Abstract
Necroptosis is a recently discovered programmed necrosis, regulated by receptor interacting protein kinase 1 (RIP1) and RIP3 after death signal stimulation and could be specifically inhibited by necrostatin-1. The aim of this study was to investigate the role of RIP1 and RIP3 signal pathways in a mouse model of collagenase-induced intracerebral hemorrhage (ICH) and assess the effect of necrostatin-1 on brain injury after ICH. We found that RIP1 and RIP3 proteins were abundantly expressed and increased in mice brain after ICH. Necrostatin-1 pretreatment improved neurological function and attenuated brain edema in mice after ICH. Moreover, necrostatin-1 reduced RIP1–RIP3 interaction and propidium iodide (PI) positive cell death, and further inhibited microglia activation and pro-inflammatory mediator genes [tumor necrosis factor-a (TNF-α) and interleukin-1β (IL-1β)] expression after ICH. These findings indicate that RIP1/RIP3-mediated necroptosis is an important mechanism of cell death after ICH. Through inhibiting necroptosis, necrostatin-1 plays a protective role in reducing necrotic cell death after ICH. Necrostatin-1 is a promising therapeutic agent that protects cells from necroptosis and improves functional outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracerebral hemorrhage (ICH) accounts for approximately 15 % of acute strokes [1]. After ICH, complex pathophysiologic processes occured in the brain tissue around the hematoma, including brain edema formation, cytotoxic blood components releasing, oxidative stress and inflammation response [1]. These pathological factors together finally caused cell death in the brain tissue and subsequent neurological dysfunction after ICH. Previous researches in animal and human hemorrhagic brains had revealed that necrosis and apoptosis are two major kinds of cell death presenting in the brain tissue after ICH [2–4]. Little study has been done to evaluate the contribution of programmed necrosis to brain injury after ICH. Some recent studies indicated that some molecular pathways could regulate particular kind of necrosis, which was named programmed necrosis or necroptosis [5, 6].
Necroptosis is a caspase-independent type of cell death that is activated when caspases are inhibited or not activated [7, 8]. Several recent studies had showed that necroptosis is regulated by the kinase activity of receptor interacting protein kinase 1 (RIP1) and its interaction with receptor interacting protein kinase (RIP3) after death signal stimulation [5, 6, 9, 10]. And necrostatin-1 (Nec-1), which is a specific small molecule inhibitor of necroptosis, can specifically inhibit RIP1 kinase activity and RIP1–RIP3 interaction [6, 9, 11]. Necroptosis or programmed necrosis has been shown to take part in various disease models, including ischemic stroke and traumatic brain injury [11, 12]. However, it remains unknown whether RIP1/RIP3-mediated necroptosis occurs in hemorrhagic brain tissue and whether necrostatin-1 could inhibit necroptosis after ICH in mice.
In the present study, we demonstrated that RIP1/RIP3-mediated necroptosis contributed to necrotic cell death after ICH. And we further proved that RIP1 kinase inhibitor necrostatin-1 attenuated brain injury through inhibiting RIP1/RIP3 pathway after ICH in mice.
Materials and Methods
Intracerebral Hemorrhage Animal Model and Drug Administration
The research protocols were reviewed and approved by the Committee of Animal Care and Use of Nanjing University, in compliance with NIH guidelines. Male ICR mice (25–35 g) were supplied by Animal Center of Chinese Academy of Sciences (Shanghai, China). The mice were kept in a constant circumstance with free access to food and water and 12-h dark-light cycle. The researchers who participated in this study were blinded to all aspects of the study. Experimental procedures were performed following criteria derived from Stroke Therapy Academic Industry Roundtable (STAIR) group guidelines for preclinical evaluation of stroke therapeutics. Animals that died after ICH induction were excluded from experimental groups.
