAPK, which leads to DNA damage and promotes apoptosis (Yao et al. 2020). In addition, ROS activates ERK1/2, leading to PARP-1 activation and inhibiting glycolysis to promote apoptosis (Kauppinen et al. 2006). To further investigate the molecular mechanism of low glucose-induced apoptosis and the anti-apoptosis effect of BHBA, the expression of p38, p-p38, ERK, p-ERK, NF-κB, and caspase-3 were examined. Western blotting results showed that after 24 h BHBA treatment with 1 mM and 2 mM BHBA, the expression of p38, p-p38, and NF-κB in low glucose-injured cells was significantly decreased (p < 0.01) and p-ERK was significantly increased compared with those in the BHBA-free group (p < 0.01) (Fig. 5A, B). After 48 h, the expression of NF-κB in the 2 mM BHBA group was significantly decreased (p < 0.01) and p-ERK was significantly increased compared with that in BHBA-free group (p < 0.05) (Fig. 5C, D). Furthermore, most of the stress-activated MAPK eventually concentrates on caspase activation, which induces apoptosis through a variety of pathways (Green and Llambi 2015). Our results showed that at 24 h and 48 h treatment with BHBA, the expression of caspase-3 was significantly decreased in the 1 mM and 2 mM BHBA groups compared with that in BHBA-free group. These data suggest that BHBA alleviated low glucose-induced apoptosis via inhibiting p38 MAPK activation and increased phosphorylation of ERK.
Effects of BHBA on the expression of apoptosis-related proteins in low glucose-injured cells. Western Blot for apoptosis-related proteins in the low glucose-injured cells cultured with different concentrations of BHBA for 24 h A and 48 h C. Quantitative analysis of different apoptosis-related proteins in the low glucose-injured cells cultured with different concentrations of BHBA for 24 h B and 48 h D (n = 4–5 per group). *p < 0.05, **p < 0.01, and ***p < 0.001 relative to 0 mM group; #p < 0.05, ##p < 0.01, and ###p < 0.001 relative to control group
BHBA Reduced ROS Production and Apoptosis of Primary Cortical Neurons Induced by Low Glucose
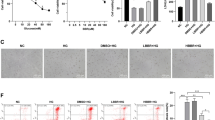
The above results demonstrate convincingly that 2 mM BHBA effectively blocks low glucose-induced apoptosis of PC12 cells. To further investigate the protective effects of BHBA treatment on neurons, a series of experiments were performed using primary cortical neurons. MAP2 is an important component of the neuron cytoskeleton, which can reflect the changes of neuron morphology and neurite. Immunofluorescence results showed that the branch of neurites decreased and the length became shorter in low-glucose group. Importantly, the reductions of neurite lengths and branches were significantly inhibited following 2 mM BHBA treatment after 24 h compared with the low-glucose group (p = 0.0003). Furthermore, the proportion c-Fos+ cells and the intracellular ROS levels were significantly lower in BHBA groups compared with that in low-glucose groups (p < 0.0001) (Fig. 6A). Apoptosis analysis by flow cytometry showed that the apoptosis rate of the low-glucose group was significantly increased than that of the control group (p < 0.0001). However, the BHBA treatment significantly inhibited apoptosis, which was close to the control group (p = 0.014) (Fig. 6B). These results suggest that BHBA can improve the damage of low glucose-induced neuron, reduce the accumulation of ROS, and inhibit the apoptosis of neuron.
BHBA reduced low glucose-induced apoptosis in primary cortical neurons. A Representative fluorescent images and quantitative analysis of MAP2 (n = 30 random fields per group), MAP2/c-Fos (n = 20 random fields per group), and ROS (n = 18 random fields per group) in neurons. B Representative image of flow cytometry and quantitative analysis of apoptotic rate (n = 4 per group). C: control group; LG: low glucose group; BHBA: BHBA-treated group. *p < 0.05, **p < 0.01, and ***p < 0.001 relative to control; #p < 0.05, ##p < 0.01, and ###p < 0.001 relative to low glucose group. Scale bar: 100 μm
BHBA Alleviated Nuclear Translocation of NF-κB and Inhibited the Activation of p38 MAPK in Low Glucose–Injured Neurons
In order to explore whether BHBA can also inhibit the apoptosis of primary cortical neurons by mediating the p38 MAPK signal pathway, the expressions of p38, p-p38, ERK, p-ERK, NF-κB, and caspase-3 were examined by immunofluorescence or Western blotting. Immunofluorescence results showed that the relative fluorescent intensity of caspase-3 and NF-κB were significantly lower in BHBA groups compared with that in low-glucose groups (p < 0.0001). In addition, the NF-κB proteins of control group was located in the cytoplasm, but was translocated into the nucleus in low-glucose group. While in BHBA group, nuclear translocation of NFKB was significantly prevented (Fig. 7A). Western blotting results showed that after 2 mM BHBA treatment, the expression of p-p38, NF-κB, and caspase-3 in low glucose-injured neurons were significantly decreased (p < 0.01) and p-ERK was significantly increased compared with those in the low-glucose group (p = 0.0006) (Fig. 7B). These results demonstrate that BHBA decreased neuronal apoptosis by mediating the p38 MAPK signaling pathway, inhibited the nucleus translocation of NF-κB, and caspase-3 apoptosis cascade.
