Abstract
Background
The involvement of malfunctioning glutamate systems in various central nervous system (CNS) disorders is widely acknowledged. Urolithin B, known for its neuroprotective and antioxidant properties, has shown potential as a therapeutic agent for these disorders. However, little is known about its protective effects against glutamate-induced toxicity in PC12 cells. Therefore, in this study, for the first time we aimed to investigate the ability of Urolithin B to reduce the cytotoxic effects of glutamate on PC12 cells.
Methods
Different non-toxic concentrations of urolithin B were applied to PC12 cells for 24 h before exposure to glutamate (10 mM). The cells were then analyzed for cell viability, intracellular reactive oxygen species (ROS), cell cycle arrest, apoptosis, and the expression of Bax and Bcl-2 genes.
Results
The results of MTT assay showed that glutamate at a concentration of 10 mM and urolithin B at a concentration of 114 μM can reduce PC12 cell viability by 50%. However, urolithin B at non-toxic concentrations of 4 and 8 μM significantly reduced glutamate-induced cytotoxicity (p < 0.01). Interestingly, treatment with glutamate significantly enhanced the intracellular ROS levels and apoptosis rate in PC12 cells, while pre-treatment with non-toxic concentrations of urolithin B significantly reduced these cytotoxic effects. The results also showed that pre-treatment with urolithin B can decrease the Bax (p < 0.05) and increase the Bcl-2 (p < 0.01) gene expression, which was dysregulated by glutamate.
Conclusions
Taken together, urolithin B may play a protective role through reducing oxidative stress and apoptosis against glutamate-induced toxicity in PC12 cells, which merits further investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases, involve the impairment of human cerebral cortex neurons. Nevertheless, the exact causes of these diseases remain poorly understood [1]. Glutamate, a vital neurotransmitter in the central nervous system (CNS), plays a role in excitatory synaptic responses and the development of neurons by activating glutamate receptors [2, 3]. Excessive levels of glutamate in extracellular brain regions can potentially cause acute damage to neural cells. In addition to Alzheimer’s and Parkinson’s diseases, excessive levels of glutamate can lead to neuronal cell death, which is closely related to a variety of CNS disorders, including cerebral ischemia, Huntington’s, hypoxia, autoimmune encephalomyelitis, and alcoholism [4,5,6].
There are two proposed mechanisms to explain how glutamate induces neuronal cell death. The first mechanism suggests that glutamate’s excitotoxicity is due to its excitatory amino acid receptors [7], which leads to an influx of extracellular calcium, ultimately resulting in neuronal cell death [8]. The second possible mechanism involves glutamate-induced cytotoxicity, where cystine uptake is competitively inhibited, leading to a decline in cellular glutathione levels and reducing the antioxidant defense against oxidative stress [9, 10]. This mechanism also activates calcium-dependent enzymes, further intensifying the oxidative stress experienced by neuronal cells [11]. The intracellular calcium levels combined with oxidative stress can cause programmed cell death and necrosis in neurons [12].
Consumption of ellagitannin-rich foods like walnuts and pomegranates can lead to the production of microbial metabolites (urolithins) by gut microbiota and possess various beneficial properties, including antioxidant, anti-inflammatory, estrogenic, hepatoprotective, and antiestrogenic effects. Urolithins have been demonstrated to be potentially effective agents for chemotherapy in laboratory experiments [13,14,15,16,17]. Additionally, urolithin B has shown significant antioxidant and neuroprotective effects by reducing the production of reactive oxygen species (ROS) and apoptosis caused by quinolinic acid in SH-SY5Y neuroblastoma cell line [18]. Rat pheochromocytoma PC12 cells provide a widely used model in neurobiology, commonly used to study neuronal cell death and neuronal injury [19, 20]. Therefore, in this study we investigated the protective effects of urolithin B against glutamate-induced toxicity in the PC12 cells, particularly focusing on intracellular ROS production and cell apoptosis.
Materials and methods
Materials
The PC12 cells were sourced from the cell bank of Pasture Institute (Tehran, Iran). Urolithin B (purity of 99.4%) was obtained from Gol Elixir Company (Iran). The MTT, RPMI 1640 medium, and fetal bovine serum (FBS) were provided from Gibco (Grand Island, NY, USA). Dimethyl sulphoxide (DMSO) and ethanol were purchased from Mojallali Co. (Iran). The ROS assay kit was purchased from Abcam (Cambridge, United Kingdom).
