Abstract
(−)-Epigallocatechin gallate (EGCG) has recently been shown to exert neuroprotection in a variety of neurological diseases; however, its role and the underlying mechanisms in cerebral ischemic injury are not fully understood. This study was conducted to investigate the potential neuroprotective effects of EGCG and the possible role of the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway in the putative neuroprotection against experimental stroke in rats. The results revealed that EGCG exhibit significant neuroprotection, as evidenced by reduced infarction size and the decrease in transferase dUTP nick end labeling-positive neurons. Furthermore, EGCG also enhanced levels of Nrf2 and its downstream ARE pathway genes such as heme oxygenase-1, glutamate-cysteine ligase modulatory subunit and glutamate-cysteine ligase regulatory subunit, as compared to control groups. In accordance with its induction of Nrf2 activation, EGCG exerted a robust attenuation of reactive oxygen species generation and an increase in glutathione content in ischemic cortex. Taken together, these results demonstrated that EGCG exerted significant antioxidant and neuroprotective effects following focal cerebral ischemia, possibly through the activation of the Nrf2/ARE signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke is a debilitating clinical disorder that affects millions of people, yet lacks effective neuroprotective treatments. There is now considerable evidence indicating that oxidative stress plays an important role in the secondary injury after acute cerebral ischemia, which was characterized by a progressive increase in reactive oxygen species (ROS) [1]. Therefore, the intervention of oxidative damage using compounds with antioxidant properties may relieve or prevent diseases in which oxidative stress is the primary cause [2]. The redox sensitive transcription factor nuclear factor erythroid-2 related factor 2 (Nrf2) plays a key role in the cellular defence against oxidative stress. Activation of Nrf2 has been implicated to protect neurons from ischemic insult [3]. Under basal conditions, Nrf2 is retained in the cytosol by binding to Kelch-like ECH-associated protein 1 (Keap1). In response to oxidative stress, Nrf2 dissociates from Keap1 and translocates to the nucleus [4–7], where it forms a heterodimer with its obligatory partner Maf and binds to the antioxidant response element (ARE) sequence to activate transcription of a large number of antioxidative and electrophile detoxification genes including heme oxygenase-1 (HO-1), NAD(P)H:quinine oxidoreductase 1 (NQO1) and glutamate-cysteine ligase (GCL) [8].
Green tea, a popular beverage derived from tea plant, contains large quantities of the biologically active polyphenols and catechins. Four types of catechins, namely (−)-epicatechin (EC) (−)-epigallocatechin (EGC) (−)-epicatechin-3-gallate (ECG), and EGCG, comprise 30 % of the dry weight of green tea. EGCG is the most abundant catechin and is mainly responsible for the biological properties of green tea such as its potent antioxidant effects [9, 10]. Several studies have indicated that EGCG have protective effects against cerebral ischemia in rats [11]. In a recent study Romeo et al [12] reported that EGCG could protect against oxidative stress-induced H19-7 cell death through its effects on Nrf2 activation. However, it was not clear whether EGCG regulate Nrf2 signaling in the brain. The present study tested the hypothesis that EGCG protect against ischemic brain injury and ameliorate cerebral ischemia-induced oxidative damage by activating the Nrf2/ARE signaling pathway.
Materials and Methods
Drugs and Reagents
(−)-Epigallocatechin gallate (EGCG) and DCFH-DA were purchased from Sigma (St. Louis, MO, USA). Antibodies to Nrf2, HO-1, GCLC, GCLM, anti-mouse-horseradish peroxide (HRP) IgG and anti-rabbit-HRP-IgG were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). In Situ Cell Death Detection Kit was purchased from Roche (Indianapolis, IN, USA). The glutathione assay kit was provided by Beyotime Institute of Biotechnology (Haimen, China).
Animal Preparation and Drug Treatment
All animal experiments were approved by Institutional Animal care and Use Committee of Shandong University. Adult male Sprague–Dawley rats weighing 270–320 g were used for the experiment. Middle cerebral artery occlusion (MCAO) was induced as described previously [13]. Briefly, rats were anesthetized with 10 % chloralhydrate (350 mg/kg, i.p). The left common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA) were isolated. An 18 mm length of nylon suture (φ 0.2 mm) was introduced into the ECA lumen and advanced into the ICA to block the origin of the MCA. Restoration of MCA blood flow in animals subjected to 2 h of MCA occlusion was achieved by withdrawing the suture to the ECA. The sham control rats received the same surgery procedures but did not have the suture inserted. EGCG (40 mg/kg) was given by intraperitoneally injection once daily for three consecutive days prior to surgery. Sham rats received vehicle (0.9 % NaCl, i.p) only. During and after the surgery, rectal temperature was controlled with a homeothermic blanket and kept at 37 °C until the complete recovery of the animal from the anesthesia. 24 h after reperfusion, animals were anesthetized and decapitated for future experiment.
