Abstract
This work investigated the antioxidant and antidepressant-like effects of ethyl acetate extract from Eugenia catharinensis in mice treated with corticosterone (20 mg/Kg). The animals received saline or corticosterone (21 days) and, in the last 7 days, they were treated with the extract (50, 125, 200 or 250 mg/Kg) or vehicle. After 24 h, the mice were submitted to the open field and forced swimming tests, after which the hippocampus and cerebral cortex were removed. Our results showed that the extract decreased the immobility time of mice in the forced swimming test and that the extract was able to reverse the effect caused by corticosterone. Corticosterone pre-treatment generated oxidative stress, altering antioxidant enzymes in the nervous tissue. The extract increased the catalase and superoxide dismutase activities and reversed the effects of corticosterone. In the hippocampus, the extract increased superoxide dismutase activity and reversed the increase in catalase activity elicited by corticosterone. We propose that the effects elicited by the Eugenia catharinensis are dependent on the presence of phenolic compounds (gallic acid, protocatechuic acid, syringic acid, 4-hydroxy methylbenzoic acid, chlorogenic acid, salicylic acid, caffeic acid, vanillic acid, p-coumaric acid, isoquercetin, rutin, ferulic acid, aromadendrin, galangin and apigenin) in this extract, as demonstrated by HPLC-ESI-MS/MS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is a debilitating disease characterized by depressed mood, diminished interests, impaired cognitive function and vegetative symptoms, such as disturbed sleep or appetite. More than 300 million people live with depression, an increase of more than 18% between 2005 and 2015 (World Health Organization 2017). According to the World Health Organization (WHO 2017), depression will be the most common mental disorder in the world by 2030, affecting more people than diseases like cancer. Although several neurobiological-based hypotheses for the cause of depression have been proposed, the underlying molecular mechanisms remain obscure (Umehara et al. 2017).
In depressive disorders, alteration in the hypothalamic-pituitary-adrenal (HPA) axis, often linked to stress, has been frequently observed. In this process, there is deregulation of negative feedback on this axis, leading to a continuous elevation of cortisol secretion. Chronic corticosterone (CORT) administration in rodents is shown to promote depressive-like behaviors (Weng et al. 2016) and repeated CORT treatment alters antioxidant enzyme activities in the brain and increases pro-oxidant markers such as lipid peroxidation (Zafir and Banu 2009). Although depression is a multifactorial disease, oxidative stress, the result of excessive reactive oxygen species (ROS) production, has been implicated in the pathogenesis of this disorder (Ng et al. 2008). ROS may cause enzyme inhibition, lipid peroxidation and mitochondrial changes. Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) decompose ROS molecules, producing less toxic compounds (Zanoveli et al. 2016).
The use of alternative therapies is highly sought after by patients with depression, especially as medicinal plants. St. John’s herb has been widely used for mild-to-moderate depression (Grundmann et al. 2010). Lavandula officinalis tincture, commonly known as English Lavender, has long been used in traditional medicine for some nervous disorders such as epilepsy and depression (Nikfarjam et al. 2017). Thus, developing safe and effective agents from traditional herbs may provide us with a good way to lessen their side effects, as well as improve their efficacy.
Eugenia catharinensisis D. Legrand is a rare Myrtaceae species, found in lower layer Atlantic Rainforest in the States of Santa Catarina, Paraná and São Paulo and is popularly called Guamirim. The genus Eugenia L. (Myrtaceae) comprises about 1000 species, being one of the largest of the Myrtaceae family and has been widely used by the population for therapeutic purposes as a diuretic, hypoglycemic, antihypertensive, and anti-inflammatory, among others (Zaki et al. 2013). Another common species, E. uniflora is used in folk medicine for the treatment of symptoms related to depression and mood disorders (Colla et al. 2012). Different Eugenia species have presented antioxidant effects, which seem to be dependent on the phenolic compounds present in this genus (Magina et al. 2010).
In view of the growing number of individuals affected by depression worldwide, an understanding of the range of pharmacological effects of species from the Myrtaceae family is of extreme importance to investigate the possible antioxidant and antidepressant-like effects of other Myrtaceae species, such as E. catharinensis. The present study aims to investigate the antioxidant and antidepressant-like effects of the ethyl acetate extract from E. catharinensis (EAE) leaves in an animal model of depressive-like behavior induced by chronic CORT administration.