Cell culture and viability assay
PC12 cells were cultured in an RPMI 1640 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin and incubated in humidified condition at 37 °C with 5% CO2. PC12 cells were passaged twice a week, and all experiments were carried out between passage 7 and 13 [21].
The MTT assay was used to assess the effect of urolithin B (dissolved in DMSO) and glutamate on PC12 cell viability. The PC12 cells at a density of 5 × 103 cell/well were seeded in a 96-well plate and incubated for 24 h at 37 °C. The cells were then treated with urolithin B (3.9–2000 µM) or glutamate (0.375–50 mM). After 24 h incubation at 37 °C, 100 µL of MTT solution (0.5 mg/mL) was added to each well, and the cells were incubated for 3 h at 37 °C. After that, 200 µL DMSO was added to each well and the optical density was recorded at a wavelength of 570 nm using a Stat FAX303 plate reader (Awareness Technology Inc., USA).
In order to determining the protective effects of urolithin B against glutamate-induced cytotoxicity in PC12 cells, 5 × 103 cell/well were seeded in a 96-well plate and incubated for 24 h at 37 °C. Then, the cells were pre-treated with non-toxic concentrations of urolithin B (4 and 8 μM) for 24 h, followed by exposure to 10 mM concentration of glutamate for further 24 h. After that, the cell viability assays were carried out in triplicate using the MTT assay.
Intracellular ROS assay
The 2,7′-dichlorofluorescein diacetate (DCFDA/H2DCFDA) assay was applied to determine the intracellular ROS level [22]. For this, 10 × 103 cells/well were seeded into a 96-well plate and incubated for 24 h. Following the pre-treatment with non-toxic concentrations of urolithin B (4 and 8 μM) for 8 h, the cells were treated with 10 mM glutamate and incubated for further 24 h. Then, the cells were washed with wash buffer (as provided in the kit) and incubated for 1 h with 20 μM DCFDA. The tert-butyl hydroperoxide (TBHP) alone was used as a positive control. Finally, fluorescence was measured (excitation/emission: 485/535 nm).
Cell‐cycle analysis
For this purpose, two different non-toxic concentrations of urolithin B (4 and 8 μM) were subjected to 5 × 105 cells/well for 24 h and then incubated with 10 mM glutamate for or an additional 24 h at 37 °C. The cell-cycle analysis was performed through propidium iodide (PI) staining using flow cytometry method as described previously [18]. In brief, following trypsinization, the cells were washed twice with cold phosphate buffered saline (PBS), fixed with 1 mL of cold 70% ethanol at 4 °C for 2 h. The cells were resuspended in PBS containing 0.1% v/v Triton X-100 and 100 μg/mL RNase A (Sigma-Aldrich) and incubated for 30 min at 37 °C. After that, the PC12 cells were stained by adding 200 μL of PI solution (1 mg/mL) and after a 20 min incubation in the dark, the cell cycle was assessed using FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). The percentage of cell-cycle distribution in the G0/G1, S, and G2/M phases was quantified using Flow Jo software version 7.6.1 (Tristar, El Segundo, CA).
Determination of apoptotic cells by flow cytometry
The protective effect of urolithin B on apoptosis caused by glutamate was assessed using an annexin V/fluorescein isothiocyanate (FITC) kit (Abcam, Cambridge, United Kingdom) and flow cytometry analysis [23]. For this, 5 × 105 PC12 cells/well were seeded into a 6-well plate, followed by overnight incubation. Subsequently, the cells were pre-treated with different non-toxic concentrations of urolithin B (4 and 8 μM) for 24 h and then treated with 10 mM glutamate for or an additional 24 h at 37 °C. After that, the cells were washed with PBS and the cell pellets were resuspended in 100 μL of binding buffer. Subsequently, the cells were stained with 5 μL of annexin V-FITC and 10 μL of PI and incubated at room temperature for 15 min in the dark place. After adding 400 μL of binding buffer, the apoptosis rate was analyzed by flow cytometry method and the results were analyzed by Flow Jo version 7.6.1 (Tristar, El Segundo, CA).
