Abstract
Evidence has shown that the activation of the autophagy pathway after experimental subarachnoid hemorrhage (SAH) protects against neuronal damage. Tert-butylhydroquinone (tBHQ), a commonly used nuclear factor erythroid 2-related factor 2 (Nrf2) activator, was found to significantly enhance autophagy activation. The aim of this study was to explore the effect of tBHQ treatment on early stage brain injury at 24 h after SAH. The results showed that tBHQ treatment failed to stimulate an effective anti-oxidative effect at 24 h after the SAH operation, but succeeded in ameliorating early brain injury, including alleviated brain edema, BBB disruption, neuronal degeneration and neurological deficits. Further exploration found that tBHQ treatment significantly increased the expression of Beclin-1 and the ratio of microtubule-associated protein 1 light chain 3 (LC3)-II to LC3-I, suggesting that autophagy was enhanced after tBHQ treatment. Moreover, tBHQ treatment restored Bcl-2 and Bax expression and reduced caspase-3 cleavage, suggesting the protective effect of tBHQ treatment in ameliorating brain injury after SAH. Furthermore, tBHQ enhanced autophagy activation, decreased neuronal degeneration and improved the neurological score after SAH in Nrf2-deficient mice. Taken together, these findings suggest that tBHQ treatment exerts neuro-protective effects against EBI following SAH by enhancing Nrf2-independent autophagy. Therefore, tBHQ is a promising therapeutic agent against EBI following SAH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is a devastating disease with high mortality and morbidity, which not only causes the immediate death of 25 % of patients, but also results in persistent cognitive, memory and functional impairments [1]. Recently, early brain injury (EBI) following SAH has been paid much attention, because EBI was suggested to contribute more to the poor outcomes than cerebral vasospasm [2]. Thus, efficient therapeutics and management for EBI is significantly important in ameliorating the serious consequences of SAH. Tert-butylhydroquinone (tBHQ), an Nrf2 activator, is an effective therapeutic molecule against secondary brain injury that is induced by traumatic brain injury (TBI), cerebral ischemia–reperfusion (CIR) and SAH via anti-oxidation or anti-inflammation [3–8]. Nonetheless, tBHQ is found to protect mouse hepatic cells from injury by enhancing the Nrf2-independent autophagy pathway [9]. Interestingly, enhanced autophagy activity is suggested to be beneficial in EBI after SAH [10–15]. Moreover, autophagy pathway activation is critical for accelerating the degradation of an Nrf2 inhibitor, kelch-like ECH-associated protein 1 (Keap1), and promoting Nrf2 and Nrf2-derived anti-oxidative enzyme activation [9, 16–19]. In this present study, we explored the effect of tBHQ treatment on the early SAH-induced secondary injury in mice.
Materials and Methods
Animals
All experimental protocols were approved by the Animal Care and Use Committee of Nanjing University and followed the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. One hundred thirty male wild type ICR mice and 46 male Nrf2-deficient mice (all weighted 30–35 g) from the Animal Center of Jinling Hospital (Nanjing, China) were used. The Nrf2-deficient mice were kindly provided by Dr. Thomas W. Kensler (Johns Hopkins University. Baltimore, MD, USA), and were bred in the Animal Center of Jinling Hospital and genetically identified by reverse transcription-polymerase chain reaction (RT-PCR) before the experiment, as described in our previous studies [20]. The primers used in RT-PCR applications are present in Supplementary Table 1. The PCR results were shown in Supplemental Fig. 1. Homozygous Nrf2 knockout mice were used in this study. The mice were housed with free accessing to food and water under environmentally controlled conditions (12/12 h light/dark cycle with a temperature of approximately 25 °C).
Experimental Design and Drug Administration
A total of 50 mg of tBHQ (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in dimethyl sulfoxide to a final concentration of 4 mg/mL in 1 % DMSO-saline. tBHQ was intraperitoneally (i.p.) injected at a dose of 50 mg/kg (12.5 ml/kg) divided into three injections at intervals of 8 h, and began immediately after SAH. In accordance with administration of tBHQ, 1 % DMSO-saline was given i.p. as a vehicle. This dose and route of tBHQ administration has been used in our previous study on TBI [8]. Because there were no significant differences in any of the measurement between the SAH and SAH + vehicle group in the wild type mice and considering the 3Rs (reduction, replacement, refinement) principle, we arranged the Nrf2-deficient mice into three groups for western blotting detection and two groups for the EBI test. All the animals were euthanized at 24 h after SAH.