BHBA reduced the expression of NF-κB and inhibited the activation of p38 MAPK in primary cortical neurons. A Representative images of immunostaining and quantitative analysis of MAP2/caspase-3 (n = 10 random fields per group) and NF-κB (n = 20 random fields per group). → : Inactive NF-κB proteins localization in the cytoplasm; triangle: the nucleus translocation of NF-κB. B Representative Western blotting and quantitative analysis of apoptosis-related proteins in the low glucose-injured neurons (n = 5 per group). C: control group; LG: low glucose group; BHBA: BHBA-treated group. *p < 0.05, **p < 0.01, and ***p < 0.001 relative to control; #p < 0.05, ##p < 0.01, and ###p < 0.001 relative to low glucose group. Scale bar: 50 μm
Discussion
The brain relies on glucose as the main metabolic substrate for systemic circulation. When hypoglycemia occurs and the blood glucose level is below 2.1 mM, the cerebral glucose level may drop to zero due to glucose consumption (Choi et al. 2001). Neurons use glucose as an energy source and are silenced at very low glucose concentrations. Glucose-sensing neurons respond sensitively to the changes in extracellular glucose concentration and modulate their firing rate (Stanley et al. 2019). Therefore, glucose concentration has very important effect on the regulation and survival of neurons. Hypoglycemia or glucose deprivation reduces the availability of glucose in the brain. The view on the mechanism and treatment of brain injury caused by hypoglycemia has been paid attention by the researchers in recent years. In the present study, we established a low glucose model and different concentrations of BHBA were given to investigate the protective effect of BHBA on low glucose-induced apoptosis of PC12 cells and primary cortical neurons as well as the potential mechanisms. Specifically, we found that the low glucose-induced apoptosis or death was associated with accumulation of the intracellular ROS. BHBA could alleviate low glucose-induced apoptosis by inhibiting the ROS-mediated p38 MAPK cascades and caspase-3 activation. In addition, BHBA was able to act as a signaling molecule to activate ERK phosphorylation that inhibits cell apoptosis. These findings provide insight into the cellular signaling and anti-apoptotic effect of BHBA in a glucose deficient condition, and suggest that BHBA has the potential to be a new candidate for the prevention or treatment of hypoglycemia.