Material and methods
Analyses of phenolic compounds in the EAE were conducted in an Agilent® 1200 chromatograph with TurbolonSpray® as an ionization source coupled to a Qtrap® 3200 mass spectrometer. Biochemical analyzes were performed using a UV-visible Shimadzu® spectrophotometer. All reagents are analytical grade and obtained from Sigma-Aldrich®. Solvents used in the extraction process were obtained from Vetec.
Preparation of Eugenia catharinensis extract
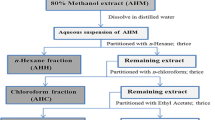
Leaves from Eugenia catharinensis were collected in Blumenau, Santa Catarina, Brazil (26o90’63” S, 49o08’01” W) on October 2016. The identification was made by the botanical André Luís de Gasper. A voucher specimen from this plant was deposited in the Dr. Roberto Miguel Klein Herbarium (FURB, http://furb.jbrj.gov.br) under registration number 14995. The collected leaves were dried at room temperature and ground in a knife mill. The pulverized sample was macerated in three different solvents of different polarity, 70% ethyl alcohol, ethyl acetate and dichloromethane, to obtain crude extracts. The maceration process was carried out for three days, after which the extract was filtered, and the procedure was repeated once more. The extracts resulting from the two macerations were pooled and concentrated with a rotary evaporator under reduced pressure until complete drying.
Analysis of phenolic compounds in the EAE by HPLC-ESI-MS/MS
EAE was analyzed by HPLC-ESI-MS/MS (High Performance Liquid Chromatography tandem Mass Spectrometry with Electrospray Ionization) in the LABEC (Laboratório de eletroforese capilar) at the Universidade Federal de Santa Catarina (UFSC), according to Pauleti et al. (2017) with slight modifications. Analyses were conducted with a Phenomenex® Synergi 4 μ Polar-RP 80A (150 mm × 2 mm ID, particle size of 4 μm) at a temperature of 30 °C. The eluents were formed by mixing solvents A (MeOH/H2O in ratio of 95:5, v v−1) and B (H2O ultrapure/formic acid (0,1%) as follows: 1st stage – 10% solvent A and 90% B (isocratic mode) for 5 min; 2nd stage – linear gradient of solvents A and B (from 10 to 90% of A) for 2 min; 3rd stage – 90% solvent A and 10% B (isocratic mode) for 3 min; 4th stage – linear gradient of solvents A and B (from 90 to 10% of A) for 7 min with a flow rate of 250 μL min−1 for the mobile phase. For the analysis, an aliquot of 50 mg of EAE was ressupended in 5 mL of HCl at pH 2. These 5 mL were extracted three times with 2 mL of ethyl ether each, which were then combined. After drying the combined extract, it was stored sealed at −20 °C. To perform the analysis, the dried material was dissolved in 1 mL of MeOH and centrifuged at 12,000 rpm for 120 s. Three parts of the supernatant was add to 7 parts of ultrapurified water and the injected volume was 5 μL.
For the identification of compounds, 47 standard phenolic compounds (4-aminobenzoic acid, 4-methyl-umbelliferone, 4-hydroxymethylbenzoic acid, p-anisic acid, caffeic acid, cinnamic acid, chlorogenic acid, ellagic acid, ferulic acid, gallic acid, mandelic acid, methoxyphenylacetic acid, p-coumaric acid, rosmarinic acid, salicylic acid, sinapic acid, syringic acid, vanillic acid, apigenin, aromadendrin, carnosol, catechin, chrysin, coniferaldehyde, epicatechin, epigallocatechin, epigallocatechin-gallate eriodictyol, scopoletin, fustin, galangin, hispidulin, isoquercetrin, kaempferol, myricetrin, naringenin, naringin, pinocembrin, protocatechuic acid, quercetin, resveratrol, rutin, sinapaldehyde, syringaldehyde, taxifolin, umbelliferone and vanillin) diluted in methanol (1 mg L−1) were analysed under the same conditions as described above. For the quantitative analysis of the identified compounds, the area of each peak was interpolated into calibration curves performed with the identified standards (r2 > 0.98), under the same conditions of analysis. The experiments were performed in duplicate.
