Abstract
Cecropia species are widely used in traditional medicine by its anti-diabetic, anti-hypertensive and anti-inflammatory properties. In the present study, we investigated the neuroprotective and antioxidant effects of the crude aqueous extract from Cecropia pachystachya leaves in a rat model of mania induced by ketamine. The results indicated that ketamine treatment (25 mg/kg i.p., for 8 days) induced hyperlocomotion in the open-field test and oxidative damage in prefrontal cortex and hippocampus, evaluated by increased lipid peroxidation, carbonyl protein formation and decreased total thiol content. Moreover, ketamine treatment reduced the activity of the antioxidant enzymes superoxide dismutase and catalase in hippocampus. Pretreatment of rats with C. pachystachya aqueous extract (200 and 400 mg/kg p.o., for 14 days) or with lithium chloride (45 mg/kg p.o., for 14 days, used as a positive control) prevented both behavioral and pro-oxidant effects of ketamine. These findings suggest that C. pachystachya might be a useful tool for preventive intervention in bipolar disorder, reducing the episode relapse and the oxidative damage associated with the manic phase of this disorder .
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bipolar disorder (BD) is a severe and chronic psychiatric condition with an estimated prevalence around 1–3 % in the worldwide population [1, 2]. The clinical course of this disease is characterized by the occurrence of one or more manic episodes that often cycles with euthymic and depressive states. It has been even more recognized that recurrent episodes influence the outcome of BD by increasing patient’s vulnerability to subsequent episodes and reducing treatment response [3].
The pathophysiological mechanisms underlying BD are still unknown and include genetic variations, dysregulation of neurotransmitter systems and neural networks, neuroinflammation and neurodegeneration [4, 5]. In this context, oxidative stress plays an important role triggering cellular process associated with the development and progression of several neurological and psychiatric disorders, including BD [4, 6].
Since the brain is particularly susceptible to oxidative stress, considerable attention has been given to the role of ROS in the development of neuropsychiatric disorders [7]. Preclinical studies conducted in animal models of mania have suggested that oxidative stress is a major feature associated with the neurodegeneration and behavioral alterations observed in these models [8–11]. Furthermore, clinical studies also indicate high systemic markers of oxidative damage in BD patients, suggesting that this biological phenomenon accompany illness etiology and progression [4, 6, 12].
Despite continued efforts to optimize pharmacological treatment for individuals with psychiatric disorders, efficacy and tolerability of medication remains highly variable. Recently, increasing amounts of preclinical and clinical studies reveal a range of complex psychotropic activity from nutriceuticals medicines potentially beneficial for treating certain psychiatric conditions [13]. In this context, there are several pharmacological studies that showed the effects of the crude extracts of Cecropia species on CNS [11, 14–17]. In addition, the aqueous extract of C. pachystachya, induced sedative effects in mice [15], as well as antioxidant [18], hypoglycemic [19] anti-inflammatory and cytotoxic [20] activities are also reported. Further, we recently reported the neuroprotective potential of the crude extract C. pachystachya against behavioral and biochemical dysfunctions induced by chronic stress [11], suggesting that C. pachystachya might represent an interesting tool to manage the neurodegeneration associated with psychiatric disorders.
Considering the impact of BD on patients and society, there is an urgent need for the development of early intervention strategies aimed to improve treatment efficacy. Prevention of future episodes with natural compounds could avoid the substantial negative consequences associated with the disease [21]. In this context, the present work hypothesized that administration of aqueous extract (AE) from C. pachystachya might prevent some of the behavioral and neurochemical modifications in a model of mania induced by ketamine in rats.
Materials and Methods
Chemical and Reagents
Acetic acid and acetonitrile (HPLC grade) were provided by Tedia® (Brazil). Water was purified on a MilliQ system (Millipore®, Bedford, USA). All solutions used in HPLC were filtered through a 0.45 µm membrane before use. Chlorogenic acid (3-O-caffeoylquinic acid, ≥98.0 %), isovitexin (4′,5,7-tetrahydroxyflavone-6-glucoside, ≥98.0 %) isoquercitrin (3′,4′,5,7-tetrahydroxyflavone-3-O-glucoside, ≥98.0 %), isoorientin (3′,4′,5,7-tetrahydroxyflavone-6-glucoside, ≥98.0 %) and orientin (3′,4′,5,7-tetrahydroxyflavone-8-glucoside, ≥98.0 %) were purchased from Sigma Aldrich® Co. (St. Louis, USA). Ketamine and Lithium Chloride (LiCl) were purchased from Sigma Aldrich® Co. (St. Louis, USA).