Glucose is an important substrate for maintaining the balance of energy metabolism in the body. Hypoglycemia or glucose deprivation is extremely damaging to the body (Amiel 2021). It not only damages the brain, but also increases the risk of cardiovascular disease, leading to coma and death during a severe episode (AAmiel et al. 2019). In addition, the blood glucose content can regulate the glucose levels in the brain. The relation between brain glucose concentration and blood glucose level was linear within the physiological range (Choi et al. 2001). The concentration of glucose in brain extracellular fluid ranged from ~ 2.5 mM in feeding to 0.7 mM in fasting animals, which is much lower than that in the systemic circulation (McNay et al. 2001). Previous study showed that glucose diffusion is variable in different brain regions, resulting in different extracellular glucose concentrations across brain regions (Stanley et al. 2019). Glucose-sensing neurons, which can detect and respond to changes in blood sugar levels, have been described in multiple brain regions. Severe hypoglycemia can affect the response mechanism of glucose-sensing neurons, which leads to the up-regulation of glucose transport and the alteration of hypothalamus signal inducing consciousness disturbance (Stanley et al. 2019). The damage of hypoglycemia or glucose deprivation to neurons is mainly manifested in the decrease of cellular viability and intracellular ATP level (Lamichhane et al. 2017). The degeneration and even loss of granule cells in the dentate gyrus of hippocampus were observed in hypoglycemic animal models (Auer 2004). Moreover, different degrees of nonspecific neuronal necrosis were also observed in hippocampal CA1 and CA3 areas (Jackson et al. 2018). However, what is the optimal glucose concentration and duration to establish hypoglycemia model in vitro? According to the reports, when glucose level dropped to 1 mM, the hippocampal CA3 rhythm changed rapidly in cultured brain sections, which may lead to seizure generation (Florez et al. 2015). In our study, to elucidate the effect of BHBA on low glucose-injured cells, it is particularly important to establish the low glucose cell model. PC12 cells have been widely used in a variety of studies as a neuronal model in vitro. In the present study, PC12 cells cultured in HG-CM were used as control. Although the density of cells cultured in MG-CM was less than that in control group, the cells grew well and fewer apoptotic or death was observed. The density of cells cultured in LG-CM was greatly reduced with poor growth, and a large number of apoptotic or death. This result is consistent with Lamichhane’s observation that 1 mM glucose-containing medium was used to simulate glucose deprivation (Lamichhane et al. 2017). In addition, we found that OD value of MTT, intracellular ROS level, and proportion of PI-positive dead cells were significantly increased after cultured in LG-CM for 24–48 h. Previous study showed that the viability of PC12 cells decreased significantly after glucose deprivation for 24 h. At the same time, ATP levels decreased significantly but remained above 50%. However, ATP levels dropped to about 20% after glucose deprivation for 48 h (Liu et al. 2003). Our data showed that the mortality of cells cultured in low glucose was up to 31.5% at 48 h, which was disadvantage for the subsequent studies. Therefore, low glucose culturing for 24 h was adopted as the model of low glucose-injured cells for the following studies.
Ketone bodies (KB) are products of lipolysis, comprising BHBA, acetoacetate, and acetone, with BHBA being the most abundant (Achanta and Rae 2017). In the case of reduced glucose utilization, KB can provide up to 50%-70% of the energy to replace glucose as the energy substrate of the central nervous system, which can reduce the energy shortage caused by mitochondrial damage and protect the brain from hypoxia, ischemia (Tajima et al. 2019), hypoglycemia (Julio-Amilpas et al. 2015), excitatory intoxication (Ułamek-Kozioł et al. 2019), and other neurotoxic injuries (White and Venkatesh 2011). In the present study, we demonstrated that OD values of MTT test in the groups treated with 0.5–2 mM BHBA for 24–48 h were significantly higher than that in BHBA-free group. Therefore, a certain concentration of BHBA plays an important role in enhancing cells viability and alleviating low glucose-induced cells death. In addition to serving as an alternative energy source, BHBA is also involved as a signaling mediator in many cellular functions, regulating intracellular signaling, mitochondrial function, and oxidative stress (Puchalska and Crawford 2017). Hypoglycemia-induced brain injury is closely related to the production of reactive oxygen species (ROS) and oxidative stress. It has been suggested that ROS increases in different brain regions after severe hypoglycemia, especially in the hippocampus, the cerebral cortex, and the striatum (Amador-Alvarado et al. 2014). In the case of glucose deprivation, combination of glutamate with NMDA or AMPA receptor induces a large calcium influx, resulting in mitochondrial membrane depolarization, calcium overload, and ROS production. The intracellular accumulation of ROS and calcium leads to destruction of neuronal structure and neuronal death (Páramo et al. 2013). Consistent with the notion that the intracellular accumulation ROS after LG-CM treatment plays a role in mediating cells damage, we noted that the relative fluorescence intensity of ROS significantly increased after 24–48 h induction with low glucose, which was positively correlated with the amount of cell mortality. The intracellular ROS levels in the groups treated with 0.5–2 mM BHBA for 24 h were significantly decreased than that in BHBA-free group. Compared with that in control group, the intracellular ROS level in BHBA-free group was significantly increased, while treatment with BHBA had no difference. After 48 h of BHBA treatment in low glucose PC12 cell model, ROS level in the 2 mM BHBA treatment group was significantly lower than that in the BHBA-free group, and the relative fluorescence intensity of ROS in the 0–1 mM groups were significantly higher than that in the control group. Specifically, ROS levels in the 0–1 mM BHBA groups were approximately 3 times higher than those in the control group. The reason may be that we treated the cells with BHBA without changing the low glucose environment. With the extension of the treatment time with BHBA, the damage effect of low glucose became more obvious. Although BHBA has some extents beneficial effects, it is difficult to reverse persistent low glucose damage. Therefore, we considered that BHBA could reduce low glucose–induced cell apoptosis by alleviating oxidative stress at appropriate concentration and time. The present data showed that the low glucose–induced cell apoptosis and death can be reduced by 1–2 mM BHBA within 24 h. On this basis, 2 mM BHBA treatment for 24 h was used as the primary cortical neuron–related experiments.