Subarachnoid Hemorrhage Model
The subarachnoid hemorrhage was produced by injecting autologous blood into the pre-chiasmatic cistern according to Sabri’s instructions and our previous study [20–22]. Briefly, the mice were anesthetized by i.p. injection of chloral hydrate (350 mg/kg). An incision was made in the right inguinal region after disinfection with 0.5 % iodine. Aided by a surgical microscope, the femoral artery was isolated and punctured using a 100 μl Hamilton microsyringe. Moreover, 100 μl of autologous blood was withdrawn from the slowly outflowing blood, hemostasis was quickly established using sterile soluble hemostatic gauze and the wounds were quickly sutured. Then, an incision was made in the midline of the anterior scalp to expose the transparent skull. A mini hole was drilled 40° caudally in the skull at 5 mm anterior to the bregma, the microsyringe was advanced to approximately 4 mm through the burr at 40° caudally. For the SAH and vehicle group, 100 μl of blood was injected into the skull base within 15 s, while the sham group animals underwent exactly the same procedure as described above, but with no blood injected. The syringe was retained for about 5 min before removal, and the hole was plugged with bone wax. After suturing the wound, 150 μl of saline was injected into the peritoneal cavity immediately after the operation. Then, the mice were returned to their cages in a temperature-controlled room.
Tissue Preparation
The brain samples were harvested at 24 h after the SAH operation. Briefly, after deep anesthesia with chloral hydrate (400 mg/kg, i.p.), the mice were exsanguinated by intracardial perfusion with 0.9 % saline (0–4 °C) and quickly decapitated. For the molecular biological and biochemical measurements, the cortex sample from both temporal lobes was removed and rinsed in 0.9 % normal saline (0–4 °C) several times to wash away blood clots and then frozen in liquid nitrogen until use. For Fluoro-Jade b staining (FJb), the mice were anesthetized and intracardially perfused with 0.9 % saline (0–4 °C) followed by 4 % paraformaldehyde (0–4 °C). Then mice were decapitated, and the obtained tissue samples were immediately frozen in liquid nitrogen, and then transferred to a −80 °C freezer until use.
Measurement of Glutathione Peroxidase (GPx) and Superoxide Dismutase (SOD) Activities
To investigate the anti-oxidant effect of tBHQ treatment at 24 h after SAH, the GPx and SOD activities were measured using a commercially available kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturers’ instructions and our previous study [8, 23–25]. Briefly, at 24 h after SAH, the inferior basal temporal lobe tissue (about 50 μg from both temporal lobes) were removed from the mice and homogenized in nine volumes (grams per liter) of ice-cold saline for 10 min using a Dounce Tissue Grinder (Kimble and Kontes, Vineland, NJ, USA). The samples were centrifuged at 12,000g for 10 min at 4 °C. The supernatant was used to measure the GPx and SOD activities using a spectrophotometer. The total protein concentrations were determined by the Bradford method. The results were expressed as units per milligram of protein in relation to the prepared standards.
Brain Edema and BBB Disruption
Brain edema was investigated by detection of the brain water content. At 24 h after SAH operation, the mice were perfused with ice-cold saline after being deeply anesthetized. The cerebral hemispheres of the forebrain were quickly isolated, separated and weighed (wet weight). The brain specimens were then dried in an oven at 85 °C for 72 h and weighed again (dry weight). The percentage of water content was calculated as follows: (wet weight-dry weight)/wet weight.
BBB disruption was investigated using Evans blue extravasation. At 21 h after the SAH operation, Evans blue dye (2 %, 4 ml/kg of body weight) was injected i.p. and allowed to absorb for 3 h. Then, the mice were deeply anesthetized and perfused with ice-cold saline. The temporal lobe tissues were collected, weighed, homogenized in PBS, and centrifuged at 15,000g for 30 min. A 0.5 ml sample of the resulting supernatant was added to an equal volume of 50 % trichloroacetic acid. After incubation overnight and centrifugation at 15,000g for 30 min at 4 °C, the supernatant was used for spectrophotometric quantification at 610 nm. The Evans blue content was expressed as μg per gram of protein.
Fluoro-Jade b (FJb) Staining
Neuronal degeneration was evaluated by FJb staining, and the procedure was performed according to the manufacturer’s protocol and previous studies [26, 27]. Briefly, 10-μm thick frozen cryostat sections mounted on gelatin-coated slides were prepared. The slides bearing the frozen tissue sections were first immersed in a basic alcohol solution consisting of 1 % sodium hydroxide in 80 % ethanol for 5 min. They were then rinsed for 2 min in 70 % ethanol, for 2 min in distilled water, and then incubated in a 0.06 % potassium permanganate solution for 10 min. Following a 1–2 min water rinse, the slides were then transferred to a 0.0004 % solution of FJb (Millipore, USA) dissolved in 0.1 % acetic acid vehicle for 10 min. The slides were then rinsed through three changes of distilled water for 1 min each. Then, the slides were air-dried, cover slipped and visualized using a microscope system. Microscopic examination of the stained tissue sections was performed by a pathologist blinded to the experimental groups. The extent of brain damage was evaluated by the number of FJb-positive cells in 10 fields (at 200× magnification) that were restricted to the temporal cortex in every coronal section.