The liquid chromatograph was coupled to a mass spectrometer with an electrospray ionization source using a negative ionization mode with the following source parameters: ion spray interface at 400 °C; ion spray voltage of 4500 V; curtain gas, 10 psi; nebulizer gas, 45 psi; auxiliary gas, 45 psi; collision gas, medium. The Analyst® (version 1.5.1) software was used for recording and processing the data. Pairs of ions were monitored in MRM (Multiple Reaction Monitoring) mode.
Animals
Male Swiss mice (30-40 g) were used in the study and maintained at a constant temperature (26 °C), under a 12:12 h light-dark cycle and with free access to water and food. Each animal was used only once and was sacrificed after the behavior tests by decapitation with no anesthesia. The “Principles of Laboratory Animal Care” (NIH publication 85–23, revised 1985) were followed in all experiments and the experimental protocol was approved by the Ethics Committee for Animal Research of the Universidade Regional de Blumenau, Blumenau, Brazil, under the protocol number 107/16. The animals were randomly divided into 10 experimental groups as follows: control 1, control 2, G1, G2, G3, G4, G5, G6, G7 and G8. The animals of the control 1, G1, G3, G5 and G7 groups were treated for 21 days, subcutaneously (sc), with saline. For the animals of the Control 2, G2, G4, G6 and G8 groups CORT (Sigma-Aldrich®) was administered for 21 days at a dose of 20 mg/kg (sc). During the last 7 days of treatment, vehicle (distilled water with tween 80 2%) (control 1, control 2 groups) or EAE were administered at doses of 50 mg/Kg (G1, G2groups), 125 mg/Kg (G3, G4groups), 200 mg/Kg (G5, G6 groups) or 250 mg/Kg (G7, G8 groups). Twenty-four hours after the last treatment, the animals were submitted to behavioral tests before being sacrificed by decapitation. The hippocampi and cerebral cortexes were removed from animals for the evaluation of oxidative stress parameters (Fig. 1).
Forced-swimming test
The test was performed in a plastic cylinder with 24 cm in height, 10 cm in diameter and 19 cm of water at 25 °C (±1 °C). The immobility time was recorded. It is known that antidepressants generate an action that reduces the time of immobility in the forced swimming test (Porsolt et al. 1977).
Open field test
To eliminate the possibility of immobility reduction in the FST due to an increase in locomotor activity but not an antidepressant effect, a session was held in the OFT. The test was done in a wooden box measuring 40 × 60 cm and 50 cm high. The floor of the box is divided into 12 squares of equal size. The number of squares that each mouse crossed with all four legs for 6 min was recorded (Pauleti et al. 2017).
Oxidative stress determination procedure
The hippocampus and cerebral cortex were removed and kept on ice-cold buffered sodium phosphate (20 mM, pH 7.4, 140 mMKCl). The organs were homogenized in ten volumes (1:10 w/v) of appropriate buffer, according to the technique to be performed. Homogenates were prepared using a Potter-Elvehjem homogenizer (Remi motors, Mumbai, India) by passing 5 pulses and centrifuging at 800 x g for 10 min at 4°C before discarding nuclei and cell debris. The pellet was discarded, and the supernatant was saved in aliquots and stored at −80°C for assaying the activity of antioxidant enzymes and damage to proteins and lipids (Delwing-de Lima et al. 2017).
Thiobarbituric acid reactive substances (TBA-RS)
TBA-RS were determined according to the method described by Ohkawa et al. (1979).
Total sulfhydryl content
The total thiol group concentration was determined by the method of Aksenov & Markesbery (Aksenov and Markesbery 2001).
Catalase assay (CAT)
CAT activity was assayed by the method described by Aebi (1984), using a UV–visible Shimadzu spectrophotometer.
Glutathione peroxidase assay (GSH-Px)
GSH-Px activity was measured by the method of Wendel (1981), using tert-butyl-hydroperoxide as substrate.
