Abstract
Transforming growth factor-β 1 (TGFβ1) has a diverse role in astrogliosis and neuronal survival, but the underlying mechanism remains to be elucidated, especially in traumatic brain injury (TBI). Here, we show that the expression of TGFβ1 was increased in the pericontusional region, accompanied with astrogliosis and neuronal loss in TBI rats. Moreover, TGFβ1 knockdown not only reduced the number of neurons and inhibited astrogliosis but also resulted in a significant neurological dysfunction in rats with TBI. Subsequently, Smad3, a key downstream signal of TGFβ1, was involved in pericontusional region after TBI. These findings therefore indicate that TGFβ1 is involved in neuroprotection and astrogliosis, via activation of down stream Smad3 signal in the brain after injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trauma is one of the leading killers under 44 years old, especially from car accidents. Among all of trauma, more than half consists of traumatic brain injury (TBI), and most of the survivors suffer from permanent disabilities. With the dramatic expansion of modern transportation and industry, the incidence of TBI caused by traffic accidents and falling is increased in recent years. About 50 million TBI patients are admitted to the hospital each year, among which 75,000–90,000 die. Therefore, a resurgence of interest to the brain injury is coming and involved mechanism needs to be investigated [1–4].
Transforming growth factor-β (TGF-β) plays several prominent roles in immune regulation [5–7]. As an important neurotrophic factor in supporting cell survival and proliferation [8–11], TGF-β was expressed in the nervous system as three distinct yet highly homologous isoforms, known as β1, β2 and β3. TGFβ1 can mediate signal transduction from cell membrane to nucleus through the activation of TGF receptors [12, 13], which in turn phosphorylate the smad family proteins [14]. In addition, TGFβ1 shares a positive role for neuronal survival in vitro, and promotes astrogliosis [8–11]. In vivo, TGFβ1 was found to express in both forebrain subventricular and hippocampal dentate gyrus [15]. Previous studies have showed that administration of TGFβ1 promotes the recovery of wound after injury. However, the role of TGFβ1 and related signal in TBI is waiting to be elucidated.
In this study, we detected the temporal change of the intrinsic TGFβ1 expression in the brain of rats with TBI, then examined its roles and associated molecular mechanism. The findings could help to understand the role of endogenous TGFβ1 and its implication that could be useful for its clinic application involved in patients suffering from TBI.
Methods
Animal Experiments
Adult Sprague–Dawley female rats (weighing 180–210 g) were obtained from the Animal Experimental Center of Kunming Medical University. This research was approved by the Kunming Medical University Animal Care and all procedures were performed according to the guidelines of Chinese Academy of Sciences. Every effort was made to reduce the number of animals used and to minimize the suffering during the experiments. The rats were performed each procedure and usage, shown in Table 1.
Animal Surgery
The modified Feeney method was used to prepare the strike injury on the zona rolandica of rats [16, 17]. In brief, the rats were anesthetized by intraperitoneal injection of 3.6 % chloral hydrate (1 ml/100 g). A “V” shaped incision was made 5 mm away from the frontal fontanel in the coronary plane. The right parietal bone was exposed. A dental drill was used to make an opening through the bone at 2.5 mm from the sagittal suture, and 1.5 mm from the arcuate suture. The “bone window” was enlarged to 5.0 mm × 5.0 mm area. The cerebral cortex was exposed and a sterile clout was placed on it. A metal cylinder weighing 60 g was dropped vertically along a metal pole to strike the clout at a 10 cm height with 50 g weight. This resulted in a moderate contusion injury of the right zona rolandica. Post-operative care included daily injection of 5 IU of penicillin, beginning from the first day of operation till 3 days afterwards. Manual evacuation of the urinary bladder had been performed until the rats were humanely euthanized. The rats were given food and drink ad libitum.
siRNA Preperation
Human herpes simplex virus (HSV) vector was prepared for the construction of shRNA-TGFβ1 [18]. shRNA targeting the rat TGFβ1 cDNA was synthesized by ShenDeYuan Biotech Company. The shRNA-TGFβ1 gene and the fluorescent marker GFP were simultaneously cloned into HSV vector; the negative control vector only expresses the GFP gene. HSV-sh TGFβ1-GFP and HSV-GFP vectors were then prepared for in vivo injection.