Neurological Function Assessments
Due to the implementation of the 3Rs principle to reduce the number of animals and expand our testing sample, the neurobehavioral examination was performed at 21 h after the SAH operation using the Garcia scoring system, as previously described [28–31]. The evaluation consists of six tests that can be scored from 0 to 3 or 1 to 3. These six tests include: spontaneous activity, symmetry in the movement of all four limbs, forelimb outstretching, climbing, side stroking, and the response to vibrissae touch (Supplemental Table 2). The animals were given a score of 3–18 in each step (higher scores indicate better function).
Western Blotting Analysis
Briefly, the frozen brain samples were homogenized in extract buffer with protease inhibitors (PMSF) and centrifuged at 12,000g for 15 min at 4 °C. Then, the protein concentrations were quantified using the Bradford Assay. Equal amounts of proteins were placed in each lane of the SDS-PAGE (10 % for Beclin-1, 12 % for Bcl-2 and Bax, and 15 % for LC3 and cleaved caspase-3), electrophoresed, and then transferred to a PVDF membrane (Millipore, USA). The membrane was blocked with 5 % non-fat milk for 2 h at room temperature, and then incubated overnight at 4 °C with primary antibodies against Beclin-1 (MW 53 kDa, 1:1000 dilution, CST, USA), LC3 (MW 14/16 kDa, 1:1000 dilution; Novus, USA), cleaved caspase-3 (MW 17 kDa, 1:1000 dilution; Novus, USA), Bcl-2 (MW 26 kDa, 1:1000 dilution, Abcam, USA), Bax (MW 20 kDa, 1:1000 dilution, Abcam, USA), and β-actin (MW 43 kDa, 1:5000 dilution; Bioworld, USA). Then, the membranes were washed with TBST for 3 × 10 min, and, subsequently, were incubated with a goat anti-rabbit IgG-horseradish (1:5000, Bioworld, USA) secondary antibody for 2 h at room temperature. Next, the membranes were washed with TBST again for 3 × 10 min. The blotted protein bands were visualized by ECL Western blot detection reagents (Millipore, USA), and were exposed to X-ray film (Kodak, China). β-actin was employed as a loading control.
Statistical Analysis
The data are expressed as the mean ± SD. The statistical differences between the controls and the other groups were compared by one-way ANOVA, and the differences between groups were determined by Bonferroni analysis. All of the data were analyzed by SPSS 16.0. Statistical significance was inferred at P < 0.05.
Results
SAH Model and Mortality
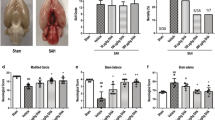
The temperature and arterial blood injection were strictly controlled throughout the experimental process. The mice that died during anesthesia or the blood injection were not included in the mortality calculations. The mortality rate in each group was 10 %, and no significant differences in mortality were found between the SAH, SAH + vehicle and SAH + tBHQ groups of wild type mice (P > 0.05, data not shown), as well as between the SAH + vehicle and SAH + tBHQ groups of Nrf2-deficient mice. None of the animals in the sham group died. General observations of the SAH mice brains and the inferior basal temporal lobe tissue taken for analysis were shown in Fig. 1.
The tBHQ Treatment Attenuated Brain Edema and the Blood–Brain Barrier Disruption at 24 h After SAH
The brain water content, an indicator of brain edema, was measured to confirm the protective effect of tBHQ at the macroscopic level. As shown in Fig. 2a, compared to the sham group, the SAH and SAH + vehicle groups showed a significant increase in the brain water content (P < 0.05), while the tBHQ-treated group showed a significant restoration in brain water content compared to the vehicle group (P < 0.05). There was no significant difference between the SAH and vehicle groups (P > 0.05). Therefore, tBHQ showed protective effects against SAH-induced brain edema.
Effect of the tBHQ treatment on the brain water content and BBB permeability. a The brain water content is significantly increased in the SAH and Vehicle groups, and the tBHQ treatment reduced this increase. b The Evans blue dye extraction is significantly increased in the SAH and Vehicle groups, while the tBHQ treatment reversed this increase (n = 6 for each group. The data represent the mean ± SD. *P < 0.05 versus the sham group; # P < 0.05 versus the SAH + vehicle group)
Blood–brain barrier (BBB) disruption or dysfunction may lead to abnormal vascular permeability for macromolecules. Therefore, we measured the cerebral Evans blue extravasation to assess the severity of the BBB damage. As is shown in Fig. 2b, extravasation of Evans blue dye was significantly increased compared to the sham group at 24 h in the SAH and SAH + vehicle group (P < 0.05). Extravasation was significantly restored in the tBHQ-treated group (P < 0.05). There were no significant differences between the SAH and vehicle groups (P > 0.05). Hence, tBHQ ameliorated the BBB dysfunction.