Superoxide dismutase assay (SOD)
The method used to assay SOD activity is based on the capacity of pyrogallol to autoxidize, a process highly dependent on superoxide (O2•-), which is a substrate for SOD (Marklund 1985).
Protein determination
Protein was measured by the Lowry et al. (1951) method, using serum bovine albumin as standard (Lowry et al. 1951).
Statistical analysis
The results were evaluated through the Kolmogorov-Smirnov normality test and the two-way ANOVA was performed afterwards, followed by the Newman-Keuls test, when necessary. The effects of CORT administration in the CAT, SOD and GSH-Px activities, TBA-RS levels and total sulfhydryl content were analyzed by the student-t test. The results were considered significant when p < 0.05.
Results and discussion
The mass spectra data and results of quantification of the compounds identified are shown on Table 1. Results indicate that from the 47 phenolic standards, 15 of them could be identified in the EAE extracts, of which 9 were phenolic acids and 5 were flavonoids. 14 of these compounds could be quantified, with the exception of rutin, whose concentration was below the limit of quantification of the methodology applied.
In order to verify the antidepressant-like effect of the EAE, the animals were treated with the extract at doses of 50, 125, 200 and 250 mg/Kg. As shown in Fig. 2, EAE (50, 125 and 200 mg/Kg), decreased the immobility time of mice in the FST, when compared to the control group, and CORT (20 mg/Kg) treatment provoked a depressive-like behavior (Fig. 2a). EAE was able to reverse the effect caused by CORT, without altering the animals’ locomotion at OFT (Fig. 2b) indicating that the reduction in the animals’ immobility time in the FST is not due to a psychostimulant effect. Several species belonging to the Eugenia genus have shown antidepressant-like effects in mice; these species include E. brasiliensis, E. catharinae, E. umbelliflora (Colla et al. 2012) and E. uniflora (Novack et al. 2013).
Although depression has been strongly associated with genetic causes, other factors, such as the activation of the hypothalamic-pituitary-adrenal (HPA) axis, especially the release of glucocorticoids, have been associated with increased risk of depression development. Some animal models used to study depression have focused on chronic CORT administration, which increases the animal’s immobility time in the FST (Levinstein and Samuels 2014). Our results are consistent with this model, since CORT treatment increased the immobility time in the FST. Furthermore, EAE reversed the effect exerted by CORT in the FST. Some antidepressant treatments reverse the effects of CORT on the depression-like behaviour of animals (Rainer et al. 2012). The increase in immobility time in the FST is characteristic of a depressive-like behaviour, while the inverse corresponds to an antidepressant-like effect (Porsolt et al. 1977).
CORT is toxic for neurons and causes an increase in ROS, which, when elevated due to an imbalance in the production and efficiency of antioxidant defences, leads to oxidative stress. Due to high rate of oxygen consumption, the central nervous system (CNS) is more vulnerable to free radical formation and consequently neuronal damage has been implicated in depression (Zafir and Banu 2009). Antioxidant enzymes, such as CAT, SOD and GSH-Px, are essential for neutralizing these free radicals; however, these enzymes are altered in depression (Thakare et al. 2017). During oxidative stress, the amount of ROS produced promotes lipid peroxidation, which causes injury to tissue cells. This process can be illustrated by the increase in the TBA-RS levels, a parameter of lipid oxidation that reflects the amount of malondialdehyde formation, a product of membrane fatty acid peroxidation. Moreover, experimental animals subjected to CORT treatment (20 mg/Kg, 21 days) exhibit elevated TBA-RS levels in the brain (Zeni et al. 2017).