Injection of HSV-sh TGFβ1 into Pericontusional Regions
After brain contusion injury, 10 µl of HSV-sh TGFβ1-GFP or the control HSV-GFP were immediately injected into the pericontusional regions (2sites located in inside and outside of epicenter, 5 µl for each site) with the help of a Hamilton micro syringe. The silencing effects of TGFβ1 interference by the above stated agents were determined by IHC, RT-PCR and Western blotting. The neurological function was evaluated with neurological severity scores at 1, 7, 14, and 28 days post operation (dpo).
Neurological Function Evaluation
The neurological severity scores (NSS) is a composite of motor, sensory, reflex and balance tests. One point was scored for the inability to perform the test or for the lack of a tested reflex; Therefore, the higher the score, the more severe the neurological defects. Neurological function was graded on a scale of 0–18 (normal score, 0; maximal deficit score, 18). NSS evaluation was carried out in all testing animals 1 week after injury and lasted for one additional week, by a three researchers blinded to injury and treathment condition.
Immunohistochemistry (IHC)
Rats were perfused with 500 ml of cold Phosphate-buffered saline (PBS) for 5 min and 500 ml cold 4 % paraformaldehyde solution for 30 min. The pericontusional tissues in each group were harvested, post-fixed for 6–12 h, and immersed in 0.1 M PBS containing 20 % sucrose overnight. Immunohistochemistry was performed as described previously [19, 20]. Sections of 10 μm thickness were cut on a freezing microtome for free floating TGFβ1 immunostaining. After rinsed with 0.01 M PBS and soaked in PBS containing 3 % H2O2 for 30 min at room temperature to quench the endogenous peroxidase activity, sections were immersed in PBS containing 5 % goat serum and 0.3 % Triton at room temperature for 2 h. This was followed by the incubation with anti-TGFβ1 (1:200,abcam) rabbit polyclonal antibody at 4 °C for 24 h and rinsed again. Then they were incubated with biotinylated goat anti-rabbit IgG antibody (1:100 dilution Vector Labs, USA) for 1.5 h at room temperature and followed by avidin-biotinylated peroxidase complexes (1:250, ABC Elite, Vector Labs). TGFβ1 immunoreactivity was visualized using diaminobenzidine and H2O2 as substrates for 5 min. All sections from the sham-operated and experimental rats were treated under identical conditions. Negative control experiments in which normal goat serum was used to substitute for the primary antibody were performed to ascertain the specificity of antibody staining. In order to compare the change on the TGFβ1 expression in each group, the number of TGFβ1 positive neurons and astrocytes from three sections of each animal was counted. 5 animals of each group were used in the study to compare the number of cells for TGFβ1 by a stereology approach.
Immunofluorescence
To detect the cell types and amount of TGFβ1 transfection in the brain, immunofluorecent staining was performed by a routing method. Briefly, sections from each group were harvested and washed with PBS, then fixed with 4 % paraformaldehyde for 10 min. The sections were then by permeabilized with 0.1 % Triton X-100 at room temperature for another 30 min. Then sections were incubated with 5 % normal goat serum for 30 min. Subsequently, rabbit anti TGFβ1 antibody (1:200, Abcam) and mouse anti-NeuN (Zhongshan company, 1:500) or mouse anti-GFAP (Milipore, 1:500) were added on sections, respectively, and sections were kept overnight at 4 °C. Then sections were washed with PBS three times, and incubated with Cy3 and 488 conjugated secondary antibody (1:200; millpore) in the dark at 37 °C for 1 h. This was followed by last washing, the DAPI was added to counterstain nuclei for 10 min. Images of stained cells from 5 fields in each sections were harvested and counted by using a Leica microscope.
