Abstract
In the brain, glutamine synthetase (GS), which is located predominantly in astrocytes, is largely responsible for the removal of both blood-derived and metabolically generated ammonia. Thus, studies with [13N]ammonia have shown that about 25 % of blood-derived ammonia is removed in a single pass through the rat brain and that this ammonia is incorporated primarily into glutamine (amide) in astrocytes. Major pathways for cerebral ammonia generation include the glutaminase reaction and the glutamate dehydrogenase (GDH) reaction. The equilibrium position of the GDH-catalyzed reaction in vitro favors reductive amination of α-ketoglutarate at pH 7.4. Nevertheless, only a small amount of label derived from [13N]ammonia in rat brain is incorporated into glutamate and the α-amine of glutamine in vivo. Most likely the cerebral GDH reaction is drawn normally in the direction of glutamate oxidation (ammonia production) by rapid removal of ammonia as glutamine. Linkage of glutamate/α-ketoglutarate-utilizing aminotransferases with the GDH reaction channels excess amino acid nitrogen toward ammonia for glutamine synthesis. At high ammonia levels and/or when GS is inhibited the GDH reaction coupled with glutamate/α-ketoglutarate-linked aminotransferases may, however, promote the flow of ammonia nitrogen toward synthesis of amino acids. Preliminary evidence suggests an important role for the purine nucleotide cycle (PNC) as an additional source of ammonia in neurons (Net reaction: l-Aspartate + GTP + H2O → Fumarate + GDP + Pi + NH3) and in the beat cycle of ependyma cilia. The link of the PNC to aminotransferases and GDH/GS and its role in cerebral nitrogen metabolism under both normal and pathological (e.g. hyperammonemic encephalopathy) conditions should be a productive area for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
I am honored to contribute an article to the special volume dedicated to Professor Leif Hertz. I have known Leif for over 25 years and we have collaborated on several occasions in the past. Leif is an expert neurochemist and an inspiration for younger neurochemists. In this article I will outline work that I began more than 30 years ago with a group of biophysicists at Memorial Sloan Kettering Cancer Center (MSKCC), New York. The results from this work are still highly relevant and interdigitate with recent concepts of nitrogen metabolism in the brain—an area to which Leif has contributed considerably.
Historical: Discovery of Compartmentation of Ammonia Metabolism in the Brain
In a classic series of experiments carried out more than half a century ago, Berl and colleagues showed that cerebral ammoniaFootnote 1 metabolism is compartmented [1]. [15N]Ammonia was continuously infused into anesthetized cats via a carotid artery. After 25 min of infusion, the cats were sacrificed and the 15N content of selected brain metabolites was determined. Nitrogen in the biosphere consists of ~99.63 % 14N and ~0.37 % 15N. The relative enrichment above background of 15N in these metabolites was found to be in the order: amide group of l-glutamine > amine group of l-glutamine > l-glutamate [1]. The only known route for synthesis of glutamine in mammals is via the GS reaction (Eq. 1). Moreover, at least a portion of the amine moiety of the glutamate needed for glutamine synthesis must have been derived from reductive amination of α-ketoglutarate catalyzed by GDH (Eq. 2). Thus, if the brain had acted as a single metabolic compartment, a simple product-precursor relationship dictates that the relative enrichment of 15N in the amine group of glutamine cannot exceed that of glutamate. Because 15N enrichment was greater in the amine group of glutamine than in glutamate Berl et al. [1] proposed that ammonia entering the brain from the blood is rapidly incorporated into glutamine in a small pool of rapidly turning over glutamate (small compartment). This pool is kinetically distinct from a much larger, more slowly turning over pool of glutamate (large compartment) [1]. The authors estimated that the turnover of glutamate in the large compartment of the cat brain is of the order of hours, but less than 1 h in the small compartment. Metabolic compartmentation of glutamate metabolism in liver could not be discerned in the experiments of Berl et al. [1]. However, as an interesting aside, it is now well established that ammonia/glutamate metabolism in the liver is indeed strongly compartmented. For example, GS in the liver is confined to a small ring of perivenous hepatocytes (zone 1 of the acinus), whereas glutaminase and urea cycle enzymes are strongly represented in perivenous hepatocytes (zone 3 of the acinus) [e.g. 2, 3].
The background level of 15N in ambient nitrogen and relative insensitivity of the analytical techniques available in the late 1950s and early 1960s for isotope measurement necessitated the infusion of massive amounts of ammonia into the cats in the experiments of Berl et al. [1] in order to obtain measurable enrichments of 15N in glutamine and glutamate. This non-physiological treatment resulted in large increases in brain ammonia and glutamine, such that the animals were comatose at time of sacrifice [1]. Nevertheless, the work of Berl et al. [1] contributed substantially to our understanding of metabolic compartmentation in the brain.
Despite the large increase in brain glutamine after the infusion of [15N]ammonia there was no measurable change in the concentration of glutamate in the cat brain [1]. Part of the reason for the inability to detect a change in brain glutamate concentration in this experiment may have been due to the conversion of glutamate to glutamine in the small compartment. Changes in glutamate concentration in this compartment may have been obscured by the much larger, more slowly turning glutamate pool in the large compartment. In an accompanying publication, Berl et al. [4] showed that the brain possesses a remarkable capacity to fix CO2, which is apparently stimulated by elevated ammonia levels. The ability of brain to anaplerotically fix substantial amounts of CO2 (as bicarbonate) is now known to be primarily catalyzed by pyruvate carboxylase (Eq. 3), an enzyme predominantly localized to astrocytes in brain [5–10]. The findings of Berl et al. [4] have been amply verified by modern nuclear magnetic resonance (NMR) techniques. For example, Sibson et al. [11] used 13C-NMR to study cerebral metabolic compartmentation in rats following infusion of [2-13C]glucose or [5-13C]glucose. These authors noted that under normoammonemic conditions anaplerosis accounted for 19–26 % of the carbon flux through the GS reaction, but increased to 32 % during hyperammonemia [11]. In a more recent study Duarte et al. [12] used 13C-NMR techniques to trace the cerebral metabolic fate of [1,6-13C]glucose in anesthetized rats. Glia were found to account for ~30 % of total cerebral tricarboxylic acid (TCA) flux [12]. The anaplerotic flux through the pyruvate carboxylase reaction was estimated to be about 25 % of the flux through the glial TCA cycle in these animals [12].
The brain possesses additional enzymes that are theoretically able to anaplerotically fix CO2. These enzymes include the malic enzyme, phosphoenolpyruvate carboxykinase and propionyl carboxylase [13 and references cited therein]. However, the contribution of these enzymes to net CO2 fixation under normoammonemic and hyperammonemic conditions in the brain remains uncertain and the malic enzyme and phosphenolpyruvate carboxykinase may be a net source of CO2 in the brain, rather than a net means of removal [13]. Notwithstanding these caveats, the now well established ability of the brain to fix CO2 contributes substantially to the ability of the brain to maintain relatively constant or only slightly depressed levels of glutamate during very large increased synthesis of glutamine.
Brain Uptake Index (BUI) of [13N]ammonia in the Rat
Beginning in the late 1970s my colleagues and I used the MSKCC medical cyclotron to produce [13N]ammonia for metabolic studies. 13N is a positron-emitting isotope with a t1/2 of 9.96 min. Annihilation of a nearby electron by the emitted positron results in the production of two coincident, or almost coincident, 511 keV γ rays. The major disadvantage of 13N is its short half life. This is not, however, a problem for short-term studies, because as I shall continuously emphasize throughout this review the turnover of ammonia and certain amino acids in our experimental animal of choice (the rat) is extremely rapid. Moreover, the γ rays emitted as a consequence of 13N decay are easily quantitated. Finally, very high specific activities of 13N are theoretically possible. For comparison, the theoretical maximal specific activity of carrier-free 13N is 4.2 × 108 times greater than that of carrier-free 14C [14]. Ammonia is a ubiquitous contaminant, however. Nevertheless, we were able to generate [13N]ammonia of extremely high specific activity.
As noted above, infusion of large quantities of [15N]ammonia into cats led to a marked disturbance of cerebral nitrogen homeostasis. Because of this limitation we decided to investigate ammonia metabolism and compartmentation in brain using [13N]ammonia instead of [15N]ammonia. We also chose rats instead of cats as the experimental animal of choice. The [13N]ammonia generated in the MSKCC cyclotron was of very high specific activity, such that truly tracer quantities of labeled ammonia could be administered to the rats. For some historical perspectives on the use of 13N as a tracer see references [14] and [15].
In our first series of experiments we administered a bolus of [13N]ammonia via one carotid artery and measured the BUI of [13N]ammonia in awake, but restrained rats [16]. The BUI is the single pass extraction by the brain of the test substance (expressed as a percentage) relative to a marker that is completely extracted by the brain. The BUI for ammonia was found to be about 25 % and independent of the concentration of added, unlabeled ammonia in the bolus. The BUI was found, however, to increase with increasing pH of the bolus [16]. Similar findings were reported by Lockwood et al. [17]. The relative proportion of diffusible NH3 to NH4 + (pK a ~ 9.2) will increase with increasing pH. Therefore, our findings [16] and those of Lockwood et al. [17] indicate that ammonia enters the rat brain largely by diffusion of the free base (NH3). The pH dependence of [13N]ammonia uptake has also been demonstrated for rhesus monkey brain [18] and dog brain [19]. Raichle and Larson [18] used positron emission tomography (PET) to study uptake of [13N]ammonia in rhesus monkey brain and concluded that uptake is largely by diffusion of the free base, but that a small portion enters as ammonium ion.