The tBHQ Treatment Alleviated Neuronal Degeneration at 24 h After SAH
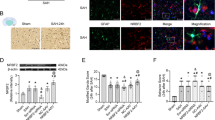
Fluoro-Jade b (FJb) staining is a method to explore neuronal degeneration. As shown in Fig. 3a, a very small amount of FJb-positive neurons were found in the brains of the mice in the sham group (a). Compared to the sham group, the number of FJb-positive cells was significantly increased in the SAH and SAH + vehicle group (b and c, P < 0.05), while the tBHQ treatment reduced the number of FJb-positive neurons in the cortex compared to the SAH + vehicle group (d, P < 0.05). There was no statistically significant difference between the SAH group and the vehicle group (P > 0.05).The result showed that the tBHQ treatment reduced neuronal degeneration in the cortex after SAH.
FJb staining. A The FJb staining shows rare degenerating neurons in the cortex in the sham group (a), while obvious FJb-positive neurons are observed in the SAH (b) and SAH + vehicle groups (c). Fewer FJb-positive neurons appeared in the tBHQ-treated group (d). B Quantification of the number of FJb-positive neurons in (A) (n = 6 for each group. The data represent the mean ± SD. *P < 0.05 versus the sham group; # P < 0.05 versus the SAH + vehicle group. Scale bar 20 μm)
The tBHQ Treatment Ameliorated the Neurological Deficits at 24 h After SAH
The neurological deficits were evaluated by the behaviors and neurological function of the mice. As is shown in Fig. 4, the neurological scores observed in the SAH and SAH + vehicle group were significantly lower than the sham group (P < 0.01), whereas a small improvement of neurological outcomes was observed in the tBHQ-treated group. Thus, tBHQ is effective in improving the SAH-induced neurological deficits in mice.
Neurological evaluation. The neurological scores in the SAH and SAH + vehicle groups were significantly lower than the sham group, and were partially restored by the tBHQ treatment (n = 6 for each group. The data represent the mean ± SD. *P < 0.05 versus the sham group; # P < 0.05 versus the SAH + vehicle group)
The tBHQ Treatment Failed to Increase the Antioxidant Capacity at 24 h After SAH in the Wild Type Mice
Because tBHQ is a commonly used Nrf2 activator that exhibited potent anti-oxidant properties in previous studies [5, 8], we measured the GPx and SOD activities to investigate the antioxidant effect of tBHQ at 24 h after tBHQ treatment following the SAH operation. As shown in Fig. 5, SAH induced a significant decrease in both the GPx and SOD activities (P < 0.01). However, the tBHQ treatment did not statistically increase the GPx and SOD activities at 24 h after SAH (P > 0.05). Thus, tBHQ failed to provide anti-oxidant neuro-protection in a relatively short time after treatment.
Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity levels. Both the GPx (a) and SOD (b) activities in the SAH and SAH + vehicle groups were significantly lower than the sham group. Although they were partially restored by the tBHQ treatment, there were no significant differences compared to the SAH or SAH + vehicle group. n = 6 for each group. The data represent the mean ± SD. *P < 0.05 versus the sham group
The tBHQ Treatment Enhanced Autophagic Marker Expression at 24 h After SAH in Wild Type Mice
Autophagy, a cellular process that recycles cellular constituents, was suggested to be neuro-protective against EBI after SAH [11, 14, 32]. As shown in Fig. 6a, western blots showed that the expression of Beclin-1 and the ratio of the conversion of light chain-3 II (LC3-I) to the light chain-3 I (LC3-I) were significantly increased after SAH. Moreover, SAH-induced autophagy activation in the SAH and SAH + vehicle group was further enhanced by the tBHQ treatment, as indicated by the additional increase in Beclin-1 expression and the LC3-II/LC-3I ratio. There was no significant difference between the SAH group and the vehicle-treated group (P > 0.05).