Western Blotting Analysis
Western blotting analysis was performed as described in previous studies [21, 22]. To investigate the level of TGFβ1 and Smad3, the downstream signaling molecules, the rats from respective group were killed. After carefully removing the brain meninges, the brain tissues from pericontusional region were homogenized on ice in Lysis Buffer containing 0.05 M Tris–HCl (pH 7.4, Amresco), 0.5 M EDTA (Amresco), 30 % TritonX-100 (Amresco), NaCl (Amresco), 10 % SDS (Sigma) and 1 mM PMSF (Amresco), and centrifuged at 12,000 rpm for 30 min. The supernatant was stored at −80 °C until analysis. Protein concentration was determined with BCA reagent (Sigma, St. Louis, MO, USA). Protein samples were electrophoresed on 12 % SDS–polyacrylamide gel (SDS-PAGE) and transferred to the nitrocellulose membrane. The membrane was blocked with phosphate-buffered saline containing 0.05 % Tween-20 (PBST) and 10 % nonfat dry milk overnight at 4 °C for 12 h. It was washed three times for 10 min each time, then rinsed with PBST and incubated with the primary antibodies specific for TGFβ1 and Smad3 (1:200, abacam, Rabbit monoclonal antibody) at 4 °C for 24 h. After washing three times for 10 min each, the membrane was incubated with a HRP-conjugated goat anti-rabbit Ig G (1:500; Vector Laboratories, CA) for 2 h at room temperature, and washed as described above. It was then developed with an ECM kit and visualized by a Bio-Gel Imagining system equipped with Genius synaptic gene tool software. Densitometry analysis for TGFβ1 and Smad3 were performed respectively, using β-actin (1:500, Santa Cruz) as internal control.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
RT-PCR was used to determine the expression level of TGFβ1 and Smad3 in pericontusional tissues, as referenced in previous reports [23, 24]. Total RNA was isolated from brain sample (weighing 50 mg), using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. The concentration was measured using a Nanodrop spectrophotometer (ND-1000) and the total RNA was subjected to a reverse transcription procedure using the Revert Aid according to the manufacturer’s recommendation. PCR primers are as follows: β-actin (227 bp) sense: 5′ -GTAAAGACCTCTATGCCAACA-3′; and antisense: 5′ GGACTCATCGTACTCCTGCT 3′; TGFβ1 (332 bp) sense: 5′GTGAGCACTGAAGCGAAAGC3′ and antisense: 5′ TAATGGTGGACCGCAACAAC 3′. Smads (413 bp) Sense: 5′ CTGGCTACCTGAGTGAAGATG 3′;antisense: 5′ GTTGGGAGACTGGACGAAA 3′. PCR was performed using 2 PCR Master Mix (Fermentas Company) in 0.2 ml thin walled reaction tubes, using the “hot-start” method. Reagents were assembled in a final volume of 25 ml, and the final concentrations of reagents were as follows: 1.5 ml first strand cDNA, 0.5 ml forward primer, 0.5 ml reverse primer, 12.5 ml 2 PCR Master Mix, and 10 ml RNase-free water to 25 ml. Samples were initially denatured at 94 °C for 5 min, then 1 min denaturation at 94 °C, 1 min annealing at 52.5 °C for β-actin and 54 °C for TGFβ1, and Smad3 1 min extension at 72 °C. The whole reverse transcription procedure was repeated for 30 cycles with a final extension step at 72 °C for 10 min. Aliquots (25 ml) of the PCR reaction were run on 1 % agarose gels, and the size of the reaction products was determined after ethidium bromide staining. Rat β-actin was amplified as an internal control, using rat-specific primer.
Statistic Analysis
All statistical analyses were performed using the statistical software SPSS11.0. A student t test was performed for two sets of data, while one-way ANOVA with a LSD-t (equal variance assumed) or Dunnett’s T3 (equal variance not assumed) post hoc test was performed for the comparison of three sets of data. A p value less than 0.05 was used to denote statistical significance.
Results
Changes of Neurological Scores After TBI
A significant neurological dysfunction, as indicated by increased NSS scores, was seen in rats at 7 days after TBI (dpo) (Fig. 1). Despite NSS scores was slightly reduction in rats at 14 dpo compared to 7 dpo, it was also obvious abnormality, compared to sham control rats. These showed that TBI caused a persistent neurological deficit in rats.