Metabolism of Blood-Derived [13N]ammonia in Rat Brain: Validation of the Metabolic Compartmentation of Cerebral Ammonia Metabolism
After determining the BUI for [13N]ammonia we next investigated the metabolic fate of tracer quantities of [13N]ammonia in rat brain. We used a rapid isolation procedure coupled with HPLC and analysis of a portion of rat brain homogenate with glutaminase to calculate the relative amount of label in the amine versus amide positions of glutamine. We found that about 60 % of the label was already in a metabolized form 5 s after carotid artery administration of a bolus of [13N]ammonia [16]. Because residual [13N]ammonia is likely to be present in the cerebral blood supply at time of sacrifice (5 s), our findings indicated that the t½ for metabolism of ammonia entering the rat brain from the blood into the small compartment must have been of the order of seconds. More than 95 % of the label in metabolites was found to be present in the amide position of glutamine with much smaller amounts in the amine position of glutamine and glutamate. Importantly, the relative specific activities were found to be in the order: l-[amide-13N]glutamine > l-[amine-13N]glutamine > l-[13N]glutamate. Similar results were obtained when tracer quantities of [13N]ammonia were infused via a carotid artery cannula for 10 min (Table 1). Thus, our results, which were obtained under truly tracer conditions, corroborated the previous findings of Berl et al. [1, 4] that ammonia metabolism is compartmented in brain. The results also highlighted the previously unsuspected speed with which nitrogen can move among different metabolites in the brain.
Another example of rapid nitrogen flux is briefly mentioned here. We showed that within 20 s after femoral artery injection of a bolus of [13N]ammonia into adult male rats almost all the label had disappeared from the circulation [20]. Moreover, of the label remaining, most was in a metabolized form [20]. The rapid exchange of nitrogen is of fundamental importance in any discussions of mechanisms of nitrogen partitioning among different amino acid pools, yet is not widely appreciated.
In other experiments [13N]ammonia was continuously infused for 20 min into adult male rats via a carotid artery cannula [16, 21]. An HPLC profile of 13N metabolites in brain obtained after a 20-min infusion is shown in Fig. 1. This figure dramatically emphasizes the importance of the GS reaction in the brain. Note the relatively small amount of radioactivity in glutamate, aspartate and GABA. The very small amount of label in urea presumably reflects the fact that a large portion of the administered [13N]ammonia circumvented the brain and entered the general circulation to be converted to [13N]urea in the liver, some of which was released to the general circulation.
HPLC profile of labeled metabolites in rat brain after a 20-min infusion of [13N]ammonia. The [13N]ammonia in isotonic saline was administered intracarotidly at a rate of 0.15 ml/min into an adult male rat. At the end of the infusion the rat was sacrificed and the label in a deproteinized fraction (0.02 ml) of the brain was analyzed by HPLC by means of a cation exchange column as described in [21]. All counts in the HPLC profile were decay corrected back to an arbitrary time (start of HPLC). Modified from [21]
Metabolism of Cerebrospinal Fluid (CSF)-Derived [13N]ammonia in Rat Brain
When tracer quantities of [13N]ammonia were administered to the CSF of an adult male rat via an indwelling cannula metabolic compartmentation of cerebral ammonia metabolism was again observed [16]. The specific activity of brain metabolites was in the order: l-[amide-13N]glutamine > l-[amine-13N]glutamine > l-[13N]glutamate. Thus, ammonia entering the brain from the CSF, in addition to that entering from the blood, is metabolized in the glutamine-synthetase containing small compartment.
Anatomical Basis for Compartmentation of Cerebral Ammonia Metabolism
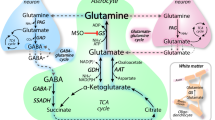
Brain has been known for over 50 years to be a rich source of GS [22]. Moreover, sheep brain is often used as a convenient source of GS [23]. But where is the enzyme located in the brain? This question was answered by Norenberg and associates who convincingly showed that GS is largely, if not exclusively, located in astrocytes in the brain [24, 25]. This work showed that, to an approximation, the small and large compartments, as defined by prior tracer and kinetic experiments, are represented by astrocytes and neurons, respectively. Astrocytic end feet surround brain capillaries and underlie the ependyma. Thus, ammonia diffusing into the brain across the blood–brain barrier (BBB) or from the CSF enters the astrocytes (small compartment) where it is efficiently incorporated into glutamine before it has a chance to diffuse further and act as a substrate of GDH in neurons (large compartment). Compartmentation of ammonia metabolism in brain would also occur if GDH occurred exclusively, or almost exclusively, in neurons rather than in astrocytes. However, as reviewed by Hertz and Schousboe GDH is well represented in both astrocytes and neurons in culture [26]. A simplified compartmentation model of cerebral ammonia metabolism is shown in Fig. 2.
Compartmentation of ammonia metabolism in the brain. Ammonia entering the brain by diffusion from either the blood or CSF is rapidly metabolized in the small compartment (astrocytes) predominately to glutamine. Ammonia generated endogenously in both the neuronal and astrocytic compartments is also rapidly incorporated into glutamine within the astrocytic compartment. For convenience the protonated form of ammonia (ammonium, NH4 +) is not shown in the brain compartments. The thickness of the arrows indicates the relative contribution of the GS reaction [NH3 → glutamine Gln)] and GDH reaction [NH3 → glutamate (Glu)] to the metabolism of endogenously produced ammonia and blood/CSF-derived ammonia. Under normal conditions, the GDH reaction plays a minor role in metabolizing brain ammonia. In the neurons the GDH reaction is a mechanism for net oxidative deamination of glutamate under normal conditions. The GDH reaction is therefore not shown as a means of removing ammonia in the neuronal compartment
Estimation of Rat Brain GS Activity In Vivo
Although our studies with 13N demonstrated unequivocally the importance of GS in the metabolism of ammonia in the brain [16], they did not provide a numerical estimate of the rate of cerebral glutamine synthesis. Several subsequent NMR studies using [15N]ammonia have, however, been used to calculate the GS flux in rat brain during hyperammonemia [e.g. 27–31]. Estimates of rat brain glutamine synthesis in these hyperammonemic animals ranged from 0.06 to 0.2 μmol/min/g [27–31]. Other estimates using 13C MR techniques suggested a rate in the hyperammonemic rat brain of 0.29–0.43 μmol/min/g [11, 32]. Recent NMR studies from Gruetter and colleagues suggest a rate of 0.18 μmol/min/g [33] under normoammonemic conditions and 0.30 μmol/min/g under hyperammonemic conditions. Some of the differences in reported rates may be due to differences in the blood ammonia concentrations in the experimental animals. For example, in the work of Kanamori et al. [27–30] the blood ammonia levels reported were from a low of 0.2–0.3 mM to a high of 0.8–1.3 mM. At the higher blood ammonia levels the brain GS reaction apparently reached saturation. In other studies, reported blood ammonia levels were 0.95 mM [33] and maximally 0.35–0.40 mM [11, 31, 32].
Different methodologies may also account for some of the apparent discrepancies in reported cerebral glutamine synthesis rates. For example, in some studies measurements were carried out in acutely hyperammonemic rats in which the brain glutamine level was rising [31, 33]. In other studies, Kanamori et al. [27–30] and Sibson et al. [11, 32] used a steady state hyperammonemia model in which brain glutamine levels were maintained at a constant, but elevated level. For example, in the Sibson et al. [32] study, blood ammonia levels were raised from ~0.05 mM (baseline) to 0.35 mM by infusion of ammonium acetate. Once a steady state level of brain glutamine was established [1-13C]glucose was infused and NMR analysis of 13C-labeled metabolites was measured. Flux through the rat brain TCA cycle under normoammonemic and hyperammonemic conditions was estimated to be 0.46 ± 0.16 and 0.57 ± 0.16 μmol/min/g, respectively (not significantly different) [32]. The rates of cerebral glutamine synthesis in anesthetized normoammonemic and hyperammonemic rats in these experiments were calculated to be 0.21 ± 0.04 and 0.43 ± 0.14 μmol/min/g, respectively (p < 0.01)–a two-fold increase [32]. The authors suggested that under normal conditions most of the glutamine flux is accounted for by neurotransmitter cycling between glutamate and glutamine (the glutamine cycle; see below). In contrast, during hyperammonemia, increased synthesis of glutamine leads to an increase in cerebral glutamine superimposed upon that required for neurotransmitter glutamate cycling and to increased egress of glutamine from the brain [32]. In a later report Sibson et al. [11] again used NMR and [1-13C]glucose infusion to study cerebral glutamine metabolism in anesthetized rats and reaffirmed their previous estimate of glutamine synthesis in the brain of about 0.2 μmol/min/g [11]. As noted above, anaplerosis was estimated to contribute 19–26 % of carbon flux in the glutamine synthesis reaction. This value increased to about 32 % in the hyperammonemic animals [32]. This finding again emphasizes the remarkable ability of the brain to utilize anaplerosis and to increase this process during hyperammonemia, as first revealed in the pioneering studies of Berl et al. [4].
It should also be noted that estimates of glutamine synthesis in the brain may vary depending on how the data were analyzed (e.g. a one compartment model versus a two compartment model; parameters iterated or assumed; K. L. Behar, personal communication). A detailed discussion of these parameters is beyond the scope of the present article. However, a few interesting points emerge from the NMR data. The best estimates of GS activity in the anesthetized rat brain in vivo under normoammonemic conditions are ~0.18–0.2 μmol/min/g [11, 12, 32]. Under hyperammonemic conditions this value may rise to ~0.3–0.4 μmol/min/g [11, 33]. These calculations show that despite a modest decrease in the maximal capacity of rat brain GS in rat models of chronic [34] and acute [35, 36] hyperammonemia (as determined by enzyme activity measured under assay optimal conditions) the brain enzyme is not normally saturated with ammonia and has the capacity to considerably increase the rate of production of glutamine under hyperammonemic conditions. This hypothesis is consistent with the average concentration of ammonia in normal rat brain of ~0.18 mM [16], a presumed lower concentration of ammonia in the small compartment, and the K m value exhibited by brain GS for ammonia of 0.18 mM [23]. However, there is a limit to this capacity. Increased rates of glutamine synthesis cannot prevent increased accumulation of brain ammonia in various animal models of hyperammonemia [e.g. 34–37] and at end stage coma glutamine synthesis rates may reach saturation [30] or actually begin to decrease [30, 36–38].