The growth of neurite is an important process in brain development. The neurite connects with each other to form highly specialized networks that make synaptic connections with other neurons. Abnormal neurites growth and neuronal migration can lead to impaired brain function, resulting in cognitive and motor dysfunction (Pierozan et al. 2020). Our study found that BHBA significantly inhibited the reduction and shortening of neurites induced by low glucose. These results suggest that BHBA plays an important role in the maintenance of neural networks and protection of neuronal morphology and function during glucose deprivation. Previous studies have shown that c-Fos plays an important role in neuronal activation, synaptic plasticity, and neuronal apoptosis (Sheng and Greenberg 1990). The expression of c-Fos was significantly up-regulated in apoptotic hippocampal neurons (Wu et al. 2018). Consistent with these reports, we observed glucose deprivation can significantly increase the number of c-Fos+ cells and the rate of apoptosis. BHBA significantly decrease the number of c-Fos+ cells and improve survival rate of primary cortical neurons.
Previous reports have shown that low glucose induces mitochondrial ROS (mtROS) production, resulting in activation of NF-κB in endothelial cells (Nishikawa et al. 2000; Yoshinaga et al. 2021). ROS-mediated signaling pathways stimulate multiple transcription factors, such as Akt, p38, STAT, and NF-κB, which regulate the expression of proteins involved in cell survival or death (Chen et al. 2011). P38 MAPK can be activated by environmental factors, genotoxicity, and oxidative stress, and plays an important regulatory role in cell cycle arrest, apoptosis, growth inhibition, and differentiation (Sui et al. 2014). It has been demonstrated that ROS inhibitors can suppress the activation of p38 MAPK signaling pathway, thereby reduce the apoptosis induced by endoplasmic reticulum stress and oxidative stress (Shen et al. 2020; Qi et al. 2018). The present results showed that intracellular ROS level was significantly increased after cultured with LG-CM for 24–48 h in PC12 cells. Similarly, the ROS of the primary neurons induced by low glucose also increased compared with control group, while this increase was higher that PC12 cells. This is probably that cortical neurons are more sensitive to glucose deprivation. Importantly, a certain concentration of BHBA can significantly reduce intracellular ROS level and apoptosis rate under low glucose condition. Then, does BHBA also play an anti-apoptosis role by inhibiting ROS-mediated p38 MAPK signaling pathway? To further explore the molecular mechanism of BHBA inhibiting low glucose-induced apoptosis, we examined the expression of p38, p-p38, NF-κB, and caspase-3 by using immunofluorescence or western blot. The results showed that BHBA effectively inhibited the activation of p38, p-p38, and NF-κB by blocking low glucose-induced accumulation of intracellular ROS. In conclusion, BHBA mediates the transcriptional regulation of nuclear NF-κB by inhibiting p38 phosphorylation, thus exerting an anti-apoptosis role. Under the low glucose condition, ROS production increased, that altered redox state of cells and triggered the downstream apoptotic cascade of caspase activation (Moley and Mueckler 2000). Caspase-3 has been identified as a key mediator of inflammation and oxidative stress-induced apoptosis. It has been reported that decreasing caspase-3 activity can reduce inflammation, oxidative stress, and apoptosis (Kuo et al. 2019; Broughton et al. 2009). Inhibition of ROS production reduced cleaved caspase-3 levels and the proportion of apoptosis in cultured astrocytes (Ma et al. 2017). As an energy metabolite, BHBA is increasingly recognized to have cellular signaling functions. The utilization of BHBA increases the efficiency of ATP production from the mitochondrial proton gradient, which affecting mitochondrial redox status by reducing the production of free radicals. Our data are consistent with the notion that BHBA inhibited low glucose-induced ROS production and thus alleviated apoptosis, and that this effect appeared to be related to mtROS-mediated p38 MAPK signaling pathway and caspase-3 apoptosis cascade. We hypothesize that low glucose-induced apoptosis is caused by intracellular ROS accumulation, which activates p38 MAPK signaling pathway and nuclear NF-κB. On the other hand, the accumulation of ROS can directly stimulate the increase of caspase-3, and then induce apoptosis. BHBA directly inhibits ROS activation and plays an anti-apoptotic role.