Assessment of Functional Outcome and Cerebral Infarction
Functional outcome was evaluated using modified neurological severity score (mNSS) [14]. The rats were evaluated 24 h after MCAO. 2,3,5-triphenyltetrazolium chloride (TTC) staining was used to check the brain infarction size 24 h after reperfusion [15]. The colorless TTC was reduced to a red formazan product by dehydrogenases, which were abundant in mitochondria [16]. After anesthesia, the brains were removed and sectioned into 2 mm slices, which were then incubated in a 0.2 % solution of TTC at 37 °C for 30 min. Infarct volume was measured by a blinded observer using digital imaging (digital camera, Olympus MDF-382E) and image analysis software (C imaging 1280 system). The infarct area was calculated by Swanson’s method [17] to correct for edema. The total volumes of both contralateral (VC) and ipsilateral hemisphere (VL) were measured, and the infarct percentage (I) was calculated as % I = 100 × \( \frac{{({\text{V}}_{\text{c}} \text{ - }{\text{V}}_{\text{L}} )}}{{{\text{V}}_{\text{c}} }} \) to avoid mismeasurement secondary to edema.
Hematoxylin and Eosin (HE) Staining
Rats were sacrificed and fixed with formaldehyde perfusion 24 h after reperfusion. Paraffin blocked brains were cut into 4 μM sections. The sections were stained with HE and observed under a light microscope. The representative photographs were captured to determine the neuronal morphology in the cerebral cortex as described [18].
TUNEL Staining
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed to visualize DNA fragmentation using an In Situ Cell Death Detection Kit according to the manufacturer’s protocols (Roche Diagnostic, Indianapolis, IN). Briefly, the sections were post-fixed in ethanol-acetic acid (2:1) and then rinsed. Next, sections were incubated with proteinase K (100 µg/ml), rinsed, incubated in 3 % H2O2, permeabilized with 0.5 % Triton X-100, rinsed again, and then incubated in the TUNEL reaction mixture. Sections were then rinsed and visualized using a Converter- horse-radish peroxidase (POD) with 0.02 % 3, 3′-diaminobenzidine (DAB). Sections were then counterstained with Mayer’s hematoxylin and then mounted onto gelatin-coated slides. Slides were air dried overnight at room temperature [19].
Western Blot Analysis for Nrf2, HO-1, GCLC and GCLM
Protein extracts were prepared from ischemic cortex or control brains by homogenization in a RIPA buffer. Protein concentration was determined by bicinchonininc acid (BCA) method. Samples were mixed with loading buffer and boiled for 5 min. Equal amounts of protein were separated by Tris–glycine-SDS-PAGE. The membranes were blocked in 5 % non-fat dried milk in TBS-T, and then were incubated with primary antibody (anti-Nrf2 antibody 1:200; anti-HO-1 antibody 1:100; anti-GCLC antibody 1:100; anti-GCLM antibody 1:100; anti-actin antibody 1:1,000) in blocking buffer overnight at 4 °C. Signals from peroxidase-conjugated secondary antibody were visualized by chemiluminescence, and data, within a linear range, were quantified with Image J software [20].
Measurement of Glutathione Levels
Glutathione (GSH) levels were determined by using Glutathione Quantification Kit. 5, 5′-Dithiobis (2-nitrobenzoic acid) (DTNB) and GSH reacted to generate 2-nitro-5-thiobenzoic acid and glutathione disulfide (GSSG). Since 2-nitro-5-thiobenzoic acid is a yellow colored product, GSH concentration in the sample was determined by reading 412 nm absorbance with a multi-well plate reader.
ROS Determination
The ROS level was determined using a ROS-sensitive fluorescent probe, 2, 7-dichlorodihydro fluorescent diacetate (DCFH-DA). The fluorescence intensity of brain tissues was measured with a fluorescence spectrophotometer (Thermo Scientific Varioskan Flash), with excitation and emission wavelengths of 488 and 525 nm respectively.
Statistical Analysis
All values are presented as the mean ± SD unless otherwise specified. An unpaired t test or analysis of variance (ANOVA) was used for the comparison of means between groups. P < 0.05 was considered statistically significant.