In order to determine the effects of CORT administration on CAT, SOD and GSH-Px activities, TBA-RS levels and total sulfhydryl content in the cerebral cortex and hippocampus, mice were treated with CORT (20 mg/Kg) or saline. CORT treatment increased CAT and SOD activities in the cerebral cortex (Fig. 3a) and hippocampus (Fig. 4a) of mice. However, the GSH-Px activity was decreased only in the hippocampus (Fig. 4a) after CORT administration. Figures 3b and 4b show the effects of CORT on TBA-RS levels, which were increased only in the cerebral cortex. CORT treatment did not change the sulfhydryl content in the cerebral structures of mice (Figs. 3c and 4c). These findings can be explained by the fact that CORT leads to overproduction of ROS, resulting in an increase in antioxidant enzymes and lipid peroxidation. In animal models of stress, CORT levels appear elevated in the brain, in association with an increase in lipid peroxidation (Thakare et al. 2017). An overproduction of ROS has been reported, as well as an increase in the antioxidant enzyme activities in patients with depression, besides lipid peroxidation (Bilici et al. 2001). Furthermore, our results show that CORT decreased GSH-Px activity in the hippocampi of mice, which may indicate the consumption of this enzyme during removal of excess ROS or a decrease in its co-factor, glutathione (GSH). A study, conducted by Abuelezz and Hendawy (2017), showed a decreased in the GSH levels in the hippocampus of rats submitted to chronic restraint stress (CRS) with the elevation of CORT levels.
Since oxidative stress and depression have been correlated, we evaluated the antioxidant effect of EAE. Based on previous results, we measured only the oxidative stress markers that were altered in the cerebral cortex and hippocampus of mice (Figs. 5 and 6). CORT pre-treatment generated oxidative stress and altered antioxidant enzymes in the cerebral cortex. The administration of EAE for 7 consecutive days, at doses of 200 mg/Kg and 250 mg/Kg, led to significant increases in the CAT and SOD activities and reversed the effect exerted by CORT at the dose of 50 mg/Kg. Two-way ANOVA demonstrated that EAE per se did not alter TBA-RS levels and reversed the effect elicited by CORT pre-treatment (Fig. 5c).
Effects of EAE administration on corticosterone-induced alterations in the antioxidant enzymes in the cerebral cortex of mice. CAT (a) and SOD (b) activities and TBA-RS (C) levels. Values are expressed as means ± S.E.M., *** p < 0.001, as compared with vehicle-treated group (control); ##p < 0.01, ###p < 0.001, as compared with the same group that was pretreated with CORT (n = 7)
Effects of EAE administration on corticosterone-induced alterations in antioxidant enzymes in the hippocampus of mice. CAT (a), SOD (b) and GSH-Px activities. Values are expressed as means ± S.E.M., **p < 0.01, ***p < 0.001 as compared with vehicle-treated group (control); ##p < 0.01, ###p < 0.001 as compared with the same group that was pretreated with CORT (n = 6)
In the hippocampus of mice, the activities of both, CAT and SOD increased, and the activity of GSH-Px decreased, after the CORT administration (Fig. 6). In addition, EAE treatment at doses of 50, 125, 200 and 250 mg/Kg was able to increase SOD activity (Fig. 6b) without altering CAT (Fig. 6a), when compared to the control group. EAE treatment reversed the increased in CAT activity elicited by CORT. However, at doses of 200 and 250 mg/Kg, when administrated in animals that received CORT, EAE enhanced the SOD activity when compared to the CORT-treated group. With regard to GSH-Px, CORT decreased its activity, and the EAE treatment, at doses of 125, 200 and 250 mg/Kg, reversed the effect of CORT on GSH-Px activity (Fig. 6c).
These findings indicate that EAE improves antioxidant defences in the brain areas, since it was able to increase the activities of antioxidant enzymes. Ethyl acetate fractionation from different species of Eugenia, such as E. brasiliensis and E. beaurepaireana, has demonstrated antioxidant potential, due to the high 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical uptake of these extracts (Magina et al. 2010).
There is a well-documented association between the appearance of depressive behaviour and an increase in oxidative stress in specific brain areas, including the hippocampus and cerebral cortex (de Morais et al. 2014); our data show that EAE, at all doses tested, can reverse the lipid peroxidation promoted by CORT in the cerebral cortex. In addition, EAE treatment maintained SOD and CAT activities elevated in the cerebral cortex. In the hippocampus, there was a reversal of the effect of CORT on CAT activity after EAE administration, with SOD and GSH-Px activities maintained elevated. Taken together, these data indicate an antioxidant action of EAE. A study conducted by Novack et al. (2013), in which the antidepressant-like effect of E. uniflora was observed, demonstrated that this species has an antioxidant effect in vitro in cerebral regions. The antidepressant-like effect elicited by E.uniflora seems to be related to its antioxidant effect (Novack et al. 2013). Several antidepressants used in clinical practise, including fluoxetine, possess antioxidant effects. Cilostazol, a potential antidepressant agent, prevents lipid peroxidation and the decrease in GSH levels in the brain (Abuelezz and Hendawy 2017).