In addition to inhibiting ROS-mediated activation of the p38 MAPK apoptotic pathway, cell survival also requires active apoptosis inhibition. ERK is closely related to cell survival, proliferation, differentiation, and death. Multiple studies have found that activation of the Raf1/ERK and PI3-kinase/Akt signaling pathways induces anti-apoptotic and protects brain from ischemic stroke (Uzdensky 2019). Erythropoietin mediates the activation of JAK2, ERK1/2, and Akt pathways by increasing neuronal Bcl-XL and decreasing NO synthetase levels in ischemic brain. ERK1/2 and Akt pathways inhibit Bad function and activate pro-survival signaling pathways after cerebral ischemia (Kilic et al. 2005). Activation of Akt, ERK1/2, and AMPK inhibits the apoptotic cascade in nutrient-deficient PC12 cells (Sánchez-Temprano et al. 2021). Lamichhane’s research found that BHBA increased the survival rate and ERK phosphorylation of SH-SY5Y cells in a glucose deficient condition (Lamichhane et al. 2017). Consistent with the notion that ERK plays a role in mediating anti-apoptotic and pro-survival, we observed that BHBA concentration in medium under the low glucose condition was positively correlated with the level of ERK phosphorylation in PC12 cells. Recently, reports have shown that ERK is closely related to inflammatory and cell death (Medina et al. 2005; Wesley et al. 2021). However, our results suggest that BHBA as a signaling molecule activate ERK and inhibit apoptosis. As a complex regulatory network, the role of ERK signaling pathway in hypoglycemia-induced cell injury needs to be further studied.
Conclusions
In summary, the present study provides the evidence that BHBA alleviates low glucose-induced apoptosis of PC12 cells and primary cortical neurons. The results showed that BHBA decreases the accumulation of intracellular ROS, and further inhibited cell apoptosis by mediating the p38 MAPK signaling pathway and caspase-3 apoptosis cascade. Moreover, BHBA inhibited apoptosis by activating ERK phosphorylation and alleviated the damage of low glucose (Fig. 8). This study may shed light on a previously little-explored aspect of BHBA neuroprotection and elucidate the potential related mechanism in a glucose-deficient condition.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Abbreviations
- LG-CM:
-
Low glucose complete medium
- MG-CM:
-
5 MM medium glucose complete medium
- HG-CM:
-
25 MM high glucose complete medium
- BHBA:
-
β-Hydroxybutyrate
- ROS:
-
Reactive oxygen species
- CNS:
-
Central nervous system
- AD:
-
Alzheimer’s disease
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- PI:
-
Propidium iodide
- MTT:
-
Methylthiazolyldiphenyl-tetrazolium bromide.