Impact of TBI on TGFβ1 Expression in Injury Brain
To understand the involvement of TGFβ1 in neural injury or recovery after TBI, RT-PCR and Western blotting analysis were used to determine its expressional levels in the brain of rats after TBI. The data showed that the level of TGFβ1 mRNA was upregulated at 1, 3, 7, 14 dpo, while the level of TGFβ1 protein exhibited a slight delay and reached a statistic significance at 14 dpo in the pericontusional region after TBI (Fig. 2a–d).
Effect of TBI on TGFβ1 expression and astrogliosis. A representing image shows the levels of RT-PCR products in the brain samples from different groups (a). Quantitative histograms showed the changes of mRNA levels after TBI shown in (b) (*P < 0.05, compared with other group). c showed that Western blotting analysis of TGFβ1 in the brain samples from different groups. Quantitative histograms on the changes of TGFβ1 protein levels after TBI was shown in (d) (*Compared with other group, P < 0.05)
Localization of TGF-β1 and Its Change
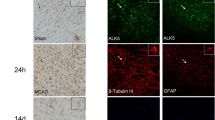
By immunofluorescent double labeling staining. We detect the localization of TGFβ1 in neurons(Fig. 3a–d) and astrocytes (Fig. 3e–h). Moreover, by immuno enzyme histochemical staining, we quantify the number of TGFβ1 positive neurons in each group. The data showed TGFβ1 immunostaining in neurons was remarkably decreased from 7 to 14 dpo, while TGFβ1 positive astrocytes exhibited a significant increase at 14 dpo in the pericontusional region of TBI, compared with sham one (Fig. 3l–m).
Localization of TGFβ1 and its change. TGFβ1 was co-localized in neurons, shown in (a–d), and TGFβ1 positive astrocytes in (e–h). Immunohistochemical enzyme-linked staining of TGFβ1 in brain section from sham, 7, and 14 dpo, shown in (i–k), respectively. l, m show quantitative data of TGFβ1 staining on the number of positive cells. The arrows highlight TGFβ1 positive cells, and scale bar is 50 μm in (g). *P < 0.05, compared with other group. Scale bar 50 μm, shown in (a)
Morphological Evidences of TGFβ1 shRNA Interference
Under fluorescence microscope, both neurons and astrocytes could emit green fluorescence, indicating that HSV shTGFβ1 has successfully transfected into host cells. These cells could be double labelled by NeuN or GFAP markers, confirming they are neurons (Fig. 4a–c) or astrocytes, respectively (Fig. 4d–g). Compared with the staining of TGFβ1 in TBI rats, HSV-shTGFβ1 decreased greatly the staining intensity of TGFβ1 in neurons and astrocytes (Fig. 4h, i).
Effect of HSV-shTGFβ1 transfection on GFP in the brain. Labeled HSV-sh TGFβ1 staining cell was shown in (a). NeuN staining was shown in (b). c is merged together with DAPI staining (blue). d–g show astrocytes transfected with TGFβ1-GFP and stained by GFAP, DAPI and merged,respectively. h showed the strong GFP expression in neurons of TBI rats, while sh-TGFβ1 transfection weakens the TGF-β expression in neurons, greatly (i). Scale bar 50 μm, shown in (a, d, h) (Color figure online)
Effect of TGFβ1 shRNA Interference on Astrogliosis and Neuronal Spared
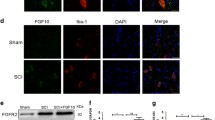
To test whether the elevated TGFβ1 expression contributes to neurological morphology after TBI, the knockdown of TGFβ1 was performed with the shRNA approach. RT-PCR (Fig. 5a) and Western blotting analysis (Fig. 5b) confirmed a significant decrease on TGFβ1 expression in HSV-shRNA injection group (Fig. 5c). Colocalization of TGFβ1 with NeuN and GFAP confirmed TGFβ1 could express both in neurons and astrocytes (Fig. 5d, e). Consistently, when compared with empty vector treated rats, the number of TGFβ1 positive neurons and astrocytes around injected site was all decreased dramatically (Fig. 5f–h).