Studies have shown that polyphenolic compounds present bioactive substances with antioxidant properties that can be used for therapeutic purposes, in order to avoid oxidative damage induced by free radicals and lipid peroxidation (Magina et al. 2010). The antioxidant and antidepressant-like effects of EAE observed in the present study may be due to the presence of phenols in this extract. At the beginning of the work, extractions of Eugenia catharinensis were performed using dichloromethane, hydroalcoholic solution at 70% and ethyl acetate as solvents. The ethyl acetate extract showed major yield, being twice in relation to the other two solvents. In addition, in the assay of phenolic contents, ethyl acetate extract presented the highest content of phenolic compounds, as well greater diversity of them (unpublished data). This fact led us to choose ethyl acetate as extraction solvent, since we were searching for compounds with antioxidant activity. Thus, these results guided the continuation and zooming the studies using the ethyl acetate extract. A number of phenols have been identified in different Eugenia species, such as E. elliptica, E. orbiculata and E. tinifolia, and these compounds all exhibited free radical scavenging activity and were able to inhibit microsomal lipid peroxidation (Neergheen et al. 2006). Furthermore, antidepressant-like (Pauleti et al. 2017) and antioxidant (Espinosa et al. 2015) activities have been reported for p-coumaric acid.
Conclusion
We, herein, show for the first time that EAE exerts antidepressant-like effects on a depression model induced by chronic CORT treatment, in association with antioxidant effects on CORT-induced oxidative stress. Additionally, we postulate that the effects demonstrated by EAE are dependent on the presence of phenolic compounds in this extract. However, further studies need to be conducted to confirm these data.
References
Abuelezz SA, Hendawy N (2017) Insights into the potential antidepressant mechanisms of cilostazol in chronically restraint rats: impact on the Nrf2 pathway. Behav Pharmacol 1:1–13
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Bilici M, Efe H, Koroglu MA, Uydu A, Bekaroglu M, Deger O (2001) Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord 64(1):43–51
Colla RS, Machado DG, Bettio LEB, Colla G, Magina MDA, Brighente MC, Lu A (2012) Involvement of monoaminergic systems in the antidepressant-like effect of Eugenia brasiliensis lam. (Myrtaceae) in the tail suspension test in mice. J Ethnopharmacol 143(2):720–731
de Morais H, de Souza CP, da Silva LM, Ferreira DM, Werner MF, Andreatini R, da Cunha JM, Zanoveli JM (2014) Increased oxidative stress in prefrontal cortex and hippocampus is related to depressive-like behavior in streptozotocin-diabetic rats. Behav Brain Res 1(258):52–64
Delwing-de Lima D, Fröhlich M, Dalmedico L, Gruenwaldt J, Aurélio M, Delwing Dal Magro D, Pereira EM, Wyse ATS (2017) Galactose alters markers of oxidative stress and acetylcholinesterase activity in the cerebrum of rats: protective role of antioxidants. Metab Brain Dis 32:359–368
Espinosa RR, Inchingolo R, Alencar SM, Rodriguez-Estrada MT, Castro IA (2015) Antioxidant activity of phenolic compounds added to a functional emulsion containing omega-3 fatty acids and plant sterol esters. Food Chem 1(182):95–104
Grundmann O, Lv Y, Kelber O, Butterweck V (2010) Mechanism of St. John's wort extract (STW3-VI) during chronic restraint stress is mediated by the interrelationship of the immune, oxidative defense, and neuroendocrine system. Neuropharmacology 58:767–773
Levinstein MR, Samuels BA (2014) Mechanisms underlying the antidepressant response and treatment resistance. Front Behav Neurosci 27(8):1–12
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-phenol reagents. J Biol Chem 193:265–275