References
AAmiel S, Aschner P, Childs B et al (2019) Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabet Endocrinol 7:385–396. https://doi.org/10.1016/s2213-8587(18)30315-2
Achanta LB, Rae CD (2017) β-Hydroxybutyrate in the Brain: One Molecule. Multiple Mech Neurochem Res 42:35–49. https://doi.org/10.1007/s11064-016-2099-2
Amador-Alvarado L, Montiel T, Massieu L (2014) Differential production of reactive oxygen species in distinct brain regions of hypoglycemic mice. Metab Brain Dis 29:711–719. https://doi.org/10.1007/s11011-014-9508-5
Amiel SA (2021) The consequences of hypoglycaemia. Diabetologia 64:963–970. https://doi.org/10.1007/s00125-020-05366-3
Auer RN (2004) Hypoglycemic brain damage. Metab Brain Dis 19:169–175. https://doi.org/10.1023/b:mebr.0000043967.78763.5b
Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40:e331-339. https://doi.org/10.1161/strokeaha.108.531632
Chen H, Yoshioka H, Kim GS et al (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14:1505–1517. https://doi.org/10.1089/ars.2010.3576
Cheng B, Lu H, Bai B et al (2013) d-β-Hydroxybutyrate inhibited the apoptosis of PC12 cells induced by H2O2 via inhibiting oxidative stress. Neurochem Int 62:620–625. https://doi.org/10.1016/j.neuint.2012.09.011
Choi IY, Lee SP, Kim SG et al (2001) In vivo measurements of brain glucose transport using the reversible Michaelis-Menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. J Cereb Blood Flow Metab 21:653–663. https://doi.org/10.1097/00004647-200106000-00003
Dąbek A, Wojtala M, Pirola L et al (2020) Modulation of cellular biochemistry, epigenetics and metabolomics by ketone bodies. Implications of the ketogenic diet in the physiology of the organism and pathological states. Nutrients. 12. https://doi.org/10.3390/nu12030788
De Angelis LC, Brigati G, Polleri G et al (2021) Neonatal hypoglycemia and brain vulnerability Front Endocrinol (lausanne) 12 634305. https://doi.org/10.3389/fendo.2021.634305
Florez CM, Lukankin V, Sugumar S et al (2015) Hypoglycemia-induced alterations in hippocampal intrinsic rhythms: decreased inhibition, increased excitation, seizures and spreading depression. Neurobiol Dis 82:213–225. https://doi.org/10.1016/j.nbd.2015.06.005
Fu SP, Wang JF, Xue WJ et al (2015) Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J Neuroinflammation 12:9. https://doi.org/10.1186/s12974-014-0230-3
Green DR, Llambi F (2015) Cell death signaling. Cold Spring Harb Perspect Biol 7. https://doi.org/10.1101/cshperspect.a006080
Isaev NK, Stel’mashuk EV, Zorov DB (2007) Cellular mechanisms of brain hypoglycemia. Biochemistry (mosc) 72:471–478. https://doi.org/10.1134/s0006297907050021
Jackson DA, Michael T, Vieira de Abreu A et al (2018) Prevention of severe hypoglycemia-induced brain damage and cognitive impairment with verapamil. Diabetes 67:2107–2112. https://doi.org/10.2337/db18-0008
Julio-Amilpas A, Montiel T, Soto-Tinoco E et al (2015) Protection of hypoglycemia-induced neuronal death by β-hydroxybutyrate involves the preservation of energy levels and decreased production of reactive oxygen species. J Cereb Blood Flow Metab 35:851–860. https://doi.org/10.1038/jcbfm.2015.1
Kauppinen TM, Chan WY, Suh SW et al (2006) Direct phosphorylation and regulation of poly(ADP-ribose) polymerase-1 by extracellular signal-regulated kinases 1/2. Proc Natl Acad Sci U S A 103:7136–7141. https://doi.org/10.1073/pnas.0508606103
Kilic E, Kilic U, Soliz J et al (2005) Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. Faseb j 19:2026–2028. https://doi.org/10.1096/fj.05-3941fje
Kittah NE, Vella A (2017) Management of endocrine disease: pathogenesis and management of hypoglycemia. Eur J Endocrinol 177:R37-r47. https://doi.org/10.1530/eje-16-1062
Kuo WT, Shen L, Zuo L et al (2019) Inflammation-induced occludin downregulation limits epithelial apoptosis by suppressing caspase-3 expression. Gastroenterology 157:1323–1337. https://doi.org/10.1053/j.gastro.2019.07.058
Lacy ME, Gilsanz P, Eng C et al (2020) Severe hypoglycemia and cognitive function in older adults with type 1 diabetes: the study of longevity in diabetes (SOLID). Diabetes Care 43:541–548. https://doi.org/10.2337/dc19-0906