Quantitative real‐time PCR (qRT-PCR)
In order to evaluate the Bax and Bcl-2 apoptosis-related gene expression, the qRT-PCR method was performed. Briefly, 5 × 105 PC12 cells were seeded into a 6-well plate and incubated for 24 h at 37 °C. Cells were then pretreated with 8 μM urolithin B for 24 h, followed by 24 incubations with 10 mM glutamate. Total RNA was isolated from PC12 cells using a RNA Extraction Kit (Pars Tous Co., Iran) and subsequently reversely transcribed to cDNA by Easy cDNA Synthesis Kit (Pars Tous Co., Iran) according to the manufacturer’s instructions. Next, qRT-PCR amplifications for Bax and Bcl-2 genes were carried out using SYBR® Select Master Mix (Applied Biosystems) and ABI StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA). The relative gene expression was calculated using the 2−ΔΔCt method [24]. The primers used for Bax, Bcl-2, and GAPDH are listed in Table 1.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 software (San Diego, CA, USA). One-way ANOVA and Tukey’s post-hoc test was used to calculate the statistical differences between the groups. The results were reported as mean ± SD and the p value < 0.05 was considered statistically significant.
Results
Glutamate decreased the PC12 cell viability
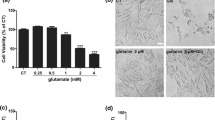
According to the findings of glutamate toxicity to the PC12 cell line, glutamate decreased cell viability dose-dependently. The 24 h exposure to glutamate at a concentration of 10 mM resulted in a 50% decrease in cell viability. Therefore, the IC50 value of 10 mM was chosen for use in subsequent investigations (Fig. 1A). Following treatment with various urolithin B concentrations, the viability of the cells was measured using MTT assay. Based on the results, none of the concentrations of 4, 8, and 16 μM of urolithin B did not reduced PC12 cells viability after 24 h. Thus, the non-toxic concentrations of 4 and 8 μM of urolithin B were used for further experiments (Fig. 1B). Next, we evaluated the urolithin B’s protective properties against glutamate-induced toxicity in PC12 cells. According to the results, urolithin B significantly attenuated glutamate-induced toxicity in PC12 cells at 4, 8 and 16 μM (p < 0.001) (Fig. 1C).
The effect of glutamate (A) and urolithin B (B) on PC12 cells viability. C The protective effects of pre-treatment with urolithin B against 10 mM glutamate-induced cytotoxicity. Results are presented as the mean ± SD of three independent experiments. (***p < 0.001 as compared to the untreated control group. ##p < 0.01 and ###p < 0.001 as compared to the glutamate-treated group)
Urolithin B reduced intracellular ROS levels induced by glutamate
To determine the effects of urolithin B on intracellular ROS levels caused by glutamate, DCFDA fluorescent dye was used. As shown in Fig. 2, the level of ROS increased significantly in the group treated with 10 mM glutamate compared to the control group (p < 0.001). Interestingly, the intracellular ROS induced by glutamate in the groups pretreated with non-toxic concentrations of urolithin B showed a significant decrease compared to the glutamate-treated group (p < 0.01). It should be noted that treatment TBHP alone as a positive control led to a substantial elevation of intracellular ROS levels in comparison with the control (p < 0.001).
The effect of urolithin B on the intracellular ROS levels induced by glutamate in PC12 cells. The tert-butyl hydroperoxide (TBHP) alone was used as a positive control, which significantly increased the formation of ROS. The results are presented as the mean ± SD of triplicate tests. (***p < 0.001 as compared to the control group, and ##p < 0.01 as compared to glutamate-treated group)
Urolithin B decreased the G0–G1 cell population induced by glutamate treatment
The results have shown a significant rise in apoptotic cells in PC12 cells following treatment with glutamate at a concentration of 10 mM, in comparison to the control group (p < 0.01). Notably, pre-treatment with urolithin B has reduced the cytotoxic effect of glutamate. The percentage of apoptotic cells in the glutamate-treated group has decreased from 93% to 80.4% and 92.2% in the groups pre-treated with 8 μM (p < 0.001) and 4 μM (p < 0.05) urolithin B, respectively (Fig. 3).