Increased cerebral glutamine is a hallmark of hyperammonemia (discussed below). Butterworth and colleagues [37] have pointed out that there is a discrepancy, however, between the accumulation of cerebral glutamine in hyperammonemic animals and rate of cerebral glutamine synthesis reported in the literature [30, 35, 38]. Therefore, other factors besides increased rates of glutamine synthesis may contribute to increased cerebral glutamine in hyperammonemic animals [37]. These authors have pointed out that hyperammonemia results in inhibition of the astrocyte glutamine transporter SNAT5 [37]. Thus, restricted egress of glutamine from astrocytes may account in large part for the increased brain glutamine and cell swelling/brain edema that are well established features in hyperammonemic animals. The authors also propose that such a mechanism could lead to decreased excitatory neurotransmission—a characteristic feature of the encephalopathy associated with acute liver failure [37]. This is an interesting and novel idea, and certainly an inhibition of the glutamine transporter could contribute to increased glutamine in astrocytes. However, it is probable that the glutamine synthesis reaction is still active even at end stage acute liver failure. Nevertheless, the exact roles that glutamine synthesis and inhibition of glutamine egress play in the accumulation of glutamine in astrocytes and associated cell swelling/edema during hyperammonemia must await further study.
Comments on the Use of Labeled Ammonia to Investigate Brain GS In Vivo
Since the GS reaction requires ATP (Eq. 1) it is generally assumed that the reaction is irreversible. However, Levintow and Meister [39] showed that the reaction is, in fact, readily reversible. Thus, under optimal conditions, the back reaction (i.e. glutamine → glutamate) is about 12 % as rapid as the forward direction (i.e. glutamate → glutamine) [39]. This reversibility could theoretically lead to an underestimate of the rate of cerebral GS when incorporation of labeled ammonia into glutamine (amide) is measured. On the other hand, another mechanism may theoretically lead to an overestimation. Thus, it has been shown, using 15N as a tracer, that GS obtained from various sources catalyzes the non-productive exchange of ammonia with the amide group of glutamine (i.e. [15NH3 + l-[amide-14N]glutamine ⇆ l-[amide-15N]glutamine + 14NH3) [40]. Wedler showed that in the presence of 0.25 mM ammonium chloride, 4 mM l-glutamate, 2 mM ATP, 5 mM ADP, 10 mM Pi, 50 mM Mg2+ and 20 mM glutamine the rate of exchange between ammonia and glutamine (amide) catalyzed by sheep brain GS is 70 % that of the rate of exchange between glutamate and glutamine (i.e. the glutamine synthesis reaction) (Table 1 in ref. 40). This finding suggests that non-productive GS-catalyzed exchange of nitrogen between ammonia and glutamine (amide) has the potential to be substantial, resulting in considerable overestimation of net glutamine synthesis rates in rat brain. However, the concentrations of glutamate and glutamine in normal rat brain are of the order 11 and 5 mM, respectively [e.g. [41]]. Thus, since the K m exhibited by brain GS for glutamate is 2.5 mM [23] and the affinity for glutamine is considerably less than that for glutamate (high concentrations of glutamine are required for maximal activity of glutamine as a substrate, typically 50 mM [42–44]), under normal conditions the non-productive exchange of nitrogen between ammonia and glutamine (amide) is likely to be much less than 70 % relative to the net glutamine synthesis reaction. But what is the situation during hyperammonemia? During chronic hyperammonemia the concentration of brain glutamate may be unchanged [1] or decrease only slightly to moderately under a variety of hyperammonemic conditions (e.g. [41, 45]; see also a review by Vaquero and Butterworth [46]), but the concentration of brain glutamine can increase by 2 to threefold e.g. [41, 45]. Thus, during hyperammonemia the concentration of glutamine in the astrocyte compartment may be considerable. These considerations would suggest that exchange of label between [15N]ammonia and the amide position of astrocytic glutamine may not be inconsequential during hyperammonemia. Nevertheless, the observation that the cerebral GS rate as measured from carbon (13C) flux is similar to that of the rate of label incorporation from [15N]ammonia into the amide position of glutamine [11, 32] argues that under hyperammonemic conditions the exchange of ammonia at the amide position of glutamine is likely small relative to net glutamine synthesis in vivo even under hyperammonemic conditions.
At present it is not possible to estimate the correction needed to obtain the “true” rates of GS in vivo from studies with labeled ammonia, but it is likely that the two above mentioned opposing processes offset each other to some extent and that the overall correction needed is probably small. Nevertheless, investigators interested in modeling glutamine synthesis in brain should be aware of the reversibility of the GS reaction and possible non-productive exchange between ammonia and the amide nitrogen of glutamine.
Studies of Ammonia Uptake and Metabolism in Human Brain
Our findings of rapid trapping of [13N]ammonia in rat brain in the amide position of glutamine, and some earlier PET studies using [13N]ammonia by Lockwood and colleagues [47] have provided the basis, in part, for a recent resurgence of interest in using PET to study [13N]ammonia metabolism and trapping in human brain under normal and pathological (hyperammonemic) conditions [48–52]. For example, Keiding et al. [49] showed that net metabolic flux of ammonia from blood to brain (and presumably trapping of most of the label as intracellular glutamine) in the cortex of patients with liver cirrhosis with accompanying hepatic encephalopathy (HE) was 13.4 μmol/min/L tissue. The corresponding values in patients with liver cirrhosis but without HE and in healthy controls were 7.4 and 2.6 μmol/min/L tissue, respectively [49]. The authors concluded that “increased trapping of ammonia in patients with cirrhosis with acute HE was primarily attributable to increased blood ammonia and to a minor extent to changed ammonia kinetics in the brain” [49]. These findings provide additional evidence that the GS reaction in normal brain is not saturated with ammonia and that cerebral glutamine synthesis is increased during hyperammonemia.
The increased cerebral ammonia uptake in the cirrhotic patients with HE compared to controls reflects the ready diffusion of ammonia (as NH3) across the BBB coupled with the higher concentration of ammonia in the blood of these patients. But how readily is ammonia metabolically trapped once it enters the human brain? In the [13N]ammonia PET studies there is apparently some back diffusion of [13N]ammonia, but the majority of the label derived from blood-borne [13N]ammonia is metabolically trapped in human brain [50, 52]. This finding is consistent with the ammonia concentration in brain and the K m value exhibited by GS toward ammonia. Reported concentrations of normal whole brain ammonia vary considerably, but, as noted above, the concentration of ammonia in rat brain is about 0.18 mM, with even lower levels probably occurring in the astrocyte compartment (reviewed in [53]). As also noted above, the K m value exhibited by highly purified ovine GS toward ammonia is 0.18 mM [23]. Assuming that the human brain enzyme is similar to the ovine enzyme, then the GS reaction is far from saturated with ammonia in normal human brain. Therefore, assuming no major changes in ATP, glutamate and GS in the small compartment, an increased influx of ammonia into the brain will result in increased synthesis of cerebral glutamine. In fact, as discussed above, increased rates of cerebral ammonia trapping (presumably as glutamine) occur in hyperammonemic HE patients [49] and in hyperammonemic rats [11, 16, 33] relative to controls.
Glutamine (or glutamine plus glutamate; Glx) can now be measured in human brain in vivo by means of NMR spectroscopy techniques. As expected from previous studies of autopsied brain material [54] and from the discussion of the kinetic properties of GS discussed above, these techniques have shown greatly increased in vivo levels of brain glutamine (or Glx) in hyperammonemic/liver disease patients [55, 56]. In a recent study, Mardini et al. [57] used NMR to quantify cerebral water content and selected amino acids in cirrhotics following induced hyperammonemia. Increased blood ammonia levels correlated with in vivo increased brain glutamine [57].
Cerebral Metabolism of [13N]ammonia in Rats Administered the GS Inhibitor l-Methionine-S,R-Sulfoximine (MSO)
MSO has been studied extensively for many years as a potent inhibitor of GS [e.g. 58]. We made use of this property to study the cerebral metabolic fate of [13N]ammonia in rats in which brain GS was inhibited. Tracer quantities of [13N]ammonia were administered to MSO-treated rats via a carotid artery cannula. After a 10-min infusion the brains were rapidly frozen and label in various metabolites was determined in a portion of the frozen brain sample (Table 1). The specific activities of GDH and GS in the MSO-treated rat brain were also determined in a separate portion of the frozen brain sample. Treatment with MSO resulted in no significant change in the specific activity of cerebral GDH, whereas the specific activity of GS was reduced by about 86 % (Table 1). The relative specific activity of cerebral l-[13N]glutamate in the MSO-treated rats was now greater than that of l-[amine-13N]glutamine (Table 1). This finding shows that compartmentation of ammonia metabolism was no longer intact and that [13N]ammonia had diffused across the small compartment (astrocyte end feet) to the large compartment (neurons) to be acted upon therein in part by GDH. Nevertheless, the amount of label in l-[amide-13N]glutamine was still considerably greater than that in glutamate (plus glutamine amine).