The procedure for inducing ICH in mice was adapted from a method that has been described before [13]. Briefly, mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and fixed in the stereotactic apparatus. Then 0.06 U of Type IV collagenase (Sigma, St. Louis, USA) in 0.5 µl of saline were injected unilaterally into the right striatum through a 0.5-mm cranial burr hole as the following coordinates (2.5 mm lateral to the bregma, 3.5 mm in depth). To prevent reflux, Collagenase was delivered over 5 min and the needle was left additional 5 min after injection. An equivalent volume of sterile saline was given as the same procedure in the Sham group. Then we closed the burr hole with bone wax, and sutured the skin. Throughout the surgery and recovery period, mice were kept at 37 °C using a warm blanket.
For drug administration, 80 nmol necrostatin-1, 5-(1H-Indol-3-ylmethyl)-(2-thio-3-methyl) hydantoin (Nec-1, Sigma, USA) was dissolved in 1 μl dimethyl sulfoxide (DMSO, Sigma, USA) based on previous studies [11, 12] and our preliminary study (data not shown). Immediately after surgery, animals were randomly assigned to the following four groups by a researcher who was blinded to the study, Sham operated group (Sham), Sham treated with necrostatin-1 group (Nec-1), ICH treated with vehicle group (ICH), ICH treated with Nec-1 group (ICH+Nec-1). The Sham group mice received an equal volume of vehicle. 1 μl Nec-1 (80 nmol) solution or 1 μl Vehicle (DMSO) was pretreated with a single intracerebroventricular injection into the ipsilateral cerebral ventricle (coordinates relative to bregma: 0.5 mm anterior, 1 mm lateral, 2 mm deep) 15 min before ICH induction. Animals were euthanized at related time points after surgery.
Neurological Deficits Assessment
Neurological deficits were first assessed using a 24-point neurological scoring system, which included body symmetry, gait, climbing, circling behavior, front limb symmetry and compulsory circling as previous reports [13]. Neurological functions were then evaluated using a wire grip test (a 5-point scoring system) as previous describe [12, 14].
A person blinded to the treatment groups performed the neurological function evaluation. The neurological function assessment was done before surgery (0 day) and at 1 day, 3 days, and 7 days after ICH. Each mouse was performed 3 times at each time point.
Brain Water Content
Brain edema was determined by the wet-dry weigh ratio method as descripted previously [13]. Briefly, brains were quickly divided into three parts, including the ipsilateral and contralateral hemisphere, and the cerebellum. Each part was immediately weighted to get wet weight. Then tissue was dried at 100 °C for 24 h to obtain dry weight. The brain water content = (wet weight–dry weight)/wet weight of brain tissue × 100 %.
Propidium Iodide Labeling
Propidium iodide (PI; Sigma, USA) was administered via intraperitoneal injection 1 h before euthanizing mice at various time points after ICH. Then the mice brains were collected and kept in liquid nitrogen. Frozen brain sections were got at every 150 µm interval through hemorrhage area from each brain. For quantification of PI positive cells, photographs were randomly taken from four individual 200× fields in the peri-hematomal region in each section. There were three sections per animal were photographed and analyzed by an investigator who blinded to the experiment condition. PI positive cells were expressed as PI+/×200 field in each group.
Immunoprecipitation and Western Blot
Frozen brain tissue was homogenized in 0.5 ml of ice-cold RIPA buffer and the supernatants were cleared by centrifugation at 12,000g for 15 min at 4 °C. Protein concentrations were determined using a BCA kit (Pierce, USA). For RIP1 immunoprecipitation, we employed Pierce Co-IP Kit (Thermo Scientific, 26149) following manufacturer’s instructions. 4 mg protein was pre-cleared with Control Agarose Resin for 2 h and then incubated with anti-RIP1 antibody or control nonspecific IgG (Santa Cruz, USA) binding AminoLink Plus Coupling Resin columns at 4 °C overnight before analysis by western blotting.
For Western blotting, aliquots of protein were boiled in denaturing sample buffer. Immunoprecipitates or Inputs were run on 12 % SDS–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. After blocking with 5 % milk in TBST for 1 h, membranes were incubated overnight at 4 °C with rabbit anti-RIP1 (Santa Cruz, USA) or anti-RIP3 antibody (Santa Cruz, USA) or β-actin (Bio-world, USA). Primary antibodies were diluted (1:1,000) in TBST. Membranes were washed with TBST three times, and then incubated for 2 h with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:10,000 in TBST) at room temperature. Blots were developed using a chemiluminescence kit (Millipore) and exposed to X-ray film. Densitometric analysis of the bands was performed with imageJ software.