Plant Material and Aqueous Extract Preparation
Aerial parts of C. pachystachya Trécul were collected in Viamão (State of Rio Grande do Sul) in March 2007. A voucher specimen (ICN 150025) was deposited in the Herbarium of Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil. The leaves of C. pachystachya were air-dried (35–40 °C) for 3 days and then extracted by infusion. Briefly, powdered leaf material (100 g) was extracted with boiled distilled water (1000 mL, 90 °C) for 30 min, filtered, freeze-dried and stored at −20 °C until use.
Chemical Characterization by High-Performance Liquid Chromatography (HPLC)
The qualitative and quantitative analyses of aqueous extract were performed as previously described [22]. Briefly, the separation was achieved on a Perkin Elmer Brownlee Choice C18 column (150 × 4.6 mm i.d.; 5 μm) and a gradient of solvent A (acetonitrile) and solvent B (acetic acid 1 %, adjusted to pH 3.0) as follows: 5–20 % A (0–30 min) and isocratic 20 % A (30–40 min) as the mobile phase. The flow rate was kept at 1.0 mL/min. The chromatograms were recorded at 340 while the UV spectra were monitored over a range of 200–450 nm. All standard solutions were analyzed in triplicate.
Animals and Drug Treatments
Female adult Wistar rats aged 11–12 weeks (250–300 g) were obtained from the Central Animal House of the Universidade Federal de Pelotas (UFPel), RS, Brazil. Animals were maintained under controlled environment (23 ± 2 °C, 12 h-light/dark cycle, free access to food and water) and handled according to the Federation of Brazilian Societies for Experimental Biology guidelines upon approval by the Ethics Committee of the UFPel (9194). The following drugs were used: (1) ketamine dissolved in saline solution (NaCl 0.9 %, w/v) administered by intraperitoneal route (i.p.); (2) AE C. pachystachya administered by oral route (p.o.); (3) LiCl dissolved in saline solution (NaCl 0.9 %, w/v) and administered by p.o. route twice a day. Appropriated vehicle groups were also assessed simultaneously. The doses of ketamine and LiCl used in the present study were chosen according to the literature [10, 23]. The C. pachystachya AE doses were previously determined by a dose response curve evaluated in the forced swim test and open-field test [17].
Experimental Protocol of Mania State
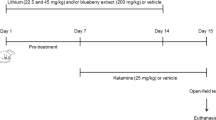
This protocol was designed to mimic the prevention protocol of the manic state, as previously proposed by Ghedim et al. [10]. Rats received saline, C. pachystachya 200 and 400 mg/kg once a day or LiCl 45 mg/kg twice a day for 14 days. From the 8th to the 14th day the animals also received saline or ketamine (25 mg/kg), once a day, totaling eight experimental groups: saline/saline, ketamine/saline, saline/LiCl 45 mg/kg, ketamine/LiCl 45 mg/kg, saline/C. pachystachya 200 mg/kg, ketamine/C. pachystachya 200 mg/kg; saline/C. pachystachya 400 mg/kg, and ketamine/C. pachystachya 400 mg/kg. On the 15th day of treatment, the animals received a single injection of ketamine or saline and the locomotor activity was assessed in the open-field apparatus after 30 min (Fig. 1).
Open-Field Test
Locomotor and anxiety-related behaviors were monitored using an open-field apparatus, as previously described [11]. The apparatus consisted of a wooden box measuring 40 × 60 × 50 cm. The floor of the arena was divided into 12 equal squares and placed in a free noise environment. Animals were placed in the rear left square and left to explore it freely for 5 min. The total number of squares crossed with all paws (crossings) was counted in order to evaluate the ambulatory behavior. The number of central crossings was the measure used to evaluate anxiety. The apparatus was cleaned up with a 10 % alcohol solution and dried after each individual animal session.
Biochemical Assay
Rats were killed by decapitation immediately after the open-field test. Prefrontal cortex (PFC) and hippocampus (HP) were manually dissected and homogenized in 10 volumes (1:10 w/v) of 20 mM sodium phosphate buffer, pH 7.4 containing 140 mM KCl. Homogenates were centrifuged at 750×g for 10 min at 4 °C, the pellet was discarded and the supernatant was immediately separated and used for the oxidative stress measurements. The protein content was quantified by the method of Lowry et al. [24], using bovine serum albumin as a standard.
Thiobarbituric Acid Reactive Species Formation (TBARS)
The measure of lipid peroxidation was determined by TBARS in according to protocol described by Esterbauer and Cheeseman [25]. Briefly, homogenates were mixed with trichloroacetic acid 10 % and thiobarbituric acid 0.67 % and heated in a boiling water bath for 25 min. TBARS was determined by the absorbance at 535 nm. Results were reported as nmol of TBARS per mg of protein.