Table 1 shows that when the GS reaction is inhibited alternative means of removing cerebral ammonia are limited. After 10 min of infusion only about 20 % of label was metabolically trapped in the brains of MSO-treated rats compared to that in the brains of control rats [16]. Thus, despite high inherent activity of the GDH reaction in brain (Table 1), this enzyme was not able to fully compensate for decreased GS activity and the level of cerebral ammonia increased by about 4.5 fold in the MSO-treated rats. Nevertheless, inspection of Table 1 indicates that about five times as much label is incorporated into glutamate in the MSO treated animals as compared to controls. But this label in cerebral glutamate [relative specific activity (1.4 %)] is still small when compared to that in glutamine amide [100 %] in untreated animals. Moreover, it should be noted that, because the GDH reaction is freely reversible incorporation of label into glutamate does not necessarily imply net synthesis.
In other experiments we showed that treatment of rats with MSO leads to a slight decrease in brain glutamate (from 12.9 mM to 10.0 mM) but to a larger decrease of brain glutamine (8.0–3.8 mM) [45]. Consider the specific activity of [13N]glutamate relative to that of [amide-3N]glutamine (i.e. 1.4 %; from Table 1) and the larger pool size of glutamate relative to glutamine (2.6; from ref. 445). From this consideration, the comparative flux of ammonia to glutamate catalyzed by the GDH reaction in the MSO-treated rats is only about 4 % (1.4 × 2.6) relative to the flux into glutamine (amide) in the brains of untreated rats. Nevertheless, as discussed below this route for ammonia assimilation may be important during maintenance of long-term nitrogen homeostasis in the hyperammonemic brain.
Cerebral [13N]ammonia Metabolism in Hyperammonemic Rats
In a separate series of experiments we investigated the short-term (5–20 s) cerebral metabolic fate of [13N]ammonia in hyperammonemic, portacaval shunted (PCS) adult male rats [34]. The results are shown in Table 2. Note that the incorporation of label into glutamine was slowed compared to that in controls. Nevertheless, incorporation of label in glutamine was still much greater than that in glutamate in these hyperammonemic animals. This was also true of rats injected with urease whether or not the animals had been subjected to a portacaval shunt (Table 3). Urease treatment is a procedure for generating acute hyperammonemia in experimental animals without portacaval shunting or inhibition of GS [59]. Although the GS reaction is still the predominant route for the metabolism of blood-borne ammonia in the hyperammonemic animals, ammonia-derived nitrogen incorporation in glutamate must have increased as a result of the larger brain ammonia pool.
Our data suggest that the GS reaction is far more important in metabolizing blood-derived ammonia in rat brain than is the GDH reaction. However, the findings do not provide qualitative data on the rate of the GDH reaction. Therefore, at this point it is worth reviewing qualitative estimates of the GDH reaction in rat brain relative to the GS reaction as obtained by 15N-NMR techniques [29, 31, 33]. Rate values using these techniques for brain GDH coupled with plasma ammonia concentrations (shown in parenthesis) have been reported to be 0.013–0.020 μmol/min/g (0.9–1.0 mM) [29], 0.065 μmol/min/g (0.35 mM) [31] and 0.033 μmol/min/g (0.95 mM) [33] in anaesthetized rats under hyperammonemic conditions. The corresponding values for the rat brain GS reaction were reported to be 0.055, 0.20 and 0.30 μmol/min/g, respectively. The corresponding ratio of the rates of the GS reaction to the GDH reaction in rat brain among the three studies were 2.7–4.1, 3.1 and 9, respectively. The apparent disparities among the various 15N-NMR studies may have been due in part to differences in plasma ammonia concentrations, experimental protocols and modeling. Nevertheless, the general conclusion that the GS reaction is the more important reaction than the GDH reaction in the hyperammonemic rat brain for removing ammonia is consistent with the earlier findings of Berl et al. [1] that relative enrichment of 15N in brains of hyperammonemic cats after administration of [15N]ammonia was in the order: glutamine (amide) > glutamine (amine) > glutamate. However, it should be noted that our 13N data suggest an even greater ratio of ammonia incorporation into glutamine (amide) relative to that in glutamate (Tables 1, 2) than that obtained from the 15N experiments. The higher ratio may be explained in part by the fact that the rats in our experiments were unanesthetized, blood ammonia levels were lower and infusion/sampling times were different. Nevertheless, a firm conclusion among our studies (Tables 1, 2), previous studies of Berl et al. [1] and later 15N-NMR studies [22, 29, 31] is that the GS reaction is a much more effective means of removing ammonia from rat brain than is the GDH reaction.
A considerable portion of the glutamate released from neurons during neurotransmission is taken up by astrocytes to be converted therein to glutamine. This flow of glutamate from neurons to astrocytes accounts for the large dilution of labeled 15N at the amine position of cerebral glutamine during infusion of [15N]ammonia noted in the 15N-NMR studies discussed above [19, 31, 33]. Glutamine is released from the astrocytes to be taken up the neurons and converted to glutamate by means of the glutaminase reaction (Eq. 4). Although probably not stoichiometric the concept of a glutamine cycle in brain is now generally accepted. See, for example, recent reviews by Albrecht et al. [60] and Hertz [61].
Major Sources of Endogenous Ammonia in the Brain
Cooper and Plum [53] have listed at least fourteen enzyme-catalyzed reactions that are capable of generating ammonia in the brain. Most of these reactions are probably quantitatively minor, but serve as a reminder of the central importance of ammonia in cerebral nitrogen metabolism. Here, I discuss two well established major routes and one likely major route for the production of ammonia in brain. Glutaminase is usually considered to be a major source of cerebral ammonia (Eq. 4). Both astrocytes and neurons contain glutaminase, but the enzyme specific activity is greater in neurons [e.g. 62]. As noted above, the enzyme is important in converting neuronal glutamine to glutamate as part of the glutamine cycle in brain.
The equilibrium position of the GDH reaction (Eq. 2) at physiological pH values favors reductive amination of α-ketoglutarate. As a consequence, and as also noted above, label derived from either [13N]ammonia or [15N]ammonia that was taken up from the blood into rat brain was incorporated in part into glutamate. However, label in glutamate was much lower than that in glutamine (amide). The very high K m value for ammonia (≥10 mM) exhibited by brain GDH [63] and much lower K m value exhibited by brain GS (0.15 mM; [23]) coupled with the very low ammonia concentration in astrocytes (<0.18 mM) [16, 53] explains in part the much less effective trapping of ammonia in glutamate as compared to trapping in glutamine (amide). Despite the fact that the equilibrium position of the GDH reaction in vitro favors glutamate formation at neutral pH strong evidence suggests that under normal circumstances the GDH reaction in the brain is drawn in the direction of glutamate oxidation by removal of ammonia as glutamine [e.g. 61, 64–66].
The purine nucleotide cycle (PNC) is likely a third major route for cerebral ammonia production. This source of ammonia is not generally considered in discussions of nitrogen metabolism in the brain, so it is important to consider the cycle here in the context of the theme of this review. This cycle was first described in detail by Lowenstein in 1972 [67]. The cycle consists of three enzyme reactions: adenylosuccinate synthetase (Eq. 5), adenylosuccinate lyase (adenylosuccinase; Eq. 6) and AMP deaminase (Eq. 7). The net reaction (Eq. 8) is the energetically favorable conversion of l-aspartate to fumarate and ammonia at the expense of GTP hydrolysis.
Lowenstein and co-workers have provided evidence that the cycle is an important source of ammonia in muscle and brain, an alternative source in kidney and of low importance in the liver [67–69]. The cycle has been suggested to act in concert with adenylate kinase (myokinase) (Eq. 9) to ensure high ratios of ATP to ADP [67, 70]. This coupling protects cells against abrupt changes in the adenylate energy charge [67, 70]. Such a safeguard may be important in muscle during bursts of exercise and in tissues such as the brain that maintain a high, sustained energy use.
Evidence for the importance of the cycle in brain is as follows: Schultz and Lowenstein [68] showed that the activity of adenylate deaminase (Eq. 7) in cell-free rat brain extracts is sufficient to account for maximal rates of ammonia production previously reported in the literature. They further showed that the activities of the other two enzymes of the cycle, namely adenylosuccinate synthetase (Eq. 5) and adenylosuccinate lyase (Eq. 6) are almost sufficient in combination with adenylate deaminase to account for this maximal ammonia production rate. Schultz and Lowenstein stated that according to the literature a) deamidation of glutamine (the glutaminase reaction; Eq. 4) can only account for ~5 % of ammonia formation noted in brain slices and b) administration of MSO to mice results in an increase in ammonia before any fall in glutamine can be observed [references quoted in 68]. In a later extension of this work, the authors showed that within 10 s after administration of electroshock treatment to rats the cerebral [ATP]/[ADP] ratio fell to a third of the pre-shock value accompanied by a three-fold increase in ammonia [69]. The total adenine nucleotide plus adenosine content of brain decreased and an equivalent amount of hypoxanthine-containing compounds appeared. Adenosine, inosine, and hypoxanthine accumulated, and there was a transitory accumulation of adenylosuccinate and IMP. The contents of ATP and creatine phosphate, and the [ATP]/[ADP] ratio, were rapidly restored to control values [69]. Schultz and Lowenstein [69] assumed that the GDH reaction is in equilibrium in rat brain. From this assumption and a calculation of the mitochondrial NADH/NAD+ ratio the authors concluded that the GDH reaction is not sufficient to account for the maximal rate of ammonia formation after electroshock treatment and may even have been a net source of ammonia removal under these conditions [69].