Iba-1 Immunohistochemistry
Iba-1 immunohistochemistry was performed as previous detailed by our laboratory [13]. Brain sections were washed with PBS, blocked in 10 % normal goat serum and then stained using rabbit anti-mouse Iba-1 antibody (1:200, Wako, Japan) at 4 °C overnight. Brain sections were washed in PBS three times for 10 min, and then labeled with Alexa-Flour tagged secondary antibody (1:200; Invitrogen, USA) for 1 h at room temperature. Immunoreactivity of Iba-1 was photographed under a fluorescence microscope. Iba-1 positive cells were counted and expressed as cells/×200 field, which was the same as above PI counting or our previous report [13].
Quantitative RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, USA). Total cDNA was synthesized as previously described [15]. Quantitative real-time polymerase chain reaction (PCR) analysis was performed using SYBR Green qRT-PCR kit (Takara Biotechnology, Japan) according to the manufacturer’s instructions. Primers (Invitrogen, Shanghai, China) were synthesized as follows: IL-1β: FP 5′-GCCCATCCTCTGTGACTCAT-3′; RP 5′-AGGCCACAGGTATTTTGTCG-3′; TNF-α: FP 5′-CGTCAGCCG ATTTGCTATCT-3′; RP 5′-CGGACTCCGCAAAGT CTAAG-3′; β-actin: FP 5′- GACAGGATGCAGAAGGAGATTACT-3′; RP 5′-TGATCCACAT TGCTGGAAGGT-3′. Total RNA concentrations in each sample were normalized to the quantity of β-actin mRNA, and the expression levels of target genes were expressed by ratio of the number of target mRNA to β-actin mRNA.
Statistical Analysis
Data were expressed as means ± SEM. The statistical analysis was determined by GraphPad Prism 5 software. For comparisons among multiple groups, one-way or two-way analysis of variance followed by a post hoc (Bonferroni) test was used to determine significant differences. Differences between two groups were determined by the Student’s t test. Statistical significance was set at p < 0.05.
Results
Temporal Course of Necrotic Cell Death in Brain Tissue After ICH
Plasmalemma permeability is a feature of necrotic cell death. ICH induced cellular plasmalemma permeability to PI. Here, we used in vivo PI labeling to identify cells with plasmalemma permeability or necrosis and studied their time course after collagenase-induced ICH in mice. Our results showed that plasmalemma permeability was detected in the insulted brain tissue after ICH, which was peak at 3 days after ICH (Fig. 1a, b). These results revealed that necrotic cell death was increased in the brain tissue after ICH.
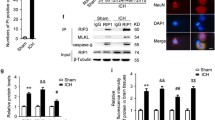
Necrotic cell death detected in vivo by propidium iodide (PI) and expression levels of RIP1 and RIP3 protein in brain tissue after intracerebral hemorrhage (ICH). a Representative photographs of PI positive cells at 0 (Sham), 1, 3 and 7 days after ICH. Scale bar 50 μm. b Quantitation of PI positive cells in ×200 fields between 0 and 7 days after ICH. c Representative Wentern blots of RIP1 and RIP3 expression. d Densitometric quantification of RIP1. e Densitometric quantification of RIP3. Values were mean ± SEM, n = 6 mice/group, *p < 0.05, **p < 0.01, ***p < 0.001 versus Sham group
Expression Levels of RIP1 and RIP3 Protein in the Mice Brain After ICH
The induction of necroptosis requires RIP1 and RIP3 kinase activity [7]. Therefore, we investigated the changes of RIP1 and RIP3 protein expression at 0–7 days after ICH. As shown in Fig. 1c, RIP1 protein was abundantly expressed in baseline and after ICH. The expression level of RIP3 was quite low in the Sham group, but dramatically increased and persisted at least 7 days after ICH induction (Fig. 1e). These results suggested that RIP1 and RIP3 mediated necroptosis was involved in the cell death after ICH.