Carbonyl Protein Formation
The oxidative damage to proteins was assessed by the determination of carbonyl groups content based on the reaction with dinitrophenylhydrazine (DNPH), as previously described [26]. Proteins were precipitated by addition of 20 % trichloroacetic acid and were re-dissolved in DNPH. The absorbance was monitored spectrophotometrically at 370 nm. Results were reported as nmol carbonyl per mg of protein.
Total Sulfhydryl Content (SH Content)
This assay was performed as described by Aksenov and Markesbery [27] which is based on the reduction of DTNB by thiols, which in turn, becomes oxidized (disulfide) generating a yellow derivative (TNB) whose absorption is measured spectrophotometrically at 412 nm. Briefly, homogenates were added to PBS buffer pH 7.4 containing EDTA. The reaction was started by the addition of 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB). Results were reported as μmol TNB per mg of protein [27].
Catalase (CAT) Assay
CAT activity was assayed by the method of Aebi [28]. H2O2 disappearance was continuously monitored during 90 s in a spectrophotometer adjusted at 240 nm. CAT specific activity was reported as units of enzyme per mg of protein [28].
Superoxide Dismutase (SOD) Assay
Total SOD activity was measured by the method described by Misra and Fridovich [29]. This method is based on the inhibition of superoxide dependent adrenaline auto-oxidation in a spectrophotometer adjusted at 480 nm. The specific activity of SOD was reported as units per mg of protein [29].
Statistical Analysis
Comparisons between experimental groups were performed by one-way or two-way analysis of variance (ANOVA) followed by Bonferroni post hoc test when appropriate. The values are expressed as mean ± SEM. p < 0.05 was considered significant.
Results
Chemical Characterization of AE by HPLC
The phenolic characterization of C. pachystachya AE was performed as described by Costa et al. [22]. It was possible to identify five compounds of the AE by comparing theirs UV spectra and the retention times (HPLC) with standard references. According to chromatogram (Fig. 2) were identified chlorogenic acid (1) isoorientin (2) orientin (3) isovitexin (4) and isoquercetrin (5).
HPLC chromatogram of AE of C. pachystachya. 1 Chlorogenic acid (27.2 ± 0.94 mg/gof extract), 2 Isoorientin (17.3 ± 0.59 mg/g of extract), 3 Orientin (17.2 ± 0.36 mg/g of extract), 4 Isovitexin (5.9 ± 0.27 mg/g of extract), 5 Isoquercitrin. Chromatographic conditions: see “Chemical Characterization by High-Performance Liquid Chromatography (HPLC)” section
Behavioral Characterization Focusing on Ambulatory Performance
The treatment with ketamine was efficient to induce hyperlocomotion in rats, as evaluated by the increase in the number of crossings in the open-field test. This locomotor parameter is an indicative of manic episode as suggested by Ghedim et al. [10]. Notably, as presented in Fig. 3, AE C. pachystachya pretreatment (200 and 400 mg/kg) and LiCl pretreatment (45 mg/kg) prevented the hyperlocomotion induced by ketamine in the open-field test (pretreatment: [F(3,40) = 3.52, p < 0.01], ketamine treatment: [F(1,40) = 16.42, p < 0.01], interaction: [F(3,40) = 7.50, p < 0.05]). No changes in the anxiety behavior, evaluated by the percentage of central crossings, were observed in the ketamine, AE C. pachystachya and/or LiCl treatment (pretreatment: [F(3,41) = 0.57, p = 0.64], ketamine treatment: [F(1,41) = 0.27, p = 0.61], interaction: [F(3,41) = 1.51, p = 0.23]) (data not shown).