The protective effect of urolithin B against cell cycle arrest induced by glutamate in PC12 cells. A Flow cytometry analysis was used to estimate the percentages of cells in each phase of the cells cycle. B The percentage of cell in the G0–G1 phase for each group. The results are expressed as the mean ± SD of triplicate tests. (**p < 0.01 as compared to the control group, #p < 0.05 and ##p < 0.01 as compared to glutamate-treated group)
Urolithin B decreased percentage of PC12 cell apoptosis induced by glutamate
Our findings demonstrated a significant increase in apoptotic cells after glutamate treatment of PC12 cells at a concentration of 10 mM compared to the control (p < 0.001). Pre-treatment with urolithin B significantly ameliorated these effects (from 12.4% of apoptotic cells in the glutamate group to 9.67% and 11.6% in the 8 μM and 4 μM urolithin B pre-treated groups, respectively; p < 0.001) (Fig. 4).
The effect of urolithin B on apoptosis rate caused by glutamate in PC12 cells. A Flow cytometry analysis was used to evaluate the apoptosis in each group. B Quantification of apoptotic PC12 cell number in each group. The results are expressed as the mean ± SD of triplicate tests. (***p < 0.001 as compared to the control group and ###p < 0.001 as compared to glutamate-treated group)
Urolithin B regulated Bax and Bcl-2 apoptosis-related genes expression altered by glutamate
The current study utilized qRT-PCR to analyze the alterations of Bax and Bcl-2 gene expression. The results showed that glutamate causes a significant increase in Bax gene expression (p < 0.01), while pre-treatment with urolithin B can reduce this alteration (p < 0.05) (Fig. 5A). Furthermore, the expression level of Bcl-2 gene exhibited a substantial increase after pre-treatment with urolithin B compared to the glutamate-treated group (p < 0.01) (Fig. 5B).
The modulatory effect of urolithin B on Bax (A) and Bcl-2 (B) gene expression altered by glutamate in PC12 cells. The results are expressed as the mean ± SD of triplicate tests. (**p < 0.01 and ***p < 0.001 as compared to the control group. #p < 0.05 and ##p < 0.01 as compared to glutamate-treated group)
Discussion
Parkinson’s disease (PD) is a neurological condition that impacts around 1% of those who are 60 years old or older. PD is characterized by the degeneration of dopaminergic neurons in the substantia nigra area of the brain, resulting in decreased dopamine levels and the emergence of motor symptoms, including tremors, stiffness, and bradykinesia. The precise etiology of PD remains uncertain. However, many pathways have been suggested to contribute to its pathophysiology. The processes involved are oxidative stress, inflammation, mitochondrial dysfunction, protein misfolding, and defective autophagy [25, 26]. The precise molecular mechanisms behind the protective properties of urolithin B are still under investigation. However, urolithin B is believed to affect several signaling pathways involved in cell survival, inflammation, and oxidative stress [27, 28]. The disruption of typical apoptosis results in the abnormal demise of dopaminergic neurons in the substantia nigra area of the brain. The precise equilibrium between pro-apoptotic and anti-apoptotic proteins is crucial in controlling apoptosis. Numerous studies have demonstrated that when these proteins are not adequately regulated, it is linked to the advancement and escalation of PD [29, 30]. The findings of our study demonstrated that pre-treatment with urolithin B led to a notable upregulation of the anti-apoptotic Bcl-2 and downregulation of the pro-apoptotic Bax gene expression compared to the glutamate-treated group. Moreover, pre-treatment with urolithin B decreased the G0–G1 cell population and percentage of PC12 cell apoptosis induced by glutamate. These results indicate that urolithin B may exercise its protective effects via modulating apoptosis. Previous research showed that applying urolithin B before treatment reduced apoptotic cells in SH-SY5Y cells [18]. Another study demonstrated that urolithin B reduced viability in U87 glioblastoma multiforme cells dose-dependently, leading to cell cycle arrest and changes in apoptotic gene expression [31]. Urolithin B significantly increased U87 cell accumulation in the Sub-G1 population, downregulated cyclin D1 expression, and increased the Bax/Bcl-2 ratio [32]. Urolithin B also hindered the growth of HCC cells by arresting the cell cycle and inducing programmed cell death. It also inhibits the growth of prostate cancer cells and induces apoptosis by impeding prostate-specific antigen expression and androgen receptor [33, 34]. Multiple investigations have demonstrated that antioxidants, which eliminate ROS and hinder oxidative harm, provide promising therapeutic advantages in PD [35]. Our findings indicated that, urolithin B pre-treatment of PC12 cells could reduce the intracellular ROS levels generated by glutamate. These results indicate that urolithin B may exercise its protective effects via modulating oxidative stress. In this line, DaSilva et al. found that optimal levels of urolithin B and its methylated derivatives effectively diminished neuroinflammation by suppressing the production of nitric oxide (NO), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), prostaglandin E2 (PGE2), and ROS levels in BV-2 microglia of mice [36]. In a study conducted by Lee et al., they found that urolithin B had vigorous antioxidant activity in microglia by decreasing the formation of ROS and reducing the expression of NADPH oxidase subunits while simultaneously increasing the expression of heme oxygenase-1 through the Nrf2/ARE signaling pathway [37]. Furthermore, Qiu et al. demonstrated that the enhanced survival of T24 cells exposed to hydrogen peroxide, as a result of urolithin B, was associated with a decrease in the levels of ROS and malondialdehyde within the cells, along with an increase in the activity of superoxide dismutase [38].