Expression of the autophagy-related proteins Beclin-1 and LC3 and apoptotic regulatory factors. a Western blot detection of the autophagy markers Beclin-1 and LC3. b Quantification of the expression of Beclin-1 and the LC3-II/LC3-I ratio in (a). Increased expression of Beclin-1 and the LC3-II/LC3-I ratio in the SAH and SAH + vehicle groups, and an additional increase in the expression of Beclin-1 and the LC3-II/LC3-I ratio in the SAH + tBHQ group were detected in the temporal cortex of mice after SAH. c Western blot of apoptotic regulatory factors Bcl-2, Bax and cleaved caspase-3. d Quantification of the expression of Bcl-2, Bax and cleaved caspase-3 in (c). Decreased expression of Bcl-2 and increased expression of Bax and cleaved caspase-3 were observed in the SAH and SAH + vehicle groups, while the expression of Bcl-2, Bax and cleaved caspase-3 were partially restored after the tBHQ treatment (n = 6 for each group. The data represent the mean ± SD. *P < 0.05 versus the sham group; # P < 0.05 versus the SAH + vehicle group)
The tBHQ Treatment Increased Bcl-2 Expression, Reduced Bax Expression and Inhibited Caspase-3 Cleavage After SAH in Wild Type Mice
As Bcl-2, Bax and cleaved caspase-3 are critical for the regulation of cell survival and apoptosis, we examined the effect of tBHQ administration on the expression of those proteins in the temporal tissue of the brain following SAH. From the Western blot analysis shown in Fig. 6b, we found that the protein level of Bcl-2 in the SAH and SAH + vehicle group was significantly decreased compared to the sham group (P < 0.05), In contrast, the tBHQ treatment partially restored the SAH-induced down-regulation of Bcl-2 in the brain tissue (P < 0.05). Moreover, SAH markedly up-regulated Bax and cleaved caspase-3 expression in the brain tissue compared to the sham mice (P < 0.05). The administration of tBHQ reduced the expression of Bax and cleaved caspase-3 in the brain tissue following SAH in mice (P < 0.05). Thus, the tBHQ treatment after SAH provided neuro-protection against SAH.
The tBHQ Treatment Enhanced Autophagy Activation and Ameliorated Early Brain Injury in the Nrf2-Deficient Mice at 24 h After SAH
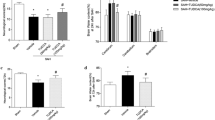
Because the study by Song et al. [9] suggested that tBHQ-enhanced autophagy is Nrf2-independent, we examined the effect of tBHQ on EBI in the Nrf2-deficient mice. Similar to the wild type mice, the SAH-induced increased expression of Beclin-1 and the LC3-II/LC-3I ratio were enhanced (Fig. 7a), indicating that the tBHQ treatment enhanced autophagy in the Nrf2-deficient mice after SAH (P < 0.05). Furthermore, we compared the neuronal degeneration and neurological deficits between the vehicle- and tBHQ-treated groups of Nrf2-deficient mice (Fig. 7c–e). The results indicated that the tBHQ treatment ameliorated EBI by attenuating the SAH-induced neuronal degeneration and neurological deficits in the Nrf2-deficient mice.
The effect of tBHQ in the Nrf2-deficient mice after SAH. A Representative Western blots of Beclin-1 and LC3. B Quantification of Beclin-1 and LC3 in (A). The expression of Beclin-1 and the LC3-II/LC3-I ratio are significantly increased after SAH in the tBHQ-treated mice. C Representative pictures of FJb staining after SAH. D Quantification of the FJb-positive cells in (A). There were fewer degenerating cells in the cortex of the tBHQ-treated Nrf2-deficient mice after SAH. E Representative neurological scores after SAH in the Nrf2-deficient mice (n = 6 for each group. The data represent the mean ± SD. *P < 0.05 versus the sham group; # P < 0.05 versus the SAH + vehicle group. Scale bar 20 μm)
Discussion
In this study, we demonstrated that tBHQ treatment is able to ameliorate EBI at 24 h after SAH in mice by attenuating brain edema, protecting the BBB from disruption, alleviating neuronal degeneration, and improving neurological deficits. Enhanced autophagy and restoration of the apoptotic regulatory proteins Bcl-2, Bax and cleaved caspase-3, but not adequate anti-oxidant enzyme activity, were explored at 24 h after tBHQ treatment as the potential mechanisms underlying the amelioration of EBI. Moreover, the tBHQ treatment exhibited neuro-protective effects after SAH in the Nrf2-deficient mice. The results revealed that tBHQ treatment ameliorated EBI after SAH in mice by enhancing Nrf2-independent autophagy. Our observations provide new insights that are pertinent to the Nrf2/Keap1-mediated oxidative autophagy, which is documented in cancer biology [18], but poorly studied in brain [19], and seemingly involved in various brain injuries.
Tert-butylhydroquinone (tBHQ) is a synthetic phenolic antioxidant, which is widely used as a food preservative to extend the shelf life of food [33–35]. A rich body of evidence has demonstrated that tBHQ may protect against cellular dysfunction that is induced by oxidative stressors, such as alcohol, hydrogen peroxide and glutamate, in various cell types [9, 36–38]. It has been well-established that tBHQ exerts its antioxidant function by increasing Nrf2 stability via inhibition of Keap1-mediated ubiquitination [38–41]. Based on these observations, tBHQ has become a widely employed Nrf2 activators in a variety of experimental settings [5, 8]. The protective role of tBHQ in neuronal injury has been documented in both cell culture and animal studies [8, 38–41]. Moreover, our previous studies showed that tBHQ administration suppressed cerebral and intestinal inflammation and reduced the secondary damage following TBI [6, 42]. Therefore, tBHQ administration could provide the neuro-protection against various types of brain injury.