Effect of AE C. pachystachya pretreatment (200 and 400 mg/kg) and lithium chloride pretreatment (45 mg/kg) on ketamine-induced hyperactivity in the open-field test. The number of crossings was recorded. Data was expressed as the mean ± SEM (n = 5–7 for group). **p < 0.01 as compared to the vehicle/saline group. # p < 0.05 as compared to the vehicle/ketamine group
Measurement of Oxidative Stress Parameters in the Prefrontal Cortex (PFC)
In order to evaluate a neuroprotective effect of AE C. pachystachya against ketamine-induced model of mania, we studied the effects of AE C. pachystachya and/or LiCl in PFC oxidative stress parameters. Figure 4a shows that AE C. pachystachya (200 and 400 mg/kg) and/or LiCl (45 mg/kg) pretreatment were able to prevent the increase in TBARS levels induced by ketamine administration in PFC (pretreatment: [F(3,41) = 9.20, p < 0.01], ketamine treatment: [F(1,41) = 0.01, p = 0.92], interaction: [F(3,41) = 10.60, p < 0.01]). Figure 4b indicates that AE C. pachystachya (200 and 400 mg/kg) and/or LiCl (45 mg/kg) pretreatment administration prevented the protein damage induced by ketamine. The two-way ANOVA revealed a significant increase in the carbonyl protein formation induced by ketamine, and a significant prophylactic effect of AE C. pachystachya and LiCl (pretreatment: [F(3,31) = 7.76, p < 0.01], ketamine treatment: [F(1,31) = 12.98, p < 0.01], interaction: [F(3,41) = 7.78, p < 0.01]). In addition, Fig. 4c shows that LiCl (45 mg/kg) alone is able to increase the SH content in when compared to control group (p < 0.01). Moreover, AE C. pachystachya (200 and 400 mg/kg) and/or LiCl (45 mg/kg) pretreatment prevented the decrease in SH content induced by ketamine in PFC (pretreatment: [F(3,39) = 53.17, p < 0.01], ketamine treatment: [F(1,39) = 6.30, p < 0.05], interaction: [F(3,39) = 6.51, p < 0.01]). Then we compared the activity of the antioxidant enzymes SOD and CAT. As shown in Fig. 4d ketamine administration did not change the activity of SOD in the PFC, as well no interaction were observed between AE C. pachystachya (200 and 400 mg/kg) and/or LiCl (45 mg/kg) (pretreatment: [F(3,34) = 1.50, p = 0.23], treatment: [F(1,34) = 0.84, p = 0.37], interaction:[F(1,34) = 2.70, p = 0.07]). There was also no evident difference in the CAT activity between groups (pretreatment: [F(3,41) = 4.95, p < 0.01], treatment: [F(1,41) = 0.33, p = 0.60], interaction: [F(3,41) = 1.19, p = 0.33]).
Effect of AE C. pachystachya pretreatment (200 and 400 mg/kg) and lithium chloride pretreatment (45 mg/kg) on TBARS formation (a); protein carbonyl (b); total SH content (c); superoxide dismutase (SOD, d); and catalase (CAT, e) activity in the prefrontal cortex (PFC) of rats. Data was expressed as mean ± SEM (n = 5–7 for group). **p < 0.01 and *p < 0.05 as compared to the vehicle/saline group. # p < 0.05 as compared to the vehicle/ketamine group
Measurement of Oxidative Stress Parameters in the Hippocampus (HP)
The effects of AE C. pachystachya in oxidative stress parameters in the HP were also evaluated. As depicted in Fig. 5a, AE C. pachystachya (200 and 400 mg/kg) and/or LiCl (45 mg/kg) pretreatment were able to prevent the increase in TBARS levels induced by ketamine administration in rats (pretreatment: [F(3,36) = 4.97, p < 0.01], treatment: [F(1,36) = 3.35, p = 0.07], interaction: [F(3,36) = 3.31, p < 0.05]). Figure 5b indicated that AE C. pachystachya (200 and 400 mg/kg) and/or LiCl (45 mg/kg) pretreatment administration prevented the carbonyl protein formation induced by ketamine, (pretreatment: [F(3,28) = 2.82, p = 0.06], ketamine treatment: [F(1,28) = 11.14, p < 0.01], interaction: [F(3,28) = 5.24, p < 0.01]).
Effect of AE C. pachystachya pretreatment (200 and 400 mg/kg) and lithium chloride pretreatment (45 mg/kg) on TBARS formation (a); protein carbonyl (b); total SH content (c); superoxide dismutase (SOD, d); and catalase (CAT, e) activity in the hippocampus (HP) of rats. Data was expressed as mean ± SEM (n = 5–7 for group). **p < 0.01 and *p < 0.05 as compared to the vehicle/saline group. # p < 0.05 as compared to the vehicle/ketamine group
The results in Fig. 5c demonstrated that ketamine treatment did not change the total SH content when compared to the control group. However, LiCl pretreatment (45 mg/kg) associated to ketamine treatment increased the SH in the hippocampus of rats (pretreatment [F(3,41) = 29.85, p < 0.01] ketamine treatment: [F(1,41) = 1.61, p = 0.21], interaction: [F(2,33) = 29.85, p < 0.01]). Notably, Fig. 5d shows that SOD activity was significantly decreased by ketamine and this effect was completely prevented in AE C. pachystachya (200 and 400 mg/kg) and/or LiCl (45 mg/kg) pretreated groups (pretreatment: [F(3,34) = 19.47, p < 0.01], ketamine treatment: [F(1,34) = 1.44, p = 0.24], interaction: [F(3,34) = 9.81, p < 0.01]). In addition, Fig. 5e also indicated that ketamine-treated rats had a decrease in CAT activity, and the pretreatment with both doses of AE C. pachystachya (200 and 400 mg/kg) and/or LiCl (45 mg/kg) were not able to prevent this effect (pretreatment: [F(3,35) = 3.19, p < 0.05], ketamine treatment: [F(1,35) = 17.87, p < 0.01], interaction: [F(3,35) = 3.60, p < 0.05]).