In summary, according to Schultz and Lowenstein [68, 69] the glutaminase and GDH reactions cannot account for maximal rates of ammonia production in brain, whereas the maximal rate of the PNC can approach maximal rates of ammonia production. However, Schultz and Lowenstein may have overstated their case. The glutaminase and GDH reactions must account for a considerable portion of endogenous cerebral ammonia production. Thus, the demonstration by NMR techniques of a functioning glutamine cycle in brain predicates an approximate stoichiometry between glutamine synthesis and glutaminase reactions. Moreover, as I have emphasized throughout this review, continuous removal of ammonia by the GS reaction will ensure a prominent role for the GDH reaction in converting glutamate to ammonia. Nevertheless, Schultz and Lowenstein make a strong case for the PNC as an important ancillary source of cerebral ammonia. If the authors are correct then the cycle is convolved with glutaminase and the GDH reaction in the production of endogenous ammonia in the brain. Thus, it is curious that the findings of these authors have been largely ignored since the original publications more than 35 years ago.
One of the very few studies published since the mid 1970s related to the PNC in brain is that of Hamprecht and colleagues [71]. AMP deaminase mRNA was detected prominently in ventricular ependymal cells, choroid plexus and some neuronal populations. Immunohistochemical staining of rat brain sections revealed a similar distribution of the protein. Staining was strikingly intense in ependymal cells and their associated cilia. No staining could be detected in astrocytes. AMP deaminase was suggested to be important for maintaining ATP for the ependyma cilia beat [71]. It will be important to follow up this study to localize and characterize the other two enzymes of the cycle in brain (i.e. adenylosuccinate synthase and adenylosuccinate lyase).
Possible metabolic connections of the PNC with aminotransferases and GDH are discussed below.
The Role of GDH in Assimilating and Dissimilating Ammonia: Transreamination and Transdeamination
I recently reviewed this subject [11], but a further discussion is provided here. The terms transreamination and transdeamination were introduced in 1957 by Braunstein [72]. These terms are not often used, but I use them here because they exemplify informative concepts. For an appreciation of Braunstein, a historical account of the discovery of transamination, and the biological roles of transamination see Cooper and Meister [73]. Braunstein pointed out that coupling of the GDH reaction (Eq. 2) to an α-ketoglutarate/l-glutamate-linked transaminase (aminotransferase) (Eq. 10) can be used to incorporate ammonia nitrogen into an amino acid (transreamination) or to remove it (transdeamination). The net reaction is shown in Eq. 11. Transreamination is the forward reaction of Eq. 11 and transdeamination is the reverse direction.
Recent work has shown that GDH is tightly associated with an aminotransferase in brain. Thus, Hutson and co-workers have shown that the mitochondrial enzyme GDH 1 (but not GDH 2) is part of a supranuclear complex (metabolon) that also includes the mitochondrial form of the branched chain amino acid (BCAA) aminotransferase [74]. Most mammals possess only the GDH 1 isoenzyme. However, some human cell types, including astrocytes, possess an additional isoenzyme–GDH 2 [75]. Most of the discussion in the present review pertains to GDH 1. According to Hutson et al. [74] the close juxtapositioning of the mitochondrial BCAA aminotransferase to GDH facilitates the channeling of nitrogen from the BCAAs toward ammonia—i.e. transdeamination, the reverse direction of Eq. 11.
In a recent review I emphasized that even under hyperammonemic conditions the GDH reaction cannot compete quantitatively over the short term with the GS reaction as a means of ammonia removal in rat brain [14], and I continue to emphasize that fact here. Recently, however, it was suggested that synthesis of alanine, presumably via a transreamination mechanism involving GDH, may be important in addition to the GS reaction for the removal of cerebral ammonia during hyperammonemia [10, 76] For example, Dadsetan et al. [10] reported that inhibition of GS with MSO in co-cultures of astrocytes and neurons resulted in increased channeling of ammonia toward alanine via coupling of alanine aminotransferase to the GDH reaction. In this case the direction of Eq. 11 is toward transreamination (Eq. 12).
In other work from the same group, Leke et al. [76] investigated metabolic processes in co-cultures of neurons and astrocytes (model of the GABAergic system) exposed to 5 mM ammonium chloride. The authors reported ammonia-stimulated increase of the TCA cycle and increased synthesis and release of alanine [76]. But, how important is alanine production for removal of ammonia in the normal and hyperammonemic brain in vivo? As indicated above, the relative short-term flux of label derived from [13N]ammonia into brain glutamate, although considerably greater in MSO-treated rats than in untreated rats, is still relatively low. Moreover, in the HPLC system shown in Fig. 1 alanine elutes at about 7 min (immediately after glutamine). In the experiment shown in Fig. 1 labeled alanine cannot be detected in the rat brain after continuous intracarotid infusion of [13N]ammonia for 20 min. Finally, the specific activity of alanine aminotransferase in rat brain (even when measured under optimal conditions) is relatively low. For example, Benuck et al. [77] reported a specific activity for transamination of l-alanine with α-ketoglutarate under optimal conditions of ~76 μmol/h/100 mg protein in adult rat brain. In contrast, the authors found maximal transamination rates with aspartate and glutamate of ~2,700 and 15,000 μmol/h/100 mg protein. Since the alanine aminotransferase reaction is freely reversible the optimal activity of alanine aminotransferase with pyruvate as substrate is not likely to be much different from that with alanine i.e. the inherent alanine aminotransferase reaction in rat brain is likely to be at least two orders of magnitude slower than that of the aspartate aminotransferase (AAT) reaction.
Measurable uptake of alanine across the rat BBB cannot be detected [78], yet l-alanine is present in rodent brain at a concentration of ~1 mM [79]. Thus, l-alanine in the rat brain is most likely synthesized de novo via transamination reactions. However, our inability to detect label in cerebral alanine after intracarotid infusion of [13N]ammonia suggests that the nitrogen for astrocytic l-alanine is not obtained from blood-derived ammonia via a transreamination pathway. But what is the fate of the ammonia generated from metabolic reactions in the large compartment (neurons) that does not readily mix with [13N]ammonia derived from the blood? Because ammonia diffuses very rapidly it is likely that the bulk of the ammonia generated in the neurons is metabolized very rapidly to glutamine in the astrocytes. However, because the neurons contain considerable GDH activity and the reaction is reversible some ammonia is likely to be incorporated into glutamate in the neuronal compartment, especially under hyperammonemic conditions [80]. Thus, as suggested by Dadsetan et al. [10] alanine could accumulate over time as a defense against persistent hyperammonemia. This is because nitrogen shuttles rapidly between glutamine and glutamate through the glutamine cycle whereas, although at a much slower rate, alanine can build up over time. In this context, it is interesting to note that in vivo cerebral microdialysis in acute liver failure (ALF) patients with persistent hyperammonemia revealed, as expected, marked increases in glutamine [81, 82], but alanine was also markedly elevated [82].
Coupling of AAT to the GDH Reaction
The AAT-catalyzed reaction is shown in Eq. 13. Most tissues contain very high levels of mitochondrial aspartate aminotransferase (mAAT) and cytosolic aspartate aminotransferase (cAAT) isozymes [83]. These isozymes, along with mitochondrial malate dehydrogenase, cytosolic malate dehydrogenase, a mitochondrial aspartate/glutamate antiporter and a malate/α-ketoglutarate antiporter, are important components of the malate-aspartate shuttle MAS). Because mitochondria are impervious to NADH generated in the cytosol during glycolysis, a shuttle such as the MAS is needed to transport reducing equivalents into the mitochondrion in lieu of NADH. Work from our laboratory, including a joint collaboration with Hertz and colleagues [84–86] has suggested an important role for the MAS in the brain (for reviews see for example [87, 88]).
In addition, mAAT and cAAT are crucial for the maintenance of nitrogen balance. As a result of the high activity of both isozymes of AAT the components of the AAT reaction are in thermodynamic equilibrium in liver [89, 90] and brain [91]. The very high activity of these enzymes is evident from our studies of the metabolism of [13N]ammonia in rat liver. After portal vein injection of a bolus of [13N]ammonia in anesthetized rats label rapidly appeared in hepatic glutamate [92]. Impressively, once label appeared in glutamate, aspartate was almost immediately labeled. However, a few minutes were required for label to totally equilibrate between glutamate and aspartate pools. This time to reach equilibrium results from the fact that label that was initially rapidly equilibrated between glutamate and aspartate in the liver mitochondria required several minutes to re-equilibrate between mitochondrial and cytosolic pools of glutamate and aspartate [92]. Our data suggest that nitrogen is extremely rapidly shared between glutamate and aspartate in both the cytosolic and mitochondrial pools in the rat liver (seconds or less).
Even though the amount of label present in cerebral glutamate after carotid artery administration of [13N]ammonia to rats was very small relative to that in glutamine (amide) we consistently noted that once label appeared in glutamate, label could also be detected in aspartate (Fig. 1). However, because the relative amount of label in glutamate was relatively small compared to that in glutamine (Fig. 1; Tables 1, 2, 3) it was difficult to estimate the rate of exchange of label between glutamate and aspartate in rat brain. Nevertheless, since the specific activities of mAAT and cAAT in rat brain are similar, or even higher, to those in rat liver [83] it is likely that equilibration of nitrogen between glutamate and aspartate, as in the rat liver, must be extremely rapid in rat brain–also seconds or lessFootnote 2.
Coupling of the GDH reaction (Eq. 2) to the AAT (Eq. 13) represents a special case of transreamination/transdeamination in which ammonia can be incorporated into aspartate or aspartate can act as a source of ammonia (Eq. 14). Since aspartate is also a component of the net reaction of the PNC (Eq. 8), it is important to emphasize at this point that both aspartate and glutamate are key components contributing to the maintenance of ammonia and nitrogen homeostasis in the brain.
The α-keto acid substrates of AAT, namely α-ketoglutarate and oxaloacetate, are components of the TCA cycle, and mAAT is of high activity in brain mitochondria [83]. It is therefore possible to combine the section of the TCA cycle that directs carbon from α-ketoglutarate to oxaloacetate (formally depicted in Eq. 15) with the AAT-catalyzed reaction (Eq. 13) such that glutamate is readily oxidized to aspartate (Eq. 16). This conversion of glutamate to aspartate was first recognized more than 60 years ago [93] and under certain circumstances may be almost stoichiometric.