Because tBHQ is suggested to be a potent antioxidant, we have detected the GPx and SOD enzyme activities to estimate the effect of tBHQ on oxidation resistance in the present study. The results showed that the GPx and SOD enzyme activities in the tBHQ-treated mice were higher than the vehicle-treated group, but were not significantly different at 24 h after the tBHQ treatment. However, our previous experiments found that tBHQ pretreatment attenuated cerebral oxidative stress in mice after TBI [8]. In addition, tBHQ pretreatment reduced the ischemia–reperfusion-induced neurological deficits in rats via the Nrf2-derived anti-oxidant pathway [40]. Therefore, we propose that the time interval of 24 h between the tBHQ treatments and the experimental detection in this study was not sufficient to activate Nrf2 and Nrf2-derived antioxidant proteins. Moreover, this may be the reason why studies on the anti-oxidant effects of tBHQ in vivo prefer “pretreatment” [5, 8, 40].
However, Taguchi and colleague found that autophagy-mediated degradation of Keap1 (an intrinsic inhibitor of Nrf2) is essential for the maintenance of cellular redox homeostasis [16]. They also found that the Keap1 protein is constitutively degraded through the autophagy pathway, and tBHQ treatment accelerated Keap1 degradation by enhancing autophagy. Moreover, a recent study on the tBHQ-mediated protection against lipotoxicity in hepatocytes uncovered that the protective effect of tBHQ is Nrf2-independent [9]. They found that tBHQ was a strong inducer of autophagy, and autophagy inhibition abolished the protective effect of tBHQ. Autophagy, which is a cellular pathway for the degradation and recycling of proteins and organelles to ensure cell survival, is suggested to play a favorable role in EBI after SAH [15, 32]. Wang and colleagues found that autophagy activity was dramatically increased in the temporal cortex after SAH [14], and they found that the rapamycin-enhanced autophagy activity significantly ameliorated the early brain damage, including brain edema, BBB impairment, cortical neuron apoptosis and neurological deficits, while it inhibited 3-MA-induced autophagy in aggravated EBI after SAH in rats [14]. Therefore, proper enhancement of the autophagy pathway is beneficial in EBI after SAH. The tBHQ-enhanced autophagy protects mouse hepatocytes from injury [9], and the autophagy-induced Keap1 degradation is important for maintaining Nrf2 activation [9, 17, 43]. Thus, we evaluate whether autophagy is involved in the protective role of tBHQ treatment in EBI after SAH in mice. As shown in the present study, increased expression of Beclin-1 and increased conversion of LC3-I to LC3-II after SAH in mice suggested the activation of autophagy after SAH, and the additional increase in Beclin-1 expression and the LC3-II/LC3-I ratio after tBHQ treatment suggested that autophagy was enhanced. Accompanying the enhanced activation of autophagy with tBHQ treatment, the secondary injury after SAH, such as brain edema, BBB disruption and neurologic deficits, is ameliorated. Thus, we are in a position to conclude that tBHQ treatment ameliorates EBI by enhancing autophagy.
Because the tBHQ-enhanced autophagy is independent of Nrf2 in hepatocytes [9], we further tested the effect of tBHQ treatment in Nrf2-deficient mice at 24 h after SAH. The result revealed that the tBHQ treatment not only enhanced autophagy, but also decreased neuronal degeneration and improved the neurological scores of Nrf2-deficient mice after SAH. Therefore, in addition to its role as an Nrf2 activator, tBHQ provides neuro-protection against EBI by enhancing autophagy after SAH.
Conclusions
In addition to its protective anti-inflammation and anti-oxidation effects after brain injury, we found a new mechanism for tBHQ in protecting against EBI after SAH by enhancing Nrf2- independent autophagy in vivo. tBHQ may be a promising therapeutic agent in the treatment of SAH patients in the future.