Effect of TGFβ1 shRNA interference on neuron and astrogliosis. Image of RT-PCR product and WB from the pericontusional regions in shRNA treatment, group and TBI control was shown in (a, b), respectively. Quantitative histograms for mRNA and protein of TGFβ1 was shown in (c). The localization of TGFβ1 in neurons (TGFβ1:red; NeuN:green; Merged) and astrocytes (TGFβ1:red; GFAP:green; Merged), respectively. f Immunostaining of TGFβ1 in the brain treated with control HSV; g Immunostaining of TGFβ1 in the brain treated with TGFβ1-shRNA HSV; h Quantitative results show the changes in TGFβ1 immunoreactive astrocytes and neurons after TBI and treatment with shRNA to TGFβ1. Thin arrows pointed astrocytes and thick arrows is for neurons. Scale bar is 50 μm in (d, e), and 100 μm in (f, g) (Color figure online)
TGFβ1 Knockdown Decreased Smad3 Expression
Consistent with the change of TGFβ1, shRNA interference for TGFβ1 significantly decrease the expression of Smad3, a downstream molecule of TGFβ1 (Fig. 6a–c).
Effect of TGFβ1 silencing on the expression of Smad3 in the brain. Representative images of RT-PCR (a) and Western blotting analysis (b) for Smad3 from the cortex at 3 and 14 dpo. Quantitative analysis of mRNA and protein levels of Smad3 is shown in Fig. 6c
Effect of TGFβ1 Knockdown on Neural Recovery in TBI Rats
To determine the role of endogenous TGFβ1 in neural recovery after TBI, we compared the behavior between the control rats and TGFβ1 silencing rats. Based on the NSS evaluation, despite no significant change could be seen on NSS scores in rats with TGFβ1 knockdown at 1, 7 and 14 dpo compared with the control, the NSS score exhibited significant increase, compared with control at 28 dpo (P < 0.05) (Fig. 7). This showed that TGFβ1 knockdown aggravates the neurological deficit in TBI rats.
Discussion
TBI remains a leading cause of mortality and immobility in the younger population and causes severe burdens for families and societies. Previous reports have found that astrocytes are activated following trauma [25], while activated actrocytes may share diverse effects in response to injury. One hand, astrogliosis may be beneficial because proliferating astrocytes could fill cavity and wound induced by injury, then diminish the lesion area [26–28], which can contribute to the trauma recovery. On the other hand, astrogliosis could induce the scar formation so as to retard the nerve regeneration, and induce a barrier of neurite growth to impair the functional recovery [29, 30]. In this study, we confirmed the decrease of neuronal number occurred following TBI, which could be related to dysfunction in TBI models. Several studies have pointed that TBI result in extensive damage in several regions like cortex, hippocampus, and even spinal cord [31, 32]. Therefore, the neurological dysfunctions may be related to the neuronal loss in these regions. Simultaneously, we found a limited improvement with the time on neurological functions in TBI rats, suggesting that neuroplasticity exists in rats after brain injury.
The mechanism underlying spontaneous neurological improvement after injury is not fully understood. Neurotrophic factors such as nerve growth factor and brain derived neurotrophic factor may play important roles in neuroplasticity after TBI [33–36], but the role of TGFβ1 in neural recovery remains to be determined. Previously, using PCR analyses, Huang found that the maximum mRNA expression for TGFβ1 was seen at 12 h and 3 days in injured cortex under hypoxia induced by TBI [37]. In addition, TGFβ1 increases at 2 h in the combined model of TBI and hemorrhage, [38]. Moreover, we elucidated the expressional change of TGFβ1 in TBI brain, which showed that the increase in TGFβ1 mRNA from 1 day to 14 days and protein exhibited a delay increase at 14 days. These suggested that role of TGFβ1 in injured brain may involve in mechanism both injury and recovery. As to talk the reason of TGFβ1 increase with delay after mRNA, there are two possibility: TGFβ1 may be degraded in the process of synthesis, which may involve the post-translation regulating mechanism. The other possibility is that synthesized TGFβ1 may be immediately transported to other region or as material for other growth factor synthesis.