Conclusion
In current study, urolithin B was evaluated for its capacity to protect against glutamate-induced toxicity in PC12 cells. Taken together, our findings demonstrates that urolithin B has potential in reducing the harmful effects of glutamate in PC12 cells. These effects include reduction of intracellular ROS level, regulation of cell cycle in G0/G1 phase, reduction of apoptosis rate, increase in expression of anti-apoptotic gene Bcl-2 and decrease in expression of pro-apoptotic gene Bax, which is dysregulated by glutamate. However, additional in vitro and in vivo studies are required to investigate the precise molecular mechanisms at the protein levels to better understanding the protective properties of urolithin B and its potential therapeutic applications in the treatment of neurological disorders.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- DMSO:
-
Dimethyl sulfoxide
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- ROS:
-
Reactive oxygen species
- CNS:
-
Central nervous system
- MTT:
-
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
- PI:
-
Propidium iodide
- FBS:
-
Fetal bovine serum
- TBHP:
-
Tert-butyl hydroperoxide
- PD:
-
Parkinson’s disease
- NO:
-
Nitric oxide
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor-alpha
- PGE2:
-
Prostaglandin E2
References
Coffey CE et al (1998) Sex differences in brain aging: a quantitative magnetic resonance imaging study. Arch Neurol 55(2):169–179
Jin R et al (2002) Mechanism of activation and selectivity in a ligand-gated ion channel: structural and functional studies of GluR2 and quisqualate. Biochemistry 41(52):15635–15643
Molnar E, Isaac JT (2002) Developmental and activity dependent regulation of ionotropic glutamate receptors at synapses. ScientificWorldJournal 2:27–47
van Os S et al (2006) Excitatory amino acid release and electrocortical brain activity after hypoxemia in near-term lambs. Brain Dev 28(6):380–388
Camins A, Pallas M, Silvestre JS (2008) Apoptotic mechanisms involved in neurodegenerative diseases: experimental and therapeutic approaches. Methods Find Exp Clin Pharmacol 30(1):43–65
Benveniste H (2009) Glutamate, microdialysis, and cerebral ischemia: lost in translation? Anesthesiology 110(2):422–425
Monaghan DT, Bridges RJ, Cotman CW (1989) The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol 29:365–402
Bleich S et al (2003) Glutamate and the glutamate receptor system: a target for drug action. Int J Geriatr Psychiatry 18(Suppl 1):S33-40
Choi DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1(8):623–634
Murphy TH et al (1989) Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 2(6):1547–1558
Zabłocka A, Janusz M (2008) The two faces of reactive oxygen species. Postepy Hig Med Dosw (Online) 62:118–124
Tan S, Schubert D, Maher P (2001) Oxytosis: a novel form of programmed cell death. Curr Top Med Chem 1(6):497–506
Cerdá B et al (2003) Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr 42(1):18–28
García-Villalba R et al (2019) Identification of novel urolithin metabolites in human feces and urine after the intake of a pomegranate extract. J Agric Food Chem 67(40):11099–11107
Espín JC et al (2013) Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: the evidence so far. Evid Based Complement Alternat Med 2013:270418
Seeram NP et al (2006) Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr 136(10):2481–2485
Olennikov DN, Kashchenko NI, Chirikova NK (2015) In vitro bioaccessibility, human gut microbiota metabolites and hepatoprotective potential of chebulic ellagitannins: a case of Padma Hepaten® formulation. Nutrients 7(10):8456–8477
Abbasinezhad-Moud F et al (2023) The effects of urolithin B and auraptene on quinolinic acid-induced toxicity in the SH-SY5Y neuroblastoma cell line. Altern Lab Anim 51(1):30–38
Wiatrak B et al (2020) PC12 cell line: cell types, coating of culture vessels, differentiation and other culture conditions. Cells 9(4):958