Discussion
Despite the challenge in replicating the complex symptoms associated with psychiatric disorders, animal models have been successfully able to mimic some of the neurochemical and physiological characteristics of these conditions [30]. In the present study, we showed at the first time that the oral administration of AE C. pachystachya prevented behavioral and biochemical changes observed in the mania model induced by the administration of ketamine in rats.
The difficulties in finding good preclinical models for psychiatric conditions lies in the lack of biological basis underlying the diagnosis categories, associated with the absence of clear boundaries between different psychiatric manifestations and heterogeneity in symptoms manifestations. In this context, non-anesthetic doses of ketamin are able to induce hyperlocomotion, stereotypy, impaired cognitive function and social interaction [31, 32]. The evaluation of the hyperlocomotion in rodents might represent a good and simple way to mimic a behavioral phenotype that is commonly associated with several psychiatric conditions. Ketamine is a dissociative anesthetic that acts as a noncompetitive antagonist of the N-methyl-d-aspartate (NMDA) glutamate receptor. High densities of this receptor—are found in the HP and cerebral cortex, critical areas involved in several behavioral functions, including emotional patterns, memory processing and cognition. The administration of ketamine mimics several behavioral (e.g., hyperactivity) and neurochemical alterations observed in individuals with BD experiencing a manic episode. Moreover, it is already described that sub-anesthetic doses of ketamine increase lipid peroxidation and tissue protein oxidation in multiple cerebral structures [33]. Ketamine is often associated with altered mitochondrial function and is capable to interfere with mitochondrial complex I function, impair oxidative phosphorylation efficiency of glutamate-malate substrate, and decrease NADH-ubiquinone oxidoreductase activity. In addition, higher doses of ketamine were already showed to increase mitochondrial nitric oxide synthase (mtNOS) activity levels of nitric oxide and hydrogen peroxide generation [34]. Previous studies have established that conventional mood stabilizer, such as lithium chloride and valproate are able to prevent behavioral and biochemical alterations observed in ketamine-treated animals, thereby indicating the predictive validity of the model [10, 11]. Moreover, preclinical studies demonstrated that glutamatergic system abnormalities are involved in the pathophysiology of BD, pointing to the potential construct validity of this model [35, 36]. Our work corroborates and extends these data suggesting that this protocol fulfills adequate characteristics of an animal model of mania, reinforcing previous proposals that both glutamatergic system and oxidative stress might be involved in this particular stage of BD [4, 37].
In fact, oxidative stress has been implicated in the pathophysiology of BD [7]. Clinical studies have been showed the central and peripheral involvement of oxidative damage in BD patients demonstrated that these markers might hold promise to reflect brain alterations [38]. Increased ROS levels generate deleterious effects on signal transduction, structural plasticity and cellular resilience, mostly by inducing lipid peroxidation in membranes, damage to proteins and nucleic acids [39]. Our results showed that ketamine model induces oxidative damage by increasing the amount of TBARS and carbonyl protein formation in the PFC and HP of rats. Moreover, ketamine administration reduced the activity of antioxidant enzymes SOD and CAT in the HP. In agreement with these findings, literature data shows that mania models increase ROS generation in several brain regions [10, 11, 40]. The mechanisms underlying these highly region-specific patterns of vulnerability against oxidative damage and other noxious stimuli are still far from being well understood. Hippocampal neurons were extensively demonstrated in the literature to be intrinsically more vulnerable to several types of insults, including mechanical insult, traumatic injury, kainic acid toxicity, stress and ischemic insults when compared to cortical neurons [41–44]. In an attempt to explain, at least partially, this selective pattern of vulnerability associated with ketamine administration, we speculated that due to increased NMDA receptor densities and lower energy capacities, as evidenced by increased susceptibility to ischemic insults, hippocampal neurons are more sensitive to ketamine in the mania model as well. Indeed, the brain NMDA receptors are unevenly distributed. Highest receptor densities are found in the hippocampus (Ca1 > Ca3) followed by cortical areas [45, 46]. As far as we aware, this is the first work reporting the preventive effects of C. pachystachya treatment in oxidative parameters induced by ketamine. These promising antioxidant properties could be explained by the accumulation of phenolic compounds and flavonoids observed in the AE of this specie. Phytochemical analysis of the AE C. pachystachya leaves showed isoorientin and chlorogenic acid as the major compounds, as well as orientin, isovitexin and isoquercetrin [22]. The biological activity of many medicinal plants and other natural products are directly related to their content of flavonoid and phenolic compounds [47]. Literature data showed that chlorogenic acid has anxiolytic, antioxidant and neuroprotective activities [48, 49]. In addition, a positive correlation between the level of flavonoids and the psychopharmacologic activity was previously described [50]. Finally flavonoids, like the isoorientin, are well known by their antioxidant properties, preventing oxidative stress, which is believed to trigger neurodegeneration in this animal model, as well as in the clinical condition [51]. In this manner, we could suggest that the neuroprotective effect of C. pachytachya might be a result of direct actions in specific cellular targets (i.e. monoaminergic receptors or transporters), combined with indirect effects mediated by the decrease in the oxidative stress and changes in the redox states of proteins, lipids and nucleic acids [52, 53].