The functional role of TGFβ1 in TBI is controversial. While studies showed that TGFβ1 is an important neurotrophic factor with neuroprotective functions, other studies reported the detrimental effect on functional recovery induced from TGF-β1 for astrogliosis [8, 36]. In addition, TGFβ1 is involving in spinal cord injury [39], neural encephalopathy, which is related to microglia activation [40, 41]. In this study, expression of TGF-β1 was located in both neurons and astrocytes, and increased mRNA level for TGFβ1 was maintained for 2 weeks after injury, This suggests that TGFβ1 is important in TBI. Perhaps, effect of TGFβ1 is not only responsible for astrocytes but also neurons. Increased TGF-β1 expression may be partially useful to the neural recovery at 14 days. The underlying mechanism may be dependent on neuronal survival. Previous have showed that TGFβ1 signal is necessary for cell survival in interneurons and spinal ganglion neurons [42, 43]. Recent study also reported TGFβ1 signal is available to generation of new neurons [44].
In order to test the exact role of TGFβ1 after TBI, we performed the RNA interference, so as to knockdown endogenous TGFβ1. TGFβ1 knockdown leads to a significant decrease in the number of neurons, which is accompanied by functional impairment in rat with TBI. Moreover, Smad3, a downstream molecule of IGFβ1 [45], has been substantially decreased in HSV-sh TGFβ1 treated animals. These showed the Smad3, known as TGFβ1 signal, has been inhibited in TGFβ1 knockdown condition. Together, we suggest that TBI could induce the expression of TGFβ1 in neurons and astrocytes in pericontusinal regions from TBI rats, while HSV-TGFβ1 isRNA injection could effectively decreased functional recovery in TBI rats and they are related to smad signal. Therefore, we conclude that endogenous TGFβ1 should be a beneficial factor in TBI condition. Our findings provide a novel evidence to understand the role of endogenous TGFβ1 and associated Smad3 signal in TBI rats. It may provide novel cues to target TGFβ1 to promote functional restoration for the treatment of TBI in future studies.
References
Harbeck-Seu A, Brunk I, Platz T, Vajkoczy P, Endres M, Spies C et al (2011) A speedy recovery: amphetamines and other therapeutics that might impact the recovery from brain injury. Curr Opin Anaesthesiol 24(2):144–153
Chen H, Epstein J, Stern E (2010) Neural plasticity after acquired brain injury: evidence from functional neuroimaging. PMR 2(12 Suppl 2):S306–S312
Bashir S, Mizrahi I, Weaver K, Fregni F, Pascual-Leone A (2010) Assessment and modulation of neural plasticity in rehabilitation with transcranial magnetic stimulation. PMR 2(12 Suppl 2):S253–S268
Warraich Z, Kleim JA (2010) Neural plasticity: the biological substrate for neurorehabilitation. PMR 2(12 Suppl 2):S208–S219
Joyce ME, Roberts AB, Sporn MB, Bolander ME (1990) Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol 110(6):2195–2207
Nathan C, Sporn M (1991) Cytokines in context. J Cell Biol 113:981–986
Alexandrow MG, Moses HL (1995) Transforming growth factor-β and cell cycle regulation. Cancer Res 55:1452–1457
Martinou JC, Le Van Thai A, Valette A, Weber MJ (1990) Transforming growth factor β1 is a potent survival factor for rat embryo motoneurons in culture. Brain Res Dev Brain Res 52:175–181
Chalazonitis A, Kalberg J, Twardzik DR, Morrison RS, Kessler JA (1992) Transforming growth factor-β has neurotrophic actions on sensory neurons in vitro and is synergistic with nerve growth factor. Dev Biol 152:121–132
Prewitt CM, Niesman IR, Kane CJ, Houle JD (1997) Activated macrophage/microglial cells can promote the regeneration of sensory axons into the injured spinal cord. Exp Neurol 148:433–443