Lamichhane S, Bastola T, Pariyar R et al (2017) ROS production and ERK activity are involved in the effects of d-β-hydroxybutyrate and metformin in a glucose deficient Condition Int J Mol Sci 18. https://doi.org/10.3390/ijms18030674
Liu Y, Song XD, Liu W et al (2003) Glucose deprivation induces mitochondrial dysfunction and oxidative stress in PC12 cell line. J Cell Mol Med 7:49–56. https://doi.org/10.1111/j.1582-4934.2003.tb00202.x
Ma YL, Zhang LX, Liu GL et al (2017) N-Myc Downstream-regulated gene 2 (Ndrg2) Is Involved in ischemia-hypoxia-induced Astrocyte Apoptosis: a Novel Target for Stroke Therapy. Mol Neurobiol 54:3286–3299. https://doi.org/10.1007/s12035-016-9814-5
Maalouf M, Sullivan PG, Davis L et al (2007) Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience 145:256–264. https://doi.org/10.1016/j.neuroscience.2006.11.065
McNay EC, McCarty RC, Gold PE (2001) Fluctuations in brain glucose concentration during behavioral testing: dissociations between brain areas and between brain and blood. Neurobiol Learn Mem 75:325–337. https://doi.org/10.1006/nlme.2000.3976
Medina MG, Ledesma MD, Domínguez JE et al (2005) Tissue plasminogen activator mediates amyloid-induced neurotoxicity via Erk1/2 activation. Embo j 24:1706–1716. https://doi.org/10.1038/sj.emboj.7600650
Mohseni S (2001) Hypoglycemic neuropathy. Acta Neuropathol 102:413–421. https://doi.org/10.1007/s004010100459
Moley KH, Mueckler MM (2000) Glucose transport and apoptosis. Apoptosis 5:99–105. https://doi.org/10.1023/a:1009697908332
Najm NA, Zimmermann L, Dietrich O et al (2020) Associations between motion activity, ketosis risk and estrus behavior in dairy cattle. Prev Vet Med 175:104857. https://doi.org/10.1016/j.prevetmed.2019.104857
Newman JC, Verdin E (2017) β-Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr 37:51–76. https://doi.org/10.1146/annurev-nutr-071816-064916
Nishikawa T, Edelstein D, Du XL et al (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790. https://doi.org/10.1038/35008121
Páramo B, Montiel T, Hernández-Espinosa DR et al (2013) Calpain activation induced by glucose deprivation is mediated by oxidative stress and contributes to neuronal damage. Int J Biochem Cell Biol 45:2596–2604. https://doi.org/10.1016/j.biocel.2013.08.013
Pierozan P, Cattani D, Karlsson O (2020) Hippocampal neural stem cells are more susceptible to the neurotoxin BMAA than primary neurons: effects on apoptosis, cellular differentiation, neurite outgrowth, and DNA methylation. Cell Death Dis 11:910. https://doi.org/10.1038/s41419-020-03093-6
Puchalska P, Crawford PA (2017) Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 25:262–284. https://doi.org/10.1016/j.cmet.2016.12.022
Qi S, Feng Z, Li Q et al (2018) Inhibition of ROS-mediated activation Src-MAPK/AKT signaling by orientin alleviates H(2)O(2)-induced apoptosis in PC12 cells. Drug Des Devel Ther 12:3973–3984. https://doi.org/10.2147/dddt.S178217
Rehni AK, Dave KR (2018) Impact of hypoglycemia on brain metabolism during diabetes. Mol Neurobiol 55:9075–9088. https://doi.org/10.1007/s12035-018-1044-6
Sánchez-Temprano A, Relova JL, Camiña JP et al (2021) Concurrent Akt, ERK1/2 and AMPK activation by obestatin inhibits apoptotic signaling cascades on nutrient-deprived PC12 cells. Cell Mol Neurobiol. Epub ahead of print 2021/01/06. DOI: https://doi.org/10.1007/s10571-020-01025-8
Shen T, Huang Z, Shi C et al (2020) Pancreatic cancer-derived exosomes induce apoptosis of T lymphocytes through the p38 MAPK-mediated endoplasmic reticulum stress. Faseb J 34:8442–8458. https://doi.org/10.1096/fj.201902186R
Sheng M, Greenberg ME (1990) The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 4:477–485. https://doi.org/10.1016/0896-6273(90)90106-p
Shimazu T, Hirschey MD, Newman J et al (2013) Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339:211–214. https://doi.org/10.1126/science.1227166
Shippy DC, Wilhelm C, Viharkumar PA et al (2020) β-hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J Neuroinflammation 17:280. https://doi.org/10.1186/s12974-020-01948-5
Stanley S, Moheet A, Seaquist ER (2019) Central mechanisms of glucose sensing and counterregulation in defense of hypoglycemia. Endocr Rev 40:768–788. https://doi.org/10.1210/er.2018-00226
Sui X, Kong N, Ye L et al (2014) p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett 344:174–179. https://doi.org/10.1016/j.canlet.2013.11.019