Necrostatin-1 Improved Neurobehavioral Performance and Attenuated Brain Edema in Mice After ICH
To test the protective effect of Nec-1 in vivo, Nec-1 was pretreated through cerebral ventricle injection to mice 15 min before ICH induction. Neurological function was assessed at 0, 1, 3 and 7 days after ICH induction or Sham-operated mice in each group. No difference in baseline neurological function before ICH was observed between any groups of mice (Fig. 2a, b). Sham-operated mice administered with Nec-1 or vehicle performed similar to intact mice (data no shown). Neurological deficits were first evaluated using a 24-point neurological scoring system. As shown in Fig. 2a, neurological deficits were significantly decreased in Nec-1-pretreated group at all assess time points versus vehicle-pretreated ICH group (n = 6 mice/group, ## p < 0.01, # p < 0.05 vs. ICH, Fig. 2a). Then, we used a wire grip test to further test the neurobehavioral performance in each group. Results showed that motor function in Nec-1-pretreated mice was significantly improved at all evaluate time points when compared with vehicle-treated ICH mice (n = 6 mice/group, ## p < 0.01, # p < 0.05 vs. ICH, Fig. 2b).
Necrostatin-1 (Nec-1) pretreatment reduced neurological function and brain edema in ICH mice. a Statistical analysis of neurological deficiency in each group at 0–7 days using a 24-point neurological scoring system. b Statistical analysis of neurobehavioral performance in each group at 0–7 days by wire grip test. c Statistical analysis of brain edema at 3 days in each group. N = 6 mice/group. ***p < 0.001 versus Sham group, #p < 0.05, ##p < 0.01 versus ICH group. Con-Hp, contralateral hemisphere; Ips-Hp, ipslateral hemisphere; Cerebellum. Values were expressed as mean ± SEM
Furthermore, we measured brain water content in each group to explore whether Nec-1 pretreatment could reduce brain edema. It showed that Nec-1 pretreatment dramatically reduced brain edema in the ipsilateral hemorrhagic brain at 3 days after ICH (n = 6 mice/group, ## p < 0.01 vs. ICH, Fig. 2c).
Necrostatin-1 Pretreatment Reduced Necrotic Cell Death Through Abolishing RIP1–RIP3 Interaction in Mice After ICH
To elucidate the effect of necrostatin-1 pretreatment on ICH-induced cell death, we counted PI-positive cells as previously described in each group. As shown in Fig. 3a, b, Nec-1 administration resulted in less PI-positive cells in the peri-hematomal region at 3 days after ICH when compared with vehicle-treated ICH mice (n = 6 mice/group, ## p < 0.01 vs. ICH, Fig. 3b), suggesting a significant reduction of necrotic cell death post ICH.
Necrostatin-1 (Nec-1) pretreatment reduced necrotic cell death and RIP1–RIP3 interaction after ICH. a Representative pictures of PI (propidium iodide) staining at 3 days after ICH. b Quantification of PI positive cells representing necrotic cell death at 3 days after ICH. Scale bar 50 μm. c Representative Immunoprecipitation (IP) and Western blot (WB) results for RIP1 and RIP3 interaction. IgG as negative control. RIP1 and β-actin input as loading control. d Quantification showing RIP1 and RIP3 interaction is significantly decreased at 1 day after ICH in Nec-1 pretreated mice. N = 6 mice/group, ***p < 0.001 versus Sham, #p < 0.05, ##p < 0.01 versus ICH
We further explored whether Nec-1 pretreatment had any effect on RIP1–RIP3 interaction after ICH. We investigated the recruitment of RIP3 to RIP1 by immunoprecipitation. Our result showed that the interaction between RIP1 and RIP3 was greatly enforced in the vehicle-treated mice at 24 h after ICH (Fig. 3c, d). However, Nec-1 pretreatment significantly reduced RIP3 recruitment to RIP1 after ICH (n = 6 mice/group, # p < 0.05 vs. ICH, Fig. 3c, d).