Results
EGCG Improved Cerebral Function after Cerebral Ischemia/Reperfusion Injury
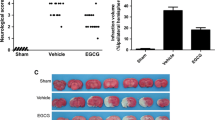
The neurological severity scores were evaluated 24 h after reperfusion. A significant increase in mNSS was found in animals 24 h after focal cerebral ischemia. Compared with cerebral ischemia/reperfusion (I/R) group, rats treated with EGCG had better cerebral functional outcome and lower scores in mNSS (*P < 0.05 vs. I/R group) (Fig. 1).
EGCG Decreased Cerebral Infarct Size after Cerebral I/R Injury
Infarct volume determined by postmortem TTC staining at 24 h were 23.4 ± 2.9 % in the I/R group (## P < 0.01 vs. sham group), while 8.95 ± 1.65 % in the EGCG group (**P < 0.01 vs. I/R group). No cerebral infarction was observed in the sham group (Fig. 2a, b).
EGCG decreases the extent of cerebral infarction in MCAO rats. a TTC staining was used to evaluate the infarction volume. The red area was healthy tissue and the white area was infarct tissue. b Infarction volume of the brain from EGCG group were less than that of the I/R group. n = 8 in each group, ## P < 0.05 versus sham; **P < 0.05 versus I/R group (Color figure online)
EGCG Attenuated Neuronal Injury in Ischemic Cortex after Cerebral I/R Injury
Hematoxylin and eosin (HE) staining was an effective and easy way to evaluate cell viability and was used in this study to evaluate neuronal cell death after MCAO. Compared with I/R group, the number of survived cells in cerebral cortex was significantly increased in rats treated with EGCG (Fig. 3a, b). The number of survived cells were 1,287.4 ± 233.6 per mm2 in the control group, while 119.4 ± 32.6 per mm2 in I/R group (## P < 0.01 vs. sham group). For the EGCG treatment group, the number was 817.7 ± 26.3 per mm2 (**P < 0.01 vs. I/R group).
EGCG reduces the neuronal death in the ischemic cortex. a Representative image of HE staining performed on sections from control and ischemic cortex. Compared with I/R group, EGCG treatment significantly increased the number of survived neurons in the ischemic cortex. The semi-quantative data were shown in (b). ## P < 0.05 versus sham; **P < 0.05 versus I/R group
The protective effect of EGCG against cerebral ischemic damage was further confirmed by TUNEL staining. Fig. 4a, b showed TUNEL staining in samples collected 24 h after reperfusion. In the MCAO group, the apoptotic cells were characterized by a round and shrunken shape with strong staining in the nucleus. Some occasional TUNEL-positive cells (4.7 ± 5.4 per mm2) were common in the sham groups. Compared with I/R group (1,002.2 ± 117.8 per mm2), number of TUNEL-positive cells (490.8 ± 73.2 per mm2) was significantly reduced with EGCG treatment (**P < 0.01 vs. I/R group).
EGCG attenuates cerebral I/R-induced neuronal apoptosis. a Representative image of TUNEL staining performed on sections from sham and ischemic cortex. b The apoptotic cells in ischemic cortex from EGCG treatment group were much less than those in I/R group. ## P < 0.05 versus sham; **P < 0.05 versus I/R group
EGCG Induced the Expression of Nrf2 and ARE-regulated Genes in Ischemic Cortex Following MCAO
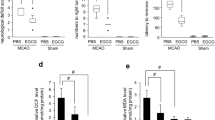
To determine whether the observed protective effect of EGCG was accompanied by activation of the Nrf2/ARE pathway in MCAO rats, we analyzed the protein levels of Nrf2 and its down-stream targets by western blot analysis. Compared with sham group, the Nrf2 expression in I/R group relatively increased about 1.5-fold 24 h after cerebral I/R, while in the EGCG treatment group it significantly increased (about 4.5-fold of the sham group) compared with I/R group (*P < 0.05 vs. I/R group) (Fig. 5a). We next determined whether EGCG also induced the expression of endogenous ARE-regulated genes. As shown in Fig. 5b, rats treated with EGCG exhibited an obvious increase in the protein levels of HO-1, GCLC and GCLM in the ischemic cortex compared to I/R group (*P < 0.05 vs. I/R group).