By coupling the AAT reaction to other enzymes of the TCA cycle it is also possible to utilize aspartate as a source of glutamate (Eq. 17). Other pathways from aspartate to glutamate that employ in part TCA cycle enzymes (and GDH) are possible (e.g. Eq. 18). For details of the enzyme-catalyzed steps involved in the overall transformations of aspartate to glutamate shown in Eqs. 13–18 see refs. [94, 95]. The important point here is that carbon metabolism through the TCA cycle is intimately connected to amino acid nitrogen metabolism via the mAAT reaction.
The respective quantitative roles of AAT and GDH in cerebral glutamate metabolism are occasionally debated (see, for example, the discussion in Ref. [96]). In my opinion the arguments are moot. The AAT isozymes are so active that their metabolic rate coefficients are virtually zero and the enzymes exert little or no directional metabolic control. Thus, these isozymes act as metabolic conduits passively directing the flow of nitrogen toward or away from glutamate/α-ketoglutarate or aspartate/oxaloacetate as dictated by ancillary enzymes such as GDH and the needs of the cells.
Pardo et al. [97] recently suggested that brain glutamine synthesis requires neuronal-derived aspartate. However, in a recent commentary Hertz [98] has pointed out that because aspartate is an excitatory neurotransmitter it is unsuitable as an agent for group transfer from neurons to astrocytes. Hertz has suggested an alternative model in which aspartate is crucial for glutamate/glutamine formation in astrocytes that circumvents the need for aspartate transport from neurons to astrocytes [98]. The points I discuss above emphasize connections between glutamate and aspartate carbon and nitrogen that can occur in the same cellular compartment.
Coupling of Aminotransferase Reactions and TCA Cycle to the PNC
Lowenstein and Goodman [99] have pointed out that coupling of the PNC (Eq. 8) to an α-ketoglutarate/l-glutamate-linked aminotransferase (Eq. 10), AAT (Eq. 13), fumarase (Eq. 19) and malate dehydrogenase (Eq. 20) results in a more favorable deamination of an amino acid (Eq. 22) as a result of the hydrolysis of GTP than the transdeamination reaction shown in the back reaction of Eq. 11.
Note that if Eq. 10 is omitted the net reaction is the oxidative deamination of glutamate coupled to the hydrolysis of GTP (Eq. 22). In this pathway the oxidative deamination of glutamate is energetically more favorable than is the GDH reaction (Eq. 2, reverse reaction) as a result of the hydrolysis of GTP [99]. These equations again serve to emphasize the interconnection of TCA cycle/energy metabolism with nitrogen metabolism.
Integration of Major Pathways of Cerebral Nitrogen Metabolism
An integration of the various metabolic pathways discussed in this review is shown in Fig. 3. The figure emphasizes the central important of ammonia in the maintenance of nitrogen homeostasis. For simplification, cellular and subcellular compartments, transporters and non-nitrogen co-substrates of key enzymes are omitted. Ammonia (shown as NH3) arising through metabolic reactions (three of which are depicted here) or entering the brain by diffusion from the blood or CSF is rapidly incorporated into the amide position of glutamine by the action of astrocytic GS (1). At the same time, glutamine is rapidly transported to the neurons and converted therein to glutamate in a reaction catalyzed by glutaminase as part of the glutamine cycle (2). Some glutamine may also be lost to the circulation and CSF as a means of maintaining nitrogen balance resulting from net cerebral uptake of ammonia and amino acids (not shown here, but discussed in Ref. [53]). Because the reaction is reversible, the GDH reaction (3) may be either a source or sink for ammonia. However, under normal conditions cerebral GDH catalyzes net ammonia production as a result of rapid removal of ammonia catalyzed by GS [glutamate → ammonia → glutamine (amide)]. Since the AAT reaction (4) is in rapid thermodynamic equilibrium aspartate nitrogen may be directed toward ammonia production via the GDH reaction coupled to GS [aspartate → glutamate → ammonia → glutamine (amide)]. Other amino acids, such as alanine and the BCAAs may also be a source of ammonia via the transdeamination pathway coupled to GS (5) [amino acid (amine) → glutamate → ammonia → glutamine (amide)]. Finally, conversion of aspartate nitrogen to ammonia by the PNC (6) is energetically favorable. Thus, association of α-ketoglutarate/glutamate-linked aminotransferases to AAT and the PNC represents an alternative to the transdeamination pathway for conversion of amino acid amine nitrogen to ammonia and hence to glutamine (amide). The sequence of nitrogen transfers is as follows: amino acid (e.g. alanine, BCAAs) → glutamate → aspartate → ammonia → glutamine.
As noted above, work by Schultz and Lowenstein [68, 69] has definitely established the occurrence of the PNC in brain. Although these workers provided strong evidence that this pathway is an important source of ammonia in brain the work has been largely ignored over the 35 years since first published. However, there is now strong evidence that the pathway may be important in neurons and ependymal cells, perhaps as a safety mechanism for maintaining ATP and adenylate energy charge [69, 70].
Under normal conditions, cycling of nitrogen among ammonia, glutamate and glutamine (amide) reaches a quasi-steady state such that whole brain values of ammonia, glutamate and glutamine are about 0.18, 10–12 and 3–5 mM, respectively. The concentrations of cerebral aspartate and alanine are about 2 and ~1 mM, respectively (see for example Ref. [100]). However, when the steady state is perturbed during acute of chronic hyperammonemia, the flow of nitrogen among the major metabolites shown in Fig. 3 may be considerably altered. For example, brain glutamine and ammonia may rise substantially, whereas glutamate levels are less affected. From tracer studies the GS reaction is still the favored route for short-term removal of ammonia under hyperammonemic conditions. However, the glutaminase reaction and the glutamine cycle are still quite active, so that ammonia nitrogen can eventually flow to glutamate and to alanine even though kinetically the alanine aminotransferase reaction is probably much slower than the GS reaction in vivo. Eventually a new steady state is reached during hyperammonemia in which some waste nitrogen is redirected toward alanine via the transreamination reaction involving alanine aminotransferase and GDH [10, 76].
Conclusions
Our previous work, beginning more than 30 years ago with [13N]ammonia showing that the major route for ammonia metabolism in normal and hyperammonemic rat brain is via the GS reaction, has stood the test of time. NMR techniques have now shown that this route for cerebral ammonia metabolism also predominates in normal and hyperammonemic human brain. The knowledge that [13N]ammonia is metabolically trapped in human brain (presumably as l-[amide-13N]glutamine) serves as a basis for new techniques of PET modeling of [13N]ammonia uptake and metabolism in normal and hyperammonemic human brain. Our original data showed that the GDH reaction is not quantitatively a major route for short-term removal of cerebral ammonia are consistent with the hypothesis that the cerebral GDH reaction normally favors oxidative deamination of glutamate. However, under conditions of prolonged hyperammonemia, it is possible that the GDH reaction contributes to a long-term increase in brain alanine as an alternative mechanism to the GS reaction as a means of removing excess nitrogen. Finally, this review emphasizes the importance of aminotransferases linked to the GDH reaction for the homeostasis of brain ammonia and the most likely important role of the PNC as a source of ammonia. The role of the PNC in brain nitrogen metabolism is a fruitful area for future research.
Notes
Ammonia free base (NH3) has a pK a of ~9.2. Thus, under normal intracellular physiological conditions (pH 7.2–7.4) ammonia exists predominantly (~99 %) as the conjugate acid, ammonium (NH4 +). For convenience, unless otherwise stated, the term ammonia is used throughout the text to indicate the sum of NH3 plus NH4 +.
This finding appears to have been the basis for the model developed by the Shulman laboratory for the NMR measurement of TCA flux using [13C]glucose cf. [11, 31, 32]. Due to the very rapid exchange of nitrogen in the AAT reaction (considerably faster than carbon flux through the TCA cycle) label in glutamate is a good proxy for that in the much smaller pool of α-ketoglutarate.