Xie D et al (2022) The cellular model for Alzheimer’s disease research: PC12 cells. Front Mol Neurosci 15:1016559
Kinarivala N et al (2017) Passage variation of PC12 cells results in inconsistent susceptibility to externally induced apoptosis. ACS Chem Neurosci 8(1):82–88
Aranda A et al (2013) Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: a quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol In Vitro 27(2):954–963
Ebrahimi S et al (2023) The in vitro anti-cancer synergy of neurokinin-1 receptor antagonist, aprepitant, and 5-aminolevulinic acid in glioblastoma. BioFactors 49(4):900–911
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408
Balestrino R, Schapira AHV (2020) Parkinson disease. Eur J Neurol 27(1):27–42
Tysnes OB, Storstein A (2017) Epidemiology of Parkinson’s disease. J Neural Transm (Vienna) 124(8):901–905
Chen P et al (2022) Recent advances and perspectives on the health benefits of urolithin B, a bioactive natural product derived from ellagitannins. Front Pharmacol 13:917266
Djedjibegovic J et al (2020) Ellagic acid-derived urolithins as modulators of oxidative stress. Oxid Med Cell Longev 2020:5194508
Moujalled D, Strasser A, Liddell JR (2021) Molecular mechanisms of cell death in neurological diseases. Cell Death Differ 28(7):2029–2044
Venderova K, Park DS (2012) Programmed cell death in Parkinson’s disease. Cold Spring Harb Perspect Med 2(8):a009365
Eidizade F et al (2023) Inhibition of glioblastoma proliferation, invasion, and migration by urolithin B through inducing G0/G1 arrest and targeting MMP-2/-9 expression and activity. BioFactors 49(2):379–389
Rahimi-Kalateh Shah Mohammad G et al (2023) Urolithin B loaded in cerium oxide nanoparticles enhances the anti-glioblastoma effects of free urolithin B in vitro. J Trace Elem Med Biol 78:127186
Lv MY et al (2019) Urolithin B suppresses tumor growth in hepatocellular carcinoma through inducing the inactivation of Wnt/β-catenin signaling. J Cell Biochem 120(10):17273–17282
Sánchez-González C et al (2014) Walnut polyphenol metabolites, urolithins A and B, inhibit the expression of the prostate-specific antigen and the androgen receptor in prostate cancer cells. Food Funct 5(11):2922–2930
Percário S et al (2020) Oxidative stress in Parkinson’s disease: potential benefits of antioxidant supplementation. Oxid Med Cell Longev 2020:2360872
DaSilva NA et al (2019) Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro. Nutr Neurosci 22(3):185–195
Lee G et al (2019) Anti-inflammatory and antioxidant mechanisms of urolithin B in activated microglia. Phytomedicine 55:50–57
Qiu Z et al (2013) In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food Chem Toxicol 59:428–437
Funding
This work’s authors recognize the following funding sources for their research, writing, and publication: Financial support for this project came from the Mashhad University of Medical Sciences (Grant No. 4011832).
Author information
Authors and Affiliations
Contributions
IA, MR, and AA contributed to the manuscript’s conception, design, acquisition, and drafting. FM, MKK, AH, and EG contributed to the interpretation and critically revised the manuscript. MS made distinctive contributions to the idea and demonstrated their specialized expertise to enhance the overall quality of the manuscript. All authors have thoroughly reviewed and provided their consent to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no known possible conflicts of interest.
Ethics approval and informed consent
The ethics committee of Mashhad University of Medical Sciences, Mashhad, Iran, approved the ethical issues of this study. It was a cell-based study, with no human sample included.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aljabouri, I., Rostami, M., Mirzavi, F. et al. Urolithin B protects PC12 cells against glutamate-induced toxicity. Mol Biol Rep 51, 360 (2024). https://doi.org/10.1007/s11033-024-09236-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09236-8