Necrostatin-1 Decreased Microglia Activation and Proinflammatory Cytokine Genes Expression After ICH
To further assess the potential effect of Nec-1 on neuroinflammation after ICH, we studied microglia activation and proinflammatory mediator genes expression at 3 days after ICH (Fig. 4). Nec-1-pretreated mice showed a significant reduction of Iba-1 positive cells compared to vehicle treatment (Fig. 4a, b, # p < 0.05 vs. ICH). In accordance with reduction of microglia activation, Nec-1 pretreatment significantly inhibited TNF-α and IL-1β mRNA levels at 3 days after ICH (Fig. 4c, d, # p < 0.05 vs. ICH). These results suggested Nec-1 inhibited inflammatory response after ICH.
Necrostatin-1 (Nec-1) pretreatment inhibited microglia activation and pro-inflammatory mediator genes expression after ICH. a Representative pictures of Iba-1 staining at 3 days after ICH. Scale bar 50 μm. b Quantification of Iba-1 positive cells representing microglia activation at 3 days after ICH. c Quantification of TNF-α mRNA expression at 3 days after ICH using quantitative RT-PCR. d Quantification of IL-1β mRNA expression at 3 days after ICH using quantitative RT-PCR. Data were normalized to β-actin. N = 6 mice/group, ***p < 0.001 versus Sham, #p < 0.05 versus ICH
Discussion
In the present study, we proved that necroptosis might be an important pathogenic mechanism of cell death after ICH. RIP1 and RIP3 proteins were abundantly expressed after ICH. Nec-1 pretreatment improved neurological function and attenuated brain edema in mice after ICH. Moreover, Nec-1 reduced RIP1–RIP3 interaction and necrotic cell death, and further inhibited inflammatory response after ICH. These results demonstrated that Nec-1 attenuated brain injury after ICH through inhibiting RIP1/RIP3-mediated necroptosis.
Necroptosis is recently discovered programmed necrosis, which is activated by TNF-α and/or Fas [8, 11]. Necroptosis has been shown to play important roles in various pathophysiological conditions, including ischemic stroke and traumatic brain injury [11, 12]. Furthermore, recent studies revealed that formation of pro-necrotic RIP1–RIP3 complex is an essential step in the RIP1 kinase activation and necroptosis induction [6, 9, 10]. Our results showed that RIP1 was abundantly expressed in the brain tissue and RIP3 expression was significantly increased after ICH (Fig. 1). These laid a foundation for the formation of RIP1–RIP3 complex and indicated that RIP1–RIP3 mediated necroptosis may be involved in the cell death after ICH.
Necrostatin-1 has been shown to play protective roles in ischemic stroke and traumatic brain injury [11, 12]. But, it’s still not well known how Nec-1 reduces ICH-induced brain injury. So, we carried out the present study. As expected, our results showed that pretreatment with Nec-1 significantly produced functional improvement (Fig. 2a, b) and reduced brain edema (Fig. 2c) after ICH. A recent similar study further proved that necrostatin-1 reduced injury volume after ICH [16]. Taken together, these data indicated that Nec-1exerted its neuroprotective effect through inhibiting secondary brain injury after ICH.
Membrane integrity disruption or plasmalemma permeability is one important feature of necrotic cell death, which can be detected using in vivo PI labeling. Our results showed that plasmalemma permeability (PI positive cells) was detected in the brain after ICH within several hours, with peak permeability at two to 3 days after ICH (Fig. 1). These results were consistent with previous report by other group [4]. Therefore, PI positive cells were counted to assess the effect of necrostatin-1 on necrotic cell death at 3 days after ICH in this study. Results showed that Nec-1 reduced necrotic cell death in the peri-hemotomal region after ICH (Fig. 3a, b). These results were consistent with previous studies which demonstrated that necrostatin-1 significantly decreased the number of PI-positive cells in TBI and ICH model [12, 16]. In addition, RIP3 knockout mice dramatically reduced necrotic cell death induced by ICH [4]. Taken together, these results and published reports linked RIP1 and RIP3 kinase activity with necrotic cell death after ICH.