Magina MA, Gilioli A, Moresco HH, Colla G, Pizzolatti MG, Brighente IMC (2010) Atividade antioxidante de três espécies de Eugenia (Myrtaceae). Lat Am J Pharm 29(3):376–382
Marklund SL (1985) Pyrogallol autoxidation. In: Greenwald RA (ed) Handbook for oxygen radical research. CRC press, Boca Raton Florida, pp 243–247
Neergheen VS, Soobrattee MA, Bahorun T, Aruoma OI (2006) Characterization of the phenolic constituents in Mauritian endemic plants as determinants of their antioxidant activities in vitro. J Plant Physiol 163(8):787–799
Ng F, Berk M, Dean O, Bush AL (2008) Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 11(6):851–876
Nikfarjam M, Rakhshan R, Ghaderi H (2017) Comparison of effect of Lavandula officinalis and venlafaxine in treating depression: a double blind clinical trial. J Clin Diagn Res 1(7):KC01–KC04
Novack F, De Siqueira A, Savegnago L, João E (2013) Involvement of serotoninergic and adrenergic systems on the antidepressant-like effect of E. uniflora L. leaves essential oil and further analysis of its antioxidant activity. Neurosci Lett 544:105–109
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pauleti NN, Melo J, Siebert DA, Micke GA, Albuquerque CAC, Alberton MD, Barauna SC (2017) Characterisation of phenolic compounds of the ethyl acetate fraction from Tabernaemontana catharinensis and its potential antidepressant-like effect. Nat Prod Res 1:1–4
Porsolt RD, Bertin A, Jalfre M (1977) Behavioural despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336
Rainer Q, Xia L, Guilloux J, Gabriel C, Mocaer E, Hen R, Enhamre E, Gardier AM, David DJ (2012) Beneficial behavioural and neurogenic effects of agomelatine in a model of depression/anxiety. Int J Neuropsychopharmacol 15(3):321–335
Thakare VN, Dhakane VD, Patel BM (2017) Attenuation of acute restraint stress-induced depressive like behavior and hippocampal alterations with protocatechuic acid treatment in mice. Metab Brais Dis 32(2):401–413
Umehara H, Numata S, Watanabe SY, Hatakeyama Y, Kinoshita M, Tomioka Y, Nakahara K, Nikawa T, Ohmori T (2017) Altered KYN/TRP, Gln/Glu, and met/methionine sulfoxide ratios in the blood plasma of medication-free patients with major depressive disorder. Sci Rep 7(1):1–8
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Weng L, Guo X, Li Y, Yang X, Han Y (2016) Apigenin reverses depression-like behavior induced by chronic corticosterone treatment in mice. Eur J Pharmacol 5(774):50–54
World Health Organization, 2017. Depression: let’s talk. Accessed on 02 September 2017, http://who.int/mental_health/management/depression/en/
Zafir A, Banu N (2009) Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress 12(2):167–177
Zaki MA, Balachandran P, Khan S, Wang M, Mohammed R, Hetta MH, Pasco DS, Muhammad I (2013) Cytotoxicity and modulation of cancer-related signaling by (Z)- and (E)-3,4,3′,5′-tetramethoxystilbene isolated from Eugenia rigida. J Nat Prod 76(4):679–684
Zanoveli JM, de Morais H, Dias IC, Schreiber AK, de Souza CP, da Cunha JM (2016) Depression associated with diabetes: from pathophysiology to treatment. Curr Diabetes Rev 12:165–178
Zeni ALB, Camargo A, Dalmagro AP (2017) Ferulic acid reverses depression-like behavior and oxidative stress induced by chronic corticosterone treatment in mice. Steroids 125:131–136
Acknowledgements
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant number 431280/2016-9), Fundação de Amparo à Pesquisa do Estado de Santa Catarina (grant number 1066/2016), Universidade da Região de Joinville and Universidade Regional de Blumenau.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Barauna, S.C., Delwing-Dal Magro, D., Brueckheimer, M.B. et al. Antioxidant and antidepressant-like effects of Eugenia catharinensis D. Legrand in an animal model of depression induced by corticosterone. Metab Brain Dis 33, 1985–1994 (2018). https://doi.org/10.1007/s11011-018-0306-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0306-3