EGCG increases the expression of Nrf2 and its downstream proteins in ischemic cortex. a Expression of Nrf2 was induced by EGCG in the ischemic cortex of MCAO rats. The results were mean ± SD of three different experiments. *P < 0.05 versus I/R group. b EGCG increased the expression of HO-1, GCLM and GCLC. The results were mean ± SD of three different experiments. # P < 0.05 versus sham; *P < 0.05 versus I/R group
EGCG Induced GSH Synthesis And Attenuated Oxidative Damage in Ischemic Cortex Following MCAO
We measured the levels of GSH and ROS at 24 h after MCAO in order to test if the in vivo activation of Nrf2/ARE signaling by EGCG was able to protect against I/R-induced oxidative damage. EGCG pretreatment protected against the I/R-associated oxidative stress by maintaining GSH levels at close to baseline values 24 h after reperfusion (**P < 0.01 vs. I/R group) (Fig. 6a). As an index of ROS formation, we utilized the fluorescent probe DCFH-DA to measure levels of ROS in the cortex of I/R-injured animals. As shown in Fig. 6b, there was a significant increase in ROS formation at 24 h after I/R injury (## P < 0.01 vs. sham group). In turn, EGCG pretreatment significantly decreased the I/R-induced ROS formation (*P < 0.05 vs. I/R group). Altogether, these results strongly suggest that the pretreatment with EGCG protected MCAO rats against I/R-associated oxidative damage in ischemic cortex.
Discussion
In the present study, we demonstrated that EGCG pretreatment has protective effects on cerebral I/R injury in a rat MCAO model demonstrated by improved neurologic scores, reduced infarct volume and ameliorated neuronal apoptosis, increased GSH biosynthesis and decreased ROS content. The mechanism was possibly attributed to activating Nrf2 expression.
Although the mechanism of cerebral I/R injury is complicated, the overproduced free radicals is one of the most important start factors, which can cause severe damage of biological macromolecules and lead to apoptosis or necrosis of cells and tissues. Therefore, antioxidants have been considered in prevention and treatment of stroke and certain agents with antioxidant effects did have neuroprotective effects [21].
Green tea is one of the most popular beverages worldwide and its habitual consumption has long been associated with health benefits. Epidemiological studies showed a protective effect of habitual green tea consumption in the cardiovascular and cerebrovascular systems with a significant reduction in the incidence of stroke. Most of the beneficial effects of green tea were attributed to its polyphenolic flavonoids, including EC, EGC, ECG and the major flavonoid EGCG [22]. Purified EGCG has been the focus of research in recent years. A number of studies on different models have demonstrated that EGCG has protective effects on intestinal I/R injury [23], spinal cord injury [24], cisplatin-induced nephrotoxicity [25] and so on. Particularly, recent years have witnessed the protective effect of EGCG on central nervous system, especially on the brain injury [26]. While several previous studies have shown that activation of Nrf2 can protect against cerebral I/R injury, it remains unclear how EGCG effectively protect neurons against oxidative stress-induced toxicity. It was hypothesized that the antioxidant effect of green tea was mainly responsible for its disease-modifying properties. In this study, we demonstrated for the first time that EGCG effectively suppressed the oxidative damage and this protective effect was dependent on stimulation of the Nrf2 mediated anti-oxidative pathways.
The results of the present study have demonstrated that the pretreatment of cerebral I/R rats by EGCG markedly increased Nrf2 expression and improved antioxidant status and histopathologic properties in brain tissue. Many questions related to the antioxidant effects of EGCG still need to be clarified. And the exact molecular mechanism needs to be further explored.
Conclusion
Taken together, the results of this study support the hypothesis that EGCG has robust protective effects and can potently protect against the cerebral I/R injury through activation of Nrf2/ARE pathway. Further studies in models of cerebral I/R injury are thus warranted to further identify other molecules involved in the protective effect and to clarify potential cross talk between upstream and downstream signaling molecules.