Recently, Gazal et al. [17] demonstrated that C. pachystachya was able to prevent both the depressive behavior and the oxidative damage induced by chronic unpredictable stress (CUS), supporting its neuroprotective potential against behavioral and biochemical dysfunctions induced by CUS. A previous work from our group showed that curcumin, another natural antioxidant agent, prevented the hyperlocomotion and the oxidative stress induced by ketamine in rats [11]. From the clinical point of view, a couple of studies investigated the potential antioxidant effect of inositol, a member of vitamin B family in BD [54, 55]. In addition, the antioxidant N-acetyl-cysteine has been extensively used in combination with BD pharmacotherapies, showing higher effectivity in the clinical trials [13]. Therefore, it is becoming clear that compounds with antioxidant properties can improve manic symptoms, counteract neurodegeneration associated with oxidative stress and should be explored as possible adjunct therapy.
The finding that AE of C. pachystachya, like LiCl, is able to prevent ketamine-induced biochemical and behavioral alterations is noteworthy. It is important to highlight that in our study AE of C. pachystachya administration produced similar protective effects to the well-established mood stabilizer lithium chloride, used as a positive control. This data may be of therapeutic relevance suggesting a potential role for C. pachystachya in the management of BD. Moreover, our findings support the hypothesis that the prevention of oxidative stress is a main mechanism that accounts for the etiology and progression of BD. However, future investigations still need evaluate the anti-inflammatory effect of the aqueous extract of C. pachystachya and their isolated compounds in the psychiatric conditions.
References
Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC (2007) Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 64:543–552
Tang V, Wang JF (2012) Oxidative stress in bipolar disorder. Biochem Anal Biochem. doi:10.4172/2161-1009.S2-002
Ketter TA, Houston JP, Adams DH, Risser RC, Meyers AL, Williamson DJ, Tohen M (2006) Differential efficacy of olanzapine and lithium in preventing manic or mixed recurrence in patients with bipolar I disorder based on number of previous manic or mixed episodes. J Clin Psychiatry 67:95–101
Andreazza AC, Kauer-Sant’anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, Yatham LN (2008) Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord 111:135–144
Martinowich K, Schloesser RJ, Manji HK (2009) Bipolar disorder: from genes to behavior pathways. J Clin Invest 119:726–736
Kapczinski F, Dal-Pizzol F, Teixeira AL, Magalhaes PV, Kauer-Sant’Anna M, Klamt F, Moreira JC, de Bittencourt Pasquali MA, Fries GR, Quevedo J, Gama CS, Post R (2011) Peripheral biomarkers and illness activity in bipolar disorder. J Psychiatr Res 45:156–161
Ng F, Berk M, Dean O, Bush AI (2008) Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 11:851–876
Valvassori SS, Petronilho FC, Reus GZ, Steckert AV, Oliveira VB, Boeck CR, Kapczinski F, Dal-Pizzol F, Quevedo J (2008) Effect of N-acetylcysteine and/or deferoxamine on oxidative stress and hyperactivity in an animal model of mania. Prog Neuropsychopharmacol Biol Psychiatry 32:1064–1068
Frey BN, Valvassori SS, Reus GZ, Martins MR, Petronilho FC, Bardini K, Dal-Pizzol F, Kapczinski F, Quevedo J (2006) Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci 31:326–332
Ghedim FV, Fraga Dde B, Deroza PF, Oliveira MB, Valvassori SS, Steckert AV, Budni J, Dal-Pizzol F, Quevedo J, Zugno AI (2012) Evaluation of behavioral and neurochemical changes induced by ketamine in rats: implications as an animal model of mania. J Psychiatr Res 46:1569–1575