Abbreviations
- AAT:
-
Aspartate aminotransferase
- AMP:
-
Adenosine monophosphate
- BBB:
-
Blood–brain barrier
- BCA:
-
Branched-chain amino acid
- BUI:
-
Brain uptake index
- cAAT:
-
Cytosolic aspartate aminotransferase
- CSF:
-
Cerebrospinal fluid
- GDH:
-
Glutamate dehydrogenase
- GS:
-
Glutamine synthetase
- HE:
-
Hepatic encephalopathy
- HPLC:
-
High performance liquid chromatography
- IMP:
-
Inosine monophosphate
- mAAAT:
-
Mitochondrial aspartate aminotransferase
- MAS:
-
Malate-aspartate shuttle
- MSKCC:
-
Memorial Sloan Kettering Cancer center
- NMR:
-
Nuclear magnetic resonance
- MSO:
-
l-Methionine-S,R-sulfoximine
- PCS:
-
Portacaval shunt
- PET:
-
Positron emission tomography
- TCA:
-
Tricarboxylic acid
References
Berl S, Takagaki G, Clarke DD, Waelsch H (1962) Metabolic compartments in vivo. Ammonia and glutamic acid metabolism in brain and liver. J Biol Chem 237:2562–2569
Gebhardt R, Ebert A, Bauer G (1988) Heterogeneous expression of glutamine synthetase mRNA in rat liver parenchyma revealed by in situ hybridization and Northern blot analysis of RNA from periportal and perivenous hepatocytes. FEBS Lett 241:89–93
Brosnan ME, Brosnan JT (2009) Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am J Clin Nutr 90:857S–861S
Berl S, Takagaki G, Clarke DD, Waelsch H (1962) Carbon dioxide fixation in the brain. J Biol Chem 237:2570–2573
Lapidot A, Gopher A (1994) Cerebral metabolic compartmentation. Estimation of glucose flux via pyruvate carboxylase/pyruvate dehydrogenase by 13C NMR isotopomer analysis of D-[U-13C]glucose metabolites. J Biol Chem 269:27198–27208
Lapidot A, Gopher A (1997) Quantitation of metabolic compartmentation in hyperammonemic brain by natural abundance 13C-NMR detection of 13C-15N coupling patterns and isotopic shifts. Eur J Biochem 243:597–604
Zwingmann C, Brand A, Richter-Landsberg C, Leibfritz D (1998) Multinuclear NMR spectroscopy studies on NH4Cl-induced metabolic alterations and detoxification processes in primary astrocytes and glioma cells. Dev Neurosci 20:417–426
Kanamatsu T, Tsukada Y (1999) Effects of ammonia on the anaplerotic pathway and amino acid metabolism in the brain: an ex vivo 13C NMR spectroscopic study of rats after administering [2-13C] glucose with or without ammonium acetate. Brain Res 84:11–19
Zwingmann C (2007) The anaplerotic flux and ammonia detoxification in hepatic encephalopathy. Metab Brain Dis 22:235–249
Dadsetan S, Bak LK, Sørensen M, Keiding S, Vilstrup H, Ott P, Leke R, Schousboe A, Waagepetersen HS (2011) Inhibition of glutamine synthesis induces glutamate dehydrogenase-dependent ammonia fixation into alanine in co-cultures of astrocytes and neurons. Neurochem Int 59:482–488
Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, Behar KL, Rothman DL, Shulman RG (2001) In vivo 13C NMR measurement of neurotransmitter glutamate cycling, anaplerosis and TCA cycle flux in rat brain during [2-13C]glucose infusion. J Neurochem 76:975–989
Duarte JMN, Lanz B, Gruetter R (2011) Compartmentalised cerebral metabolism of [1,6-13C]glucose determined by in vivo 13C NMR spectroscopy at 14.1 T. Front Neuroenergetics 3:3
Hassel B (2000) Carboxylation and anaplerosis in neurons and glia. Mol Neurobiol 22:21–40
Cooper AJL (2011) 13N as a tracer for studying glutamate metabolism. Neurochem Int 59:456–464
Cooper AJL, Gelbard AS, Freed BR (1985) Nitrogen-13 as a biochemical tracer. Adv Enzymol Relat Areas Mol Biol 57:251–356
Cooper AJL, McDonald JM, Gelbard AS, Gledhill RF, Duffy TE (1979) The metabolic fate of 13N-labeled ammonia in rat brain. J Biol Chem 254:4982–4992
Lockwood AH, Finn RD, Campbell JA, Richman TB (1980) Factors that affect the uptake of ammonia by the brain: the blood-brain pH gradient. Brain Res 181:259–266
Raichle ME, Larson KB (1981) The significance of the NH3-NH4 + equilibrium on the passage of 13N-ammonia from blood to brain. A new regional residue detection model. Circ Res 48:913–937
Carter CC, Lifton JF, Welch MJ (1973) Organ uptake and blood pH and concentration effects of ammonia in dogs determined with ammonia labeled with 10 minute half-lived nitrogen 13. Neurology 23:204–213
Cooper AJL, Freed BR (2005) Metabolism of [13N]ammonia in rat lung. Neurochem Int 47:103–118
Cooper AJL, Lai JCK, Gelbard AS (1988) Ammonia metabolism in normal and hyperammonemic rat brain. In: Norenberg MD, Hertz L, Schousboe A (eds) The Biochemical pathology of astrocytes. Alan R Liss, Inc, New York, pp 419–434
Glutamine WuC, Synthetase I (1963) A comparative study of its distribution in animals and its inhibition by DL-allo-δ-hydroxylysine. Comp Biochem Physiol 34:335–3351
Pamiljans V, Krishnaswamy PR, Dumville G, Meister A (1962) Studies on the mechanism of glutamine synthesis; isolation and properties of the enzyme from sheep brain. Biochemistry 1:153–158
Martinez-Hernandez A, Bell KP, Norenberg MD (1977) Glutamine synthetase: glial localization in brain. Science 195:1356–1358
Norenberg MD, Martinez-Hernandez A (1979) Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res 161:303–310
Hertz L, Schousboe A (1988) Metabolism of glutamate and glutamine in neurons and astrocytes in primary cultures. In: Kvamme E (ed) Glutamine and glutamate in mammals, vol II. CRC Press Inc, Boca Raton, pp 39–55
Kanamori K, Parivar F, Ross BD (1993) A 15N NMR study of in vivo cerebral glutamine synthesis in hyperammonemic rats. NMR Biomed 6:21–26
Kanamori K, Ross BD (1993) 15N n.m.r. measurement of the in vivo rate of glutamine synthesis and utilization at steady state in the brain of the hyperammonaemic rat. Biochem J 293:461–468
Kanamori K, Ross BD (1995) Steady-state in vivo glutamate dehydrogenase activity in rat brain measured by 15N NMR. J Biol Chem 270:24805–24809
Kanamori K, Ross BD, Chung JC, Kuo EL (1996) Severity of hyperammonemic encephalopathy correlates with brain ammonia level and saturation of glutamine synthetase in vivo. J Neurochem 67:1584–1594
Shen J, Sibson NR, Cline G, Behar KL, Rothman DL, Shulman RG (1998) 15N-NMR spectroscopy studies of ammonia transport and glutamine synthesis in the hyperammonemic rat brain. Dev Neurosci 20:434–443
Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, Shulman RG (1997) In vivo 13C NMR measurements of cerebral glutamine synthesis as evidence for glutamate-glutamine cycling. Proc Natl Acad Sci USA 94:2699–2704
Cudalbu C, Lanz B, Duarte JM, Morgenthaler FD, Pilloud Y, Mlynárik V, Gruetter R (2012) Cerebral glutamine metabolism under hyperammonemia determined in vivo by localized 1H and 15N NMR spectroscopy. J Cereb Blood Flow Metab 32:696–708
Cooper AJL, Mora SN, Cruz NF, Gelbard AS (1985) Cerebral ammonia metabolism in hyperammonemic rats. J Neurochem 44:1716–1723
Desjardins P, Rao KV, Michalak A, Rose C, Butterworth RF (1999) Effect of portacaval anastomosis on glutamine synthetase protein and gene expression in brain, liver and skeletal muscle. Metab Brain Dis 14:273–280
Chatauret N, Desjardins P, Zwingmann C, Rose C, Rao KV, Butterworth RF (2006) Direct molecular and spectroscopic evidence for increased ammonia removal capacity of skeletal muscle in acute liver failure. J Hepatol 44:1083–1088
Desjardins P, Du T, Jiang W, Peng L, Butterworth RF (2012) Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure: role of glutamine redefined. Neurochem Int. 2012 Feb 21. [Epub ahead of print]
Bosman DK, Deutz NE, De Graaf AA, vd Hulst RW, Van Eijk HM, Bovée WM, Maas MA, Jörning GG, Chamuleau RA (1990) Changes in brain metabolism during hyperammonemia and acute liver failure: results of a comparative 1H-NMR spectroscopy and biochemical investigation. Hepatology 12:281–290
Levintow L, Meister A (1954) Reversibility of the enzymatic synthesis of glutamine. J Biol Chem 209:265–280
Wedler FC (1974) Mechanisms of substrate binding with glutamine synthetase. Equilibrium isotope exchanges with the ovine brain, pea seed, and Escherichia coli enzymes. J Biol Chem 249:5080–5087
Hindfelt B, Plum F, Duffy TE (1977) Effect of acute ammonia intoxication on cerebral metabolism in rats with portacaval shunts. J Clin Invest 59:386–396
Meister A (1985) Glutamine synthetase from mammalian tissues. Methods Enzymol 113:185–199
Minet R, Villie F, Marcollet M, Meynial-Denis D, Cynober L (1997) Measurement of glutamine synthetase activity in rat muscle by a colorimetric assay. Clin Chim Acta 268:121–132
Santoro JC, Harris G, Sitlani A (2001) Colorimetric detection of glutamine synthetase-catalyzed transferase activity in glucocorticoid-treated skeletal muscle cells. Anal Biochem 289:18–25
Cooper AJL, Vergara F, Duffy TE (1983) Cerebral glutamine synthetase. In: Hertz L, Kvamme E, McGeer E, Schousboe A (eds) Glutamine, glutamate and GABA in the central nervous system. Alan R Liss Inc, New York, pp 77–93
Vaquero J, Butterworth RF (2006) The brain glutamate system in liver failure. J Neurochem 98:661–669
Lockwood AH, Yap EW, Wong WH (1991) Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J Cereb Blood Flow Metab 11:337–341
Ahl B, Weissenborn K, van den Hoff J, Fischer-Wasels D, Köstler H, Hecker H, Burchert W (2004) Regional differences in cerebral blood flow and cerebral ammonia metabolism in patients with cirrhosis. Hepatology 40:73–79