Abbreviations
- tBHQ:
-
Tert-butylhydroquinone
- aSAH:
-
Aneurysmal subarachnoid hemorrhage
- SAH:
-
Subarachnoid hemorrhage
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- ARE:
-
Antioxidant response elements
- Keap1:
-
Kelch-like ECH-associated protein 1
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- TBI:
-
Traumatic brain injury
- BBB:
-
Blood-brain barrier
- CSF:
-
Cerebrospinal fluid
- NF-κB:
-
Nuclear factor-kappa B
- PBS:
-
Phosphate-buffered saline
- GPx:
-
Glutathione peroxidase
- SOD:
-
Superoxide dismutase
- LC3-1:
-
Microtubule-associated protein 1 light chain 3-I
- LC3-II:
-
Microtubule-associated protein 1 light chain 3-II
- FJb:
-
Fluoro-Jade b
References
Sehba FA, Hou J, Pluta RM, Zhang JH (2012) The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol 97:14–37
Markus MA, Morris BJ (2008) Resveratrol in prevention and treatment of common clinical conditions of aging. Clin Interv Aging 3:331–339
Wang Z, Ji C, Wu L, Qiu J, Li Q, Shao Z, Chen G (2014) Tert-butylhydroquinone alleviates early brain injury and cognitive dysfunction after experimental subarachnoid hemorrhage: role of Keap1/Nrf2/ARE pathway. PLoS ONE 9:e97685
Saykally JN, Rachmany L, Hatic H, Shaer A, Rubovitch V, Pick CG, Citron BA (2012) The nuclear factor erythroid 2-like 2 activator, tert-butylhydroquinone, improves cognitive performance in mice after mild traumatic brain injury. Neuroscience 223:305–314
Nouhi F, Tusi SK, Abdi A, Khodagholi F (2011) Dietary supplementation with tBHQ, an Nrf2 stabilizer molecule, confers neuroprotection against apoptosis in amyloid beta-injected rat. Neurochem Res 36:870–878
Jin W, Kong J, Wang H, Wu J, Lu T, Jiang J, Ni H, Liang W (2011) Protective effect of tert-butylhydroquinone on cerebral inflammatory response following traumatic brain injury in mice. Injury 42:714–718
Shih AY, Li P, Murphy TH (2005) A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci 25:10321–10335
Lu XY, Wang HD, Xu JG, Ding K, Li T (2014) Pretreatment with tert-butylhydroquinone attenuates cerebral oxidative stress in mice after traumatic brain injury. J Surg Res 188:206–212
Li S, Li J, Shen C, Zhang X, Sun S, Cho M, Sun C, Song Z (2014) tert-Butylhydroquinone (tBHQ) protects hepatocytes against lipotoxicity via inducing autophagy independently of Nrf2 activation. Biochim Biophys Acta 1841:22–33
Yan F, Li J, Chen J, Hu Q, Gu C, Lin W, Chen G (2014) Endoplasmic reticulum stress is associated with neuroprotection against apoptosis via autophagy activation in a rat model of subarachnoid hemorrhage. Neurosci Lett 563:160–165
Chen J, Wang L, Wu C, Hu Q, Gu C, Yan F, Li J, Yan W, Chen G (2014) Melatonin-enhanced autophagy protects against neural apoptosis via a mitochondrial pathway in early brain injury following a subarachnoid hemorrhage. J Pineal Res 56:12–19
Liu Y, Li J, Wang Z, Yu Z, Chen G (2014) Attenuation of early brain injury and learning deficits following experimental subarachnoid hemorrhage secondary to Cystatin C: possible involvement of the autophagy pathway. Mol Neurobiol 49:1043–1054
Liu Y, Cai H, Wang Z, Li J, Wang K, Yu Z, Chen G (2013) Induction of autophagy by cystatin C: a potential mechanism for prevention of cerebral vasospasm after experimental subarachnoid hemorrhage. Eur J Med Res 18:21
Wang Z, Shi XY, Yin J, Zuo G, Zhang J, Chen G (2012) Role of autophagy in early brain injury after experimental subarachnoid hemorrhage. J Mol Neurosci: MN 46:192–202
Jing CH, Wang L, Liu PP, Wu C, Ruan D, Chen G (2012) Autophagy activation is associated with neuroprotection against apoptosis via a mitochondrial pathway in a rat model of subarachnoid hemorrhage. Neuroscience 213:144–153
Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, Motohashi H, Yamamoto M (2012) Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc Natl Acad Sci USA 109:13561–13566
Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD (2010) A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol 30:3275–3285
Nezis IP, Stenmark H (2012) p62 at the interface of autophagy, oxidative stress signaling, and cancer. Antioxid Redox Signal 17:786–793
Hensley K, Harris-White ME (2015) Redox regulation of autophagy in healthy brain and neurodegeneration. Neurobiol Dis
Li T, Wang H, Ding Y, Zhou M, Zhou X, Zhang X, Ding K, He J, Lu X, Xu J, Wei W (2014) Genetic elimination of Nrf2 aggravates secondary complications except for vasospasm after experimental subarachnoid hemorrhage in mice. Brain Res 1558:90–99