References
Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14:1505–1517
Rautiainen S, Larsson S, Virtamo J, Wolk A (2012) Total antioxidant capacity of diet and risk of stroke: a population-based prospective cohort of women. Stroke 43:335–340
Alfieri A, Srivastava S, Siow RC, Modo M, Fraser PA, Mann GE (2011) Targeting the Nrf2- Keap1 antioxidant defence pathway for neurovascular protection in stroke. J Physiol 589:4125–4136
Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P (2002) Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A 99:11908–11913
Itoh K, Wakabayashi N, Katoh Y, Ishii T, O’Connor T, Yamamoto M (2003) Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 8:379–391
Itoh K, Tong KI, Yamamoto M (2004) Molecular mechanism activating Nrf-2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med 36:1208–1213
Zhang DD (2006) Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev 38:769–789
Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P (2004) Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A 101:2040–2045
Annaba F, Kumar P, Dudeja AK, Saksena S, Gill RK, Alrefai WA (2010) Green tea catechin EGCG inhibits ileal apical sodium bile acid transporter ASBT. Am J Physiol Gastrointest Liver Physiol 298:G467–G473
Mandel SA, Amit T, Weinreb O, Reznichenko L, Youdim MB (2008) Simultaneous manipulation of multiple brain targets by green tea catechins: a potential neuroprotective strategy for Alzheimer and Parkinson diseases. CNS Neurosci Ther 14:352–365
Choi YB, Kim YI, Lee KS, Kim BS, Kim DJ (2004) Protective effect of epigallocatechin gallate on brain damage after transient middle cerebral artery occlusion in rats. Brain Res 1019:47–54
Romeo L, Intrieri M, D’Agata V, Mangano NG, Oriani G, Ontario ML, Scapagnini G (2009) The major green tea polyphenol (−)-epigallocatechin-3-gallate, induces heme oxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J Am Coll Nutr 28(Suppl):492S–499S
Lou HY, Zhang XM, Wei XB, Wang RX, Sun X (2004) Anti-inflammatory effect of hydroxyethylpuerarin on focal brain ischemia/reperfusion injury in rats. China J Physiol 47:197–201
Boltze J, Kowalski I, Geiger K, Reich D, Gunther A, Buhrle C, Egger D, Kamprad M, Emmrich F (2005) Experimental treatment of stroke in spontaneously hypertensive rats by CD34+ and CD34-cord blood cells. Ger Med Sci 3:9
Popp A, Jaenisch N, Witte OW, Frahm C (2009) Identification of ischemic regions in a rat model of stroke. PLoS ONE 4:e4764
Benedek A, Móricz K, Jurányi Z (2006) Use of TTC staining for the evaluation of tissue injury in the early phases of reperfusion after focal cerebral ischemia in rats. Brain Res 1116:159–165
Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR (1990) A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 10:290–293
Yin X, Meng F, Wang Y, Wei W, Li A, Chai Y, Feng Z (2012) Effect of hyperbaric oxygen on neurological recovery of neonatal rats following hypoxic-ischemic brain damage and its underlying mechanism. Int J Clin Exp Pathol 6:66–75
Park SW, Yi JW, Kim YM, Kang JM, Kim DO, Shin MS, Kim CJ, Lee DI, Kim DH, Lee BJ (2011) Remifentanil alleviates transient cerebral ischemia-induced memory impairment through suppression of apoptoticneuronal cell death in gerbils. Korean J Anesthesiol 61:63–68
Yang L, Calingasan NY, Thomas B, Chaturvedi RK, Kiaei M, Wille EJ, Liby KT, Williams C, Royce D, Risingsong R, Musiek ES, Morrow JD, Sporn M, Beal MF (2009) Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS ONE 4:e5757
Jing X, Ren DM, Wei XB, Shi HY, Zhang XM, Perez RG, Lou HY, Lou HX (2013) Eriodictyol-7-O-glucoside activates Nrf2 and protects against cerebral ischemic injury. Toxicol Appl Pharm 273:672–679
Graham HN (1992) Green tea composition, consumption, and polyphenol chemistry. Prev Med 21:334–350
Giakoustidis AE, Giakoustidis DE, Koliakou K, Kaldrymidou E, Iliadis S, Antoniadis N, Kontos N, Papanikolaou V, Papageorgiou G, Atmatzidis K, Takoudas D (2008) Inhibition of intestinal ischemia/repurfusion induced apoptosis and necrosis via down-regulation of the NF-κB, c-Jun and caspace-3 expression by epigallocatechin-3-gallate administration. Free Radic Res 42:180–188
Renno WM, Al-Khaledi G, Mousa A, Karam SM, Abul H, Asfar S (2013) (−)-Epigallocatechin-3-gallate (EGCG) modulates neurological function when intravenously infused in acute and chronically injured spinal cord of adult rats. Neuropharmacology 77:100–119
Sahin K, Tuzcu M, Gencoglu H, Dogukan A, Timurkan M, Sahin N, Aslan A, Kucuk O (2010) Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci 87:240–245
Park JW, Hong JS, Lee KS, Kim HY, Lee JJ, Lee SR (2010) Green tea polyphenol (−)-epigallocatechin gallate reduces matrix metalloproteinase-9 activity following transient focal cerebral ischemia. J Nutr Biochem 21:1038–1044
Acknowledgments
This work was supported by grants from the National natural science foundation (No. 81274124, No. 81200982). We are grateful to Dr. Ruth Perez for feedback on experiments and editorial assistance.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jie Han and Miaomiao Wang have contribute equally to this work.
Rights and permissions
About this article
Cite this article
Han, J., Wang, M., Jing, X. et al. (−)-Epigallocatechin Gallate Protects Against Cerebral Ischemia-Induced Oxidative Stress via Nrf2/ARE Signaling. Neurochem Res 39, 1292–1299 (2014). https://doi.org/10.1007/s11064-014-1311-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1311-5