Tajima T, Yoshifuji A, Matsui A et al (2019) β-hydroxybutyrate attenuates renal ischemia-reperfusion injury through its anti-pyroptotic effects. Kidney Int 95:1120–1137. https://doi.org/10.1016/j.kint.2018.11.034
Ułamek-Kozioł M, Czuczwar SJ, Januszewski S et al (2019) Ketogenic diet and epilepsy Nutrients 11. https://doi.org/10.3390/nu11102510
Uzdensky AB (2019) Apoptosis regulation in the penumbra after ischemic stroke: expression of pro- and antiapoptotic proteins. Apoptosis 24:687–702. https://doi.org/10.1007/s10495-019-01556-6
Wang S, Li X, Zhang Q et al (2020) Nyap1 regulates multipolar-bipolar transition and morphology of migrating neurons by fyn phosphorylation during corticogenesis. Cereb Cortex 30:929–941. https://doi.org/10.1093/cercor/bhz137
Wesley UV, Sutton IC, Cunningham K et al (2021) Galectin-3 protects against ischemic stroke by promoting neuro-angiogenesis via apoptosis inhibition and Akt/Caspase regulation. J Cereb Blood Flow Metab 41:857–873. https://doi.org/10.1177/0271678x20931137
White H, Venkatesh B (2011) Clinical review: ketones and brain injury. Crit Care 15:219. https://doi.org/10.1186/cc10020
Wiatrak B, Kubis-Kubiak A, Piwowar A et al (2020) PC12 cell line: cell types, coating of culture vessels, differentiation and other culture conditions Cells 9. https://doi.org/10.3390/cells9040958
Wu DM, Zhang YT, Lu J et al (2018) Effects of microRNA-129 and its target gene c-Fos on proliferation and apoptosis of hippocampal neurons in rats with epilepsy via the MAPK signaling pathway. J Cell Physiol 233:6632–6643. https://doi.org/10.1002/jcp.26297
Wu L, Xiong X, Wu X et al (2020a) Targeting oxidative stress and inflammation to prevent ischemia-reperfusion injury. Front Mol Neurosci 13:28. https://doi.org/10.3389/fnmol.2020.00028
Wu Y, Gong Y, Luan Y et al (2020b) BHBA treatment improves cognitive function by targeting pleiotropic mechanisms in transgenic mouse model of Alzheimer’s disease. Faseb j 34:1412–1429. https://doi.org/10.1096/fj.201901984R
Yao Y, Cui L, Ye J et al (2020) Dioscin facilitates ROS-induced apoptosis via the p38-MAPK/HSP27-mediated pathways in lung squamous cell carcinoma. Int J Biol Sci 16:2883–2894. https://doi.org/10.7150/ijbs.45710
Yoshinaga A, Kajihara N, Kukidome D et al (2021) Hypoglycemia induces mitochondrial reactive oxygen species production through increased fatty acid oxidation and promotes retinal vascular permeability in diabetic mice. Antioxid Redox Signal 34:1245–1259. https://doi.org/10.1089/ars.2019.8008
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 31802154), National Key Research and Development Program of China (2018YFE0127000), the China Postdoctoral Science Foundation funded project (No. 2019T120957), Shaanxi Provincial Regional Innovation Capability Guiding Plan Project (No. 2020QFY10-04), and General Project of Basic Research of Shaanxi Province (No. 2021JM-492).
Author information
Authors and Affiliations
Contributions
Cixia Li: writing — original draft, methodology, investigation, data curation; Xuejun Chai: writing — review and editing, validation, investigation; Jiarong Pan: methodology, visualization, investigation, data curation, Jian Huang: validation, investigation; Yongji Wu: methodology, investigation; Yuhuan Xue: investigation; Wentai Zhou: data curation; Jiping Yang: methodology; Xiaoyan Zhu: conceptualization, writing — review and editing, funding acquisition, supervision; Shanting Zhao: writing — review and editing, funding acquisition, resources, project administration.
Corresponding authors
Ethics declarations
Ethics Approval
No human studies were carried out by the authors for this article. This article does not contain any studies with human participants performed by any of the authors.
Consent to Participate
Informed consent was obtained for each participant.
Consent for Publication
All participants consented to publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, C., Chai, X., Pan, J. et al. β-Hydroxybutyrate Alleviates Low Glucose–Induced Apoptosis via Modulation of ROS-Mediated p38 MAPK Signaling. J Mol Neurosci 72, 923–938 (2022). https://doi.org/10.1007/s12031-022-01974-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-022-01974-3