Sabri M, Jeon H, Ai J, Tariq A, Shang X, Chen G, Macdonald RL (2009) Anterior circulation mouse model of subarachnoid hemorrhage. Brain Res 1295:179–185
Sabri M, Ai J, Lass E, D’Abbondanza J, Macdonald RL (2013) Genetic elimination of eNOS reduces secondary complications of experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab 33:1008–1014
Zhuang Z, Zhou ML, You WC, Zhu L, Ma CY, Sun XJ, Shi JX (2012) Hydrogen-rich saline alleviates early brain injury via reducing oxidative stress and brain edema following experimental subarachnoid hemorrhage in rabbits. BMC Neurosci 13:47
Xu J, Wang H, Ding K, Zhang L, Wang C, Li T, Wei W, Lu X (2014) Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE pathway. Free Radic Biol Med 71:186–195
Ding K, Wang H, Xu J, Li T, Zhang L, Ding Y, Zhu L, He J, Zhou M (2014) Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: the Nrf2-ARE signaling pathway as a potential mechanism. Free Radic Biol Med 73:1–11
Schmued LC, Stowers CC, Scallet AC, Xu L (2005) Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res 1035:24–31
Wang JW, Wang HD, Zhong WZ, Li N, Cong ZX (2012) Expression and cell distribution of metabotropic glutamate receptor 5 in the rat cortex following traumatic brain injury. Brain Res 1464:73–81
Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats Statistical validation. Stroke 26:627–634
McGirt MJ, Parra A, Sheng HX, Higuchi Y, Oury TD, Laskowitz DT, Pearlstein RD, Warner DS (2002) Attenuation of cerebral vasospasm after subarachnoid hemorrhage in mice overexpressing extracellular superoxide dismutase. Stroke 33:2317–2323
Sozen T, Tsuchiyama R, Hasegawa Y, Suzuki H, Jadhav V, Nishizawa S, Zhang JH (2009) Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke 40:2519–2525
Mo H, Chen Y, Huang L, Zhang H, Li J, Zhou W (2013) Neuroprotective effect of tea polyphenols on oxyhemoglobin induced subarachnoid hemorrhage in mice. Oxid Med Cell Longev 2013:743938
Lee JY, He Y, Sagher O, Keep R, Hua Y, Xi G (2009) Activated autophagy pathway in experimental subarachnoid hemorrhage. Brain Res 1287:126–135
Shahidi F, Zhong Y (2010) Novel antioxidants in food quality preservation and health promotion. Eur J Lipid Sci Technol 112:930–940
Parke D, Lewis D (1992) Safety aspects of food preservatives. Food Addit Contam 9:561–577
Madhavi D, Deshpande S, Salunkhe DK (1995) Food antioxidants: technological: toxicological and health perspectives. CRC Press, Boca Raton
Steele ML, Fuller S, Patel M, Kersaitis C, Ooi L, Munch G (2013) Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox Biol 1:441–445
Lavoie S, Chen Y, Dalton TP, Gysin R, Cuenod M, Steullet P, Do KQ (2009) Curcumin, quercetin, and tBHQ modulate glutathione levels in astrocytes and neurons: importance of the glutamate cysteine ligase modifier subunit. J Neurochem 108:1410–1422
Eftekharzadeh B, Maghsoudi N, Khodagholi F (2010) Stabilization of transcription factor Nrf2 by tBHQ prevents oxidative stress-induced amyloid beta formation in NT2N neurons. Biochimie 92:245–253
Khodagholi F, Tusi SK (2011) Stabilization of Nrf2 by tBHQ prevents LPS-induced apoptosis in differentiated PC12 cells. Mol Cell Biochem 354:97–112
Shih AY, Li P, Murphy TH (2005) A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci 25:10321–10335
Li J, Johnson D, Calkins M, Wright L, Svendsen C, Johnson J (2005) Stabilization of Nrf2 by tBHQ confers protection against oxidative stress-induced cell death in human neural stem cells. Toxicol Sci 83:313–328
Jin W, Ni H, Dai Y, Wang H, Lu T, Wu J, Jiang J, Liang W (2010) Effects of tert-butylhydroquinone on intestinal inflammatory response and apoptosis following traumatic brain injury in mice. Mediators Inflamm 2010:502564
Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, Baehrecke EH, Lenardo M (2006) Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci USA 103:4952–4957
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81070974, 81000503, 81371357), the Jiangsu Provincial Key Subject (Nos. BL2013027, xk201129).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, T., Sun, Kj., Wang, HD. et al. Tert-butylhydroquinone Ameliorates Early Brain Injury After Experimental Subarachnoid Hemorrhage in Mice by Enhancing Nrf2-Independent Autophagy. Neurochem Res 40, 1829–1838 (2015). https://doi.org/10.1007/s11064-015-1672-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1672-4