Accumulating evidence has shown that Nec-1 specifically inhibits necroptosis through suppressing RIP1 kinase activity and RIP1–RIP3 interaction in vitro studies [6, 9, 11]. In the present study, we found that RIP3 was recruited to RIP1 and formed RIP1–RIP3 complex after ICH (Fig. 3c). Nec-1 treatment markedly abolished the pro-necrotic RIP1–RIP3 complex formation after ICH (Fig. 3c, d). Our results were consistent with these in vitro studies. These results indicated that RIP1 and RIP3 were key regulators for necroptosis after ICH. Inhibition of RIP1–RIP3 interaction might be the major mechanism of necrostatin-1 mediated neuroprotection in ICH.
Necrotic cells and damaged brain tissue release a wide variety of cell components into the peri-hemotomal brain parenchyma. These “danger” components subsequently activate immune system, trigger inflammatory response and finally exacerbate brain injury after ICH [1, 17, 25]. Microglia are the resident immune cells in brain tissue and play essential pathophysiological roles in many neurological diseases, such as ICH [17]. It is a major contributor of pro-inflammatory cytokines after ICH [17]. Persistent microglial activation often activates neuroinflammation and aggravates brain injury after ICH. Meanwhile, inflammatory mediators, such as TNF-α and IL-1β, increased blood brain barrier permeability, promoted vasogenic edema, induced leukocyte infiltration into the brain, and further exacerbate brain injury after ICH [18, 19]. TNF-α was increased after ICH and neutralizing TNF-α suppressed brain injury, suggesting that TNF-α may contribute to the induction of apoptosis and necroptosis after ICH [20, 21]. A recent in vitro study showed that Toll-like receptor activated microglia could undergo necroptosis through RIP1/RIP3 pathway [22]. Toll-like receptors were also involved in inflammation and brain injury after ICH [23, 24]. So we further investigated whether necrostatin-1 had effect on microglia activation and inflammatory response in vivo ICH model. As expected, our data showed that pretreatment with Nec-1 markedly inhibited microglial activation (Fig. 4a, b) and reduced the expression of pro-inflammatory mediator genes (TNF-α and IL-1β, Fig. 4c, d) at 3 days after ICH. These results were consistent with previous reports in other disease models and in vitro primary microglia study [12, 22]. Taken together, these data suggested that RIP1/RIP3-dependent necroptosis might also contribute to microglia activation and neuroinflammation after ICH.
The present study added RIP1/RIP3-mediated necroptosis to the mechanisms of brain injury after ICH. However, further work is needed to identify whether other necroptosis signaling pathways are getting involved in ICH. Our study has some limitations. First,we pre-treated Nec-1 at 15 min before ICH. It is unlikely that Nec-1 pretreatment could apply at this time point in clinical practice, which may limit the clinical use of Nec-1. Further studies are needed to determine practical and effective regimen of Nec-1 after ICH. Second, a small number of ICR mice were included in the present study. We should use C57BL6 mice and add more animals to our future studies. Third, we used single Nec-1 concentration in this study. In a future study, multiple Nec-1 doses should be evaluated to confirm the optimal concentration of Nec-1 for maximal neuroprotection in ICH mice. In addition, the therapeutic window of Nec-1 should also be investigated in the future study.
In conclusion, our work suggests that RIP1/RIP3 may be an essential pathway for necrotic cell death and contribute to neurologic dysfunction after ICH in mice. Necrostatin-1 pretreatment exerted multifaceted neuroprotection through decreasing injury volume, brain edema, necrotic cell death, RIP1/RIP3 interaction, microglia activation and inflammatory response in ICH model. These results suggest that targeting RIP1/RIP3-mediated necroptosis may be novel strategy to prevent brain injury after ICH. Our data also indicate the potential neuroprotective value of necrostatin-1 against ICH, which deserves further study.