Gazal M, Ortmann CF, Martins FA, Streck EL, Quevedo J, de Campos AM, Stefanello FM, Kaster MP, Ghisleni G, Reginatto FH, Lencina CL (2014) Antidepressant-like effects of aqueous extract from Cecropia pachystachya leaves in a mouse model of chronic unpredictable stress. Brain Res Bull 108C:10–17
Frey BN, Andreazza AC, Kunz M, Gomes FA, Quevedo J, Salvador M, Goncalves CA, Kapczinski F (2007) Increased oxidative stress and DNA damage in bipolar disorder: a twin-case report. Prog Neuropsychopharmacol Biol Psychiatry 31:283–285
Sarris J, Mischoulon D, Schweitzer I (2011) Adjunctive nutraceuticals with standard pharmacotherapies in bipolar disorder: a systematic review of clinical trials. Bipolar Disord 13:454–465
Rocha FF, Lapa AJ, De Lima TC (2002) Evaluation of the anxiolytic-like effects of Cecropia glazioui Sneth in mice. Pharmacol Biochem Behav 71:183–190
Consolini AE, Ragone MI, Migliori GN, Conforti P, Volonte MG (2006) Cardiotonic and sedative effects of Cecropia pachystachya Mart. (ambay) on isolated rat hearts and conscious mice. J Ethnopharmacol 106:90–96
Rocha FF, Lima-Landman MT, Souccar C, Tanae MM, De Lima TC, Lapa AJ (2007) Antidepressant-like effect of Cecropia glazioui Sneth and its constituents—in vivo and in vitro characterization of the underlying mechanism. Phytomedicine 14:396–402
Gazal M, Valente MR, Acosta BA, Kaufmann FN, Braganhol E, Lencina CL, Stefanello FM, Ghisleni G, Kaster MP (2014) Neuroprotective and antioxidant effects of curcumin in a ketamine-induced model of mania in rats. Eur J Pharmacol 724:132–139
Pacheco NR, de Pinto NC, da Silva JM, de Mendes RF, da de Costa JC, Aragao DM, Castanon MC, Scio E (2014) Cecropia pachystachya: a species with expressive in vivo topical anti-inflammatory and in vitro antioxidant effects. Biomed Res Int. doi:10.1155/2014/301294
Aragao DM, Guarize L, Lanini J, da Costa JC, Garcia RM, Scio E (2010) Hypoglycemic effects of Cecropia pachystachya in normal and alloxan-induced diabetic rats. J Ethnopharmacol 128:629–633
Aragao DM, Lima IV, da Silva JM, Bellozi PM, da Costa Jde C, Cardoso GM, de Souza-Fagundes EM, Scio E (2013) Anti-inflammatory, antinociceptive and cytotoxic effects of the methanol extract of Cecropia pachystachya Trecul. Phytother Res 27:926–930
Leopold K, Pfennig A, Severus E, Bauer M (2013) Prevention of bipolar disorders. Nervenarzt 84:1310–1315
Costa GM, Ortmann CF, Schenkel EP, Reginatto FH (2011) An HPLC-DAD method to quantification of main phenolic compounds from leaves of Cecropia species. Braz Chem Soc 26:1096–1102
Bruning CA, Prigol M, Luchese C, Pinton S, Nogueira CW (2012) Diphenyl diselenide ameliorates behavioral and oxidative parameters in an animal model of mania induced by ouabain. Prog Neuropsychopharmacol Biol Psychiatry 38:168–174
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Levine RL, Williams JA, Stadtman ER, Shacter E (1994) Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol 233:346–357
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Kato T, Kubota M, Kasahara T (2007) Animal models of bipolar disorder. Neurosci Biobehav Rev 31:832–842
Lipska BK, Weinberger DR (2000) To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology 23:223–239
Bubenikova-Valesova V, Svoboda J, Horacek J, Sumiyoshi T (2010) Effect of tandospirone, a serotonin-1A receptor partial agonist, on information processing and locomotion in dizocilpine-treated rats. Psychopharmacology 212:267–276
Venancio C, Felix L, Almeida V, Coutinho J, Antunes L, Peixoto F, Summavielle T (2015) Acute ketamine impairs mitochondrial function and promotes superoxide dismutase activity in the rat brain. Anesth Analg 120:320–328
Bosnjak ZJ, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Wells CW, Corbett JA, Bai X (2012) Ketamine induces toxicity in human neurons differentiated from embryonic stem cells via mitochondrial apoptosis pathway. Curr Drug Saf 7:106–119
Kugaya A, Sanacora G (2005) Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr 10:808–819