Chin J, Angers A, Cleary LJ, Eskin A, Byrne JH (2002) Transforming growth factor-β1 alters synapsin distribution and modulates synaptic depression in Aplysia. J Neurosci 22:RC220
Flanders KC, LuÈdecke G, Engels S, Cissel DS, Roberts AB, Kondaiah P et al (1991) Immunhistochemical localization of transforming growth factor-βs in the nervous system of the mouse embryo. Development 113:183–191
Unsicker K, Flanders KC, Cissel DS, Lafyatis R, Sporn MB (1991) Transforming growth factor-β isoforms in the adult rat central and peripheral nervous system. Neuroscience 44:613–625
Massagué J (2000) How cells read TGF-β signals. Nat Rev Mol Cell Biol 1:169–178
Logan TT, Villapol S, Symes AJ (2013) TGF -β superfamily gene expression and induction of the Runx1 transcription factor in adult neurogenic regions after brain injury. PLoS ONE 8(3):e59250
Zhou HL, Zhang LS, Kang Y, Zhang W, Wang TH (2008) Effects of electro-acupuncture on CNTF expression in spared dorsal root ganglion and the associated spinal lamina II and nucleus dorsalis following adjacent dorsal root ganglionectomies in cats. Neuropeptides 42:95–106
Zhang RJ, You C, Cai BW, He M, Yang YB, Liu Z et al (2006) Changes in P-selectin expression after brain injury in rats. J South Med Univ 26(3):348–351 (Chinese)
Anesti AM, Peeters PJ, Royaux I, Coffin RS (2008) Efficient delivery of RNA Interference to peripheral neurons in vivo using herpes simplex virus. Nucleic Acids Res 36(14):1–12
Zhou X, Yang JW, Zhang W, Ou KQ, Zhou HL, Ma YQ et al (2009) Role of NGF in spared DRG following partial dorsal rhizotomy in cats. Neuropeptides 43:363–369
Liu J, Zhang Z, Li JT, Zhu YH, Zhou HL, Liu S et al (2009) Effects of NT-4 gene modified fibroblasts transplanted into AD rats. Neurosci Lett 466:1–5
Qin DX, Zou XL, Luo W, Zhang W, Zhang HT, Li XL et al (2006) Expression of some neurotrophins in the spinal motoneurons after cord hemisection in adult rats. Neurosci Lett 410(3):222–227
Zhang HT, Li LY, Zou XL, Song XB, Hu YL, Feng ZT et al (2007) The immunohistochemical distribution of NGF, BDNF, NT-3, NT-4 in the brains of adult rhesus monkeys. J Histochem Cytochem 55(1):1–19
Wang XY, Li XL, Hong SQ, Xi-Yang YB, Wang TH (2009) Electroacupuncture induced spinal plasticity is linked to multiple gene expressions in dorsal root deafferented rats. J Mol Neurosci 37(2):97–110
Liu F, Sun WW, Wang Y, Hu LQ, Dai P, Tian CF et al (2009) Effects of electro-acupuncture on NT-4 expression in spinal dorsal root ganglion and associated segments of the spinal dorsal horn in cats subjected to adjacent dorsal root ganglionectomy. Neurosci Lett 450(2):158–162
Ip NY, Wiegand SJ, Morse J, Rudge JS (1993) Injury-induced regulation of ciliary neurotrophic factor mRNA in the adult rat brain. Eur J Neurosci 5:25–33
Rudge JS, Morrissey D, Lindsay RM, Pasnikowski EM (1994) Regulation of ciliary neurotrophic factor in cultured rat hippocampal astrocytes. Eur J Neurosci 6:218–229
Kirsch M, Schneider T, Lee MY, Hofmann HD (1998) Lesion-induced changes in the expression of ciliary neurotrophic factor and its receptor in rat optic nerve. Glia 23:239–248
Zhou J, Lei P, Zhang X (2008) Resveratrol on brain injury after traumatic brain tissue in neural cell apoptosis. Trauma Surg 9(1):106–109
Hu R, Zhou J, Luo C, Lin J, Wang X, Li X et al (2010) Glial scar and neuroregeneration: histological, functional, and magnetic resonance imaging analysis in chronic spinal cord injury. J Neurosurg Spine 13(2):169–180
Kopp MA, Brommer B, Gatzemeier N, Schwab JM, Prüss H (2010) Spinal cord injury induces differential expression of the profibrotic semaphorin 7A in the developing and mature glial scar. Glia 58(14):1748–1756