Keiding S, Sørensen M, Bender D, Munk OL, Ott P, Vilstrup H (2006) Brain metabolism of 13N-ammonia during acute hepatic encephalopathy in cirrhosis measured by positron emission tomography. Hepatology 43:42–50
Sørensen M, Keiding S (2007) New findings on cerebral ammonia uptake in HE using functional 13N-ammonia PET. Metab Brain Dis 22:277–284
Sørensen M, Munk OL, Keiding S (2009) Backflux of ammonia from brain to blood in human subjects with and without hepatic encephalopathy. Metab Brain Dis 24:237–242
Keiding S, Sørensen M, Munk OL, Bender D (2010) Human 13N-ammonia PET studies: the importance of measuring 13N-ammonia metabolites in blood. Metab Brain Dis 25:49–56
Cooper AJL, Plum F (1987) Biochemistry and physiology of brain ammonia. Physiol Rev 67:440–519
Lavoie J, Giguère JF, Layrargues GP, Butterworth RF (1987) Amino acid changes in autopsied brain tissue from cirrhotic patients with hepatic encephalopathy. J Neurochem 49:692–697
Córdoba J, Sanpedro F, Alonso J, Rovira A (2002) 1H magnetic resonance in the study of hepatic encephalopathy in humans. Metab Brain Dis 17:415–429
Rovira A, Alonso J, Córdoba J (2008) MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 29:1612–1621
Mardini H, Smith FE, Record CO, Blamire AM (2011) Magnetic resonance quantification of water and metabolites in the brain of cirrhotics following induced hyperammonaemia. J Hepatol 54:1154–1160
Manning JM, Moore S, Rowe WB, Meister A (1969) Identification of l-methionine S-sulfoximine as the diastereoisomer of l-methionine SR-sulfoximine that inhibits glutamine synthetase. Biochemistry 8:2681–2685
Gibson GE, Zimber A, Krook L, Richardson EP, Visek WJ (1974) Brain histology and behavior of mice injected with urease. J Neuropathol Exp Neurol 33:201–211
Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A (2007) Glutamine in the central nervous system: function and dysfunction. Front Biosci 12:332–343
Hertz L (2011) Astrocytic energy metabolism and glutamate formation–relevance for 13C-NMR spectroscopy and importance of cytosolic/mitochondrial trafficking. Magn Reson Imaging 29:1319–1329
Hogstad S, Svenneby G, Torgner IA, Kvamme E, Hertz L, Schousboe A (1988) Glutaminase in neurons and astrocytes cultured from mouse brain: kinetic properties and effects of phosphate, glutamate, and ammonia. Neurochem Res 13:383–388
Chee PY, Dahl JL, Fahien LA (1979) The purification and properties of rat brain glutamate dehydrogenase. J Neurochem 33:53–60
Yu ACH, Schousboe A, Hertz L (1982) Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J Neurochem 39:954–960
McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR (1996) Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem 66:386–393
Hertz L, Hertz E (2003) Cataplerotic TCA cycle flux determined as glutamate-sustained oxygen consumption in primary cultures of astrocytes. Neurochem Int 43:355–361
Lowenstein JM (1972) Ammonia production in muscle and other tissues: the purine nucleotide cycle. Physiol Rev 52:382–414
Schultz V, Lowenstein JM (1976) Purine nucleotide cycle. Evidence for the occurrence of the cycle in brain. J Biol Chem 251:485–492
Schultz V, Lowenstein JM (1978) The purine nucleotide cycle. Studies of ammonia production and interconversions of adenine and hypoxanthine nucleotides and nucleosides by rat brain in situ. J Biol Chem 253:1938–1943
Chapman AG, Miller AL, Atkinson DE (1976) Role of the adenylate deaminase reaction in regulation of adenine nucleotide metabolism in Ehrlich ascites tumor cells. Cancer Res 36:1144–1150
Knecht K, Wiesmüller KH, Gnau V, Jung G, Meyermann R, Todd KG, Hamprecht B (2001) AMP deaminase in rat brain: localization in neurons and ependymal cells. J Neurosci Res 66:941–950
Braunstein AE (1957) Principal ways of assimilation & dissimilation of nitrogen in animals. Adv Enzymol Relat Sub Biochem 19:335–389 (French)
Cooper AJL, Meister A (1989) An appreciation of Professor Alexander E. Braunstein. The discovery and scope of enzymatic transamination. Biochimie 71:387–404
Hutson SM, Islam MM, Zaganas I (2011) Interaction between glutamate dehydrogenase (GDH) and l-leucine catabolic enzymes: intersecting metabolic pathways. Neurochem Int 59:518–524
Spanaki C, Plaitakis A (2012) The role of glutamate dehydrogenase in mammalian ammonia metabolism. Neurotox Res 21:117–127
Leke R, Bak LK, Anker M, Melø TM, Sørensen M, Keiding S, Vilstrup H, Ott P, Portela LV, Sonnewald U, Schousboe A, Waagepetersen HS (2011) Detoxification of ammonia in mouse cortical GABAergic cell cultures increases neuronal oxidative metabolism and reveals an emerging role for release of glucose-derived alanine. Neurotox Res 19:496–510
Benuck M, Stern F, Lajtha A (1971) Transamination of amino acids in homogenates of rat brzain. J Neurochem 18:1555–1567
Oldendorf WH, Szabo J (1976) Amino acid assignment to one of three blood-brain barrier amino acid carriers. Am J Physiol 230:94–98
Young RL, Lowry OH (1966) Quantitative methods for measuring the histochemical distribution of alanine, glutamate and glutamine in brain. J Neurochem 13:785–793
Yudkoff M, Nissim I, Hertz L (1990) Precursors of glutamic acid nitrogen in primary neuronal cultures: studies with 15N. Neurochem Res 15:1191–1196
Tofteng F, Hauerberg J, Hansen BA, Pedersen CB, Jørgensen L, Larsen FS (2006) Persistent arterial hyperammonemia increases the concentration of glutamine and alanine in the brain and correlates with intracranial pressure in patients with fulminant hepatic failure. J Cereb Blood Flow Metab 26:21–27
Bjerring PN, Hauerberg J, Frederiksen HJ, Jorgensen L, Hansen BA, Tofteng F, Larsen FS (2008) Cerebral glutamine concentration and lactate-pyruvate ratio in patients with acute liver failure. Neurocrit Care 9:3–7
Parli JA, Godfrey DA, Ross CD (1987) Separate enzymatic microassays for aspartate aminotransferase isoenzymes. Biochim Biophys Acta 925:175–184
Fitzpatrick SM, Cooper AJL, Duffy TE (1983) Use of β-methylene-dl-aspartate to assess the role of aspartate aminotransferase in cerebral oxidative metabolism. J Neurochem 41:1370–1383
Hertz L, Murthy CR, Lai JC, Fitzpatrick SM, Cooper AJL (1987) Some metabolic effects of ammonia on astrocytes and neurons in primary cultures. Neurochem Pathol 6:97–129
Fitzpatrick SM, Cooper AJL, Hertz L (1988) Effects of ammonia and β-methylene-dl-aspartate on the oxidation of glucose and pyruvate by neurons and astrocytes in primary culture. J Neurochem 51:1197–1203
Hertz L, Dienel GA (2002) Energy metabolism in the brain. Int Rev Neurobiol 51:1–102
Hertz L, Kala G (2007) Energy metabolism in brain cells: effects of elevated ammonia concentrations. Metab Brain Dis 22:199–218
Williamson DH, Lund P, Krebs HA (1967) The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 103:514–527
Treberg JR, Brosnan ME, Watford M, Brosnan JT (2010) On the reversibility of glutamate dehydrogenase and the source of hyperammonemia in the hyperinsulinism/hyperammonemia syndrome. Adv Enzyme Reg 50:34–43
Howse DC, Duffy TE (1975) Control of the redox state of the pyridine nucleotides in the rat cerebral cortex. Effect of electroshock-induced seizures. J Neurochem 24:935–940
Cooper AJL, Nieves E, Coleman AE, Filc-DeRicco S, Gelbard AS (1987) Short-term metabolic fate of [13N]ammonia in rat liver in vivo. J Biol Chem 262:1073–1080
Müller AF, Leuthardt F (1950) Die Umwandlung der Glutaminsäure in Asparaginsäure in den Mitochondrien der Leber. (mit Bemerkung über das Vorkommen einer Transaminase in Clostridium Welchii). Helv Chim Acta 33:268–273
Lowenstein JM (1967) The tricarboxylic acid cycle. In: Greenberg DM (ed) Metabolic pathways, vol 1. Academic Press, New York, pp 146–270
Cooper AJL (1981) l-Glutamate (2-oxoglutarate) aminotransferases. In: Kvamme E (ed) Glutamine and glutamate in mammals, vol I. CRC Press Inc, Boca Raton, pp 123–152
McKenna MC, Stevenson JH, Huang X, Hopkins IB (2000) Differential distribution of the enzymes glutamate dehydrogenase and aspartate aminotransferase in cortical synaptic mitochondria contributes to metabolic compartmentation in cortical synaptic terminals. Neurochem Int 37:229–241
Pardo B, Rodrigues TB, Contreras L, Garzón M, Llorente-Folch I, Kobayashi K, Saheki T, Cerdan S, Satrústegui J (2011) Brain glutamine synthesis requires neuronal-born aspartate as amino donor for glial glutamate formation. J Cereb Blood Flow Metab 31:90–101
Hertz L (2011) Brain glutamine synthesis requires neuronal aspartate: a commentary. J Cereb Blood Flow Metab 31:384–387
Lowenstein JM, Goodman MN (1978) The purine nucleotide cycle in skeletal muscle. Fed Proc 37:2308–2312
Bergmeyer HU (1974) Methods of enzymatic analysis. Academic Press Inc. New York, pp. 2284–2285
Acknowledgments
I thank Dr. Boris Krasnikov (New York Medical College) for his help in preparing this manuscript. I also thank Dr. Kevin Behar (Yale University School of Medicine) for valuable discussion. Part of the work described in this review was supported by NIH grant DK 16739.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: in honour of Leif Hertz.
Rights and permissions
About this article
Cite this article
Cooper, A.J.L. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Cerebral Ammonia Homeostasis. Neurochem Res 37, 2439–2455 (2012). https://doi.org/10.1007/s11064-012-0803-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0803-4