Maeng S, Zarate CA Jr (2007) The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep 9:467–474
Machado-Vieira R (2012) Purinergic system in the treatment of bipolar disorder: uric acid levels as a screening test in mania. J Clin Psychopharmacol 32:735–736
Brown NC, Andreazza AC, Young LT (2015) An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res 218:61–68
Mahadik SP, Evans D, Lal H (2001) Oxidative stress and role of antioxidant and omega-3 essential fatty acid supplementation in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 25:463–493
Brocardo PS, Budni J, Pavesi E, Franco JL, Uliano-Silva M, Trevisan R, Terenzi MG, Dafre AL, Rodrigues AL (2010) Folic acid administration prevents ouabain-induced hyperlocomotion and alterations in oxidative stress markers in the rat brain. Bipolar Disord 12:414–424
Zhao G, Flavin MP (2000) Differential sensitivity of rat hippocampal and cortical astrocytes to oxygen–glucose deprivation injury. Neurosci Lett 285:177–180
Candelario-Jalil E, Al-Dalain SM, Castillo R, Martinez G, Fernandez OS (2001) Selective vulnerability to kainate-induced oxidative damage in different rat brain regions. J Appl Toxicol 21:403–407
Golarai G, Greenwood AC, Feeney DM, Connor JA (2001) Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J Neurosci 21:8523–8537
Geddes DM, LaPlaca MC, Cargill RS (2003) Susceptibility of hippocampal neurons to mechanically induced injury. Exp Neurol 184:420–427
Monaghan DT, Cotman CW (1985) Distribution of N-methyl-d-aspartate-sensitive l-[3H]glutamate-binding sites in rat brain. J Neurosci 5:2909–2919
Maragos WF, Chu DC, Greenamyre JT, Penney JB, Young AB (1986) High correlation between the localization of [3H]TCP binding and NMDA receptors. Eur J Pharmacol 123:173–174
Rice-Evans C (2004) Flavonoids and isoflavones: absorption, metabolism, and bioactivity. Free Radic Biol Med 36:827–828
Bouayed J, Rammal H, Dicko A, Younos C, Soulimani R (2007) Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J Neurol Sci 262:77–84
Pathak L, Agrawal Y, Dhir A (2013) Natural polyphenols in the management of major depression. Expert Opin Investig Drugs 22:863–880
De-Paris F, Petry RD, Reginatto F, Gosmann G, Quevedo J, Salgueiro JB, Kapczinski F, González Ortega G, Schenkel EP (2002) Pharmacochemical study of aqueous extracts of Passiflora alata Dryander and Passiflora edulis Sims. Acta Farmaceutica Bonarense 21:5–7
Grosso C, Valentao P, Ferreres F, Andrade PB (2013) The use of flavonoids in central nervous system disorders. Curr Med Chem 20:4694–4719
Sena LM, Zucolotto SM, Reginatto FH, Schenkel EP, De Lima TC (2009) Neuropharmacological activity of the pericarp of Passiflora edulis flavicarpa degener: putative involvement of C-glycosylflavonoids. Exp Biol Med (Maywood) 234:967–975
Park SH, Sim YB, Han PL, Lee JK, Suh HW (2010) Antidepressant-like effect of chlorogenic acid isolated from Artemisia capillaris Thunb. Anim Cells Syst 14:253–259
Chengappa KN, Levine J, Gershon S, Mallinger AG, Hardan A, Vagnucci A, Pollock B, Luther J, Buttenfield J, Verfaille S, Kupfer DJ (2000) Inositol as an add-on treatment for bipolar depression. Bipolar Disord 2:47–55
Eden Evins A, Demopulos C, Yovel I, Culhane M, Ogutha J, Grandin LD, Nierenberg AA, Sachs GS (2006) Inositol augmentation of lithium or valproate for bipolar depression. Bipolar Disord 8:168–174
Acknowledgments
This work was supported by CNPq, FAPERGS, FAPESC and CAPES Brazil. The authors are also grateful to CNPq and CAPES for their research fellowships. We thank the team at the UFPel animal facility for managing the animals.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gazal, M., Kaufmann, F.N., Acosta, B.A. et al. Preventive Effect of Cecropia pachystachya Against Ketamine-Induced Manic Behavior and Oxidative Stress in Rats. Neurochem Res 40, 1421–1430 (2015). https://doi.org/10.1007/s11064-015-1610-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1610-5