References
Keep RF, Hua Y, Xi G (2012) Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 11:720–731
Qureshi AI, Suri MF, Ostrow PT, Kim SH, Ali Z, Shatla AA, Guterman LR, Hopkins LN (2003) Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery 52:1041–1047 discussion 1047–1048
Qureshi AI, Ling GS, Khan J, Suri MF, Miskolczi L, Guterman LR, Hopkins LN (2001) Quantitative analysis of injured, necrotic, and apoptotic cells in a new experimental model of intracerebral hemorrhage. Crit Care Med 29:152–157
Zhu X, Tao L, Tejima-Mandeville E, Qiu J, Park J, Garber K, Ericsson M, Lo EH, Whalen MJ (2012) Plasmalemma permeability and necrotic cell death phenotypes after intracerebral hemorrhage in mice. Stroke 43:524–531
Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ (2003) A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem 278:51613–51621
Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4:313–321
Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T (2010) The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal 3:re4
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11:700–714
He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137:1100–1111
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK (2009) Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137:1112–1123
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1:112–119
You Z, Savitz SI, Yang J, Degterev A, Yuan J, Cuny GD, Moskowitz MA, Whalen MJ (2008) Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab 28:1564–1573
Su X, Wang H, Zhu L, Zhao J, Pan H, Ji X (2013) Ethyl pyruvate ameliorates intracerebral hemorrhage-induced brain injury through anti-cell death and anti-inflammatory mechanisms. Neuroscience 245:99–108
Hall ED (1985) High-dose glucocorticoid treatment improves neurological recovery in head-injured mice. J Neurosurg 62:882–887
Su X, Wang H, Zhao J, Pan H, Mao L (2011) Beneficial effects of ethyl pyruvate through inhibiting high-mobility group box 1 expression and TLR4/NF-kappaB pathway after traumatic brain injury in the rat. Mediators Inflamm 2011:807142
Chang P, Dong W, Zhang M, Wang Z, Wang Y, Wang T, Gao Y, Meng H, Luo B, Luo C, Chen X, Tao L (2013) Anti-necroptosis chemical necrostatin-1 can also suppress apoptotic and autophagic pathway to exert neuroprotective effect in mice intracerebral hemorrhage model. J Mol Neurosci 52:242–249
Wang J (2010) Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol 92:463–477
Castillo J, Davalos A, Alvarez-Sabin J, Pumar JM, Leira R, Silva Y, Montaner J, Kase CS (2002) Molecular signatures of brain injury after intracerebral hemorrhage. Neurology 58:624–629
Didier N, Romero IA, Creminon C, Wijkhuisen A, Grassi J, Mabondzo A (2003) Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J Neurochem 86:246–254
Hua Y, Wu J, Keep RF, Nakamura T, Hoff JT, Xi G (2006) Tumor necrosis factor-alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery 58:542–550 discussion 542–550
Mayne M, Ni W, Yan HJ, Xue M, Johnston JB, Del Bigio MR, Peeling J, Power C (2001) Antisense oligodeoxynucleotide inhibition of tumor necrosis factor-alpha expression is neuroprotective after intracerebral hemorrhage. Stroke 32:240–248
Kim SJ, Li J (2013) Caspase blockade induces RIP3-mediated programmed necrosis in Toll-like receptor-activated microglia. Cell Death Dis 4:e716
Wang YC, Wang PF, Fang H, Chen J, Xiong XY, Yang QW (2013) Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke 44:2545–2552
Fang H, Wang PF, Zhou Y, Wang YC, Yang QW (2013) Toll-like receptor 4 signaling in intracerebral hemorrhage-induced inflammation and injury. J Neuroinflamm 10:27
Aronowski J, Zhao X (2011) Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 42:1781–1786
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China No. 81070974 and Youth Project of Fujian Provincial Department of Health (2014-1-59). The authors would like to thank Dr. Gengbao Feng for his technical assistance.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Su, X., Wang, H., Kang, D. et al. Necrostatin-1 Ameliorates Intracerebral Hemorrhage-Induced Brain Injury in Mice Through Inhibiting RIP1/RIP3 Pathway. Neurochem Res 40, 643–650 (2015). https://doi.org/10.1007/s11064-014-1510-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1510-0