Gao X, Chen J (2011) Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J Neuropathol Exp Neurol 70(3):183–191
Hagen EM, Eide GE, Rekand T, Gilhus NE, Gronning M (2010) Traumatic spinal cord injury and concomitant brain injury: a cohort study. Acta Neurol Scand Suppl 190:51–57
Wang TH, Meng QS, Qi JG, Zhang WM, Chen J, Wu LF (2008) NT-3 expression in spared DRG and the associated spinal laminae as well as its anterograde transport in sensory neurons following removal of adjacent DRG in cats. Neurochem Res 33(1):1–7
Li XL, Zhang W, Zhou X, Wang TH (2007) Temporal changes in the expression of some neurotrophins in spinal cord transected adult rats. Neuropeptides 41(3):135–143
Chen J, Qi JG, Zhang W, Zhou X, Meng QS, Zhang WM et al (2007) Electro-acupuncture induced NGF, BDNF and NT-3 expression in spared L6 dorsal root ganglion in cats subjected to removal of adjacent ganglia. Neurosci Res 59:399–405
Zhang W, Li Y, Wang ZJ, Zhou X, Ou KQ, Zhou HL et al (2010) Functional roles of intrinsic neurotrophin-3 in spinal neuroplasticity of cats following partial ganglionectomy. Growth Factors 28(5):351–358
Huang RQ, Cheng HL, Zhao XD, Dai W, Zhuang Z, Wu Y, Liu Y, Shi JX (2010) Preliminary study on the effect of trauma-induced secondary cellular hypoxia in brain injury. Neurosci Lett 473(1):22–27
Sillesen M, Johansson PI, Rasmussen LS, Jin G, Jepsen CH, Imam AM, Hwabejire J, Lu J, Duggan M, Velmahos G, deMoya M, Alam HB (2013) Platelet activation and dysfunction in a large-animal model of traumatic brain injury and hemorrhage. J Trauma Acute Care Surg 74(5):1252–1259
Chin J, Angers A, Cleary LJ, Eskin A, Byrne JH (2002) Transforming growth factor-β1 alters synapsin distribution and modulates synaptic depression in Aplysia. J Neurosci 22:RC220
Welser JV, Li L, Milner R (2010) Microglial activation state exerts a biphasic influence on brain endothelial cell proliferation by regulating the balance of TNF and TGFβ1. J Neuroinflammation 7:89
Bain JM, Ziegler A, Yang Z, Levison SW, Sen E (2010) TGF beta1 stimulates the over-production of white matter astrocytes from precursors of the “brain marrow” in a rodent model of neonatal encephalopathy. PLoS ONE 5(3):e9567
Kaiser O, Paasche G, Stöver T, Ernst S, Lenarz T, Kral A, Warnecke A (2013) TGF-beta superfamily member activin A acts with BDNF and erythropoietin to improvesurvivalof spiral ganglion neurons invitro. Neuropharmacology 75:416–425
Khodosevich K, Lazarini F, von Engelhardt J, Kaneko H, Lledo PM, Monyer H (2013) Connective tissue growth factor regulates interneuron survival and information processing in the olfactory bulb. Neuron 79(6):1136–1151
Kandasamy M, Lehner B, Kraus S, Sander PR, Marschallinger J, Rivera FJ, Trümbach D, Ueberham U, Reitsamer HA, Strauss O, Bogdahn U, Couillard-Despres S, Aigner L (2014) TGF-beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J Cell Mol Med 18(7):1444–1459
Liu Y, Liu H, Meyer C, Li J, Nadalin S, Königsrainer A, Weng H, Dooley S, ten Dijke P (2013) Transforming growth factor-β (TGF-β)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J Biol Chem 288(42):30708–30719
Acknowledgments
This research was supported by natural science foundation of Yunnan province (No. 2013FB119), and natural science foundation of Zhejiang province (No. Y2090864).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xu-Yang Wang, Ying-Chun Ba, Fang Wang, Heng-Li Tian and Jin-Tao Li have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, XY., Ba, YC., Xiong, LL. et al. Endogenous TGFβ1 Plays a Crucial Role in Functional Recovery After Traumatic Brain Injury Associated with Smad3 Signal in Rats. Neurochem Res 40, 1671–1680 (2015). https://doi.org/10.1007/s11064-015-1634-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1634-x