Abstract
To test for the prolonged consequences of a short transient exposure of astrocytes to silver nanoparticles (AgNP), cultured primary astrocytes were incubated for 4 h in the presence of AgNP and the cell viability as well as various metabolic parameters were investigated during a subsequent incubation in AgNP-free medium. Acute exposure of astrocytes to AgNP led to a concentration-dependent increase in the specific cellular silver content to up to 46 nmol/mg protein, but did not compromise cell viability. During a subsequent incubation of the cells in AgNP-free medium, the cellular silver content of AgNP-treated astrocytes remained almost constant for up to 7 days. The cellular presence of AgNP did neither induce any delayed cell toxicity nor were alterations in cellular glucose consumption, lactate production or in the cellular ratio of glutathione to glutathione disulfide observed. However, Western blot analysis and immunocytochemical staining revealed that AgNP-treated astrocytes strongly upregulated the expression of metallothioneins. These results demonstrate that a prolonged presence of accumulated AgNP does not compromise the viability and the basal metabolism of cultured astrocytes and suggest that the upregulation of metallothioneins may help to prevent silver-mediated toxicity that could be induced by AgNP-derived silver ions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanomaterials have gained large interest in recent years, since their special characteristics and properties make them useful for a wide variety of applications [1]. Due to their antiseptic properties, silver nanoparticles (AgNP) are of great interest for food products, clothing or electronics as well as for medical equipment, making silver by far the most frequently used metal for nano consumer products [2, 3]. Although the chances of human exposure to silver derived from AgNP-containing products has dramatically risen over the last decade, the toxicity of AgNP to mammals and mammalian cells became only recently a matter of interest [4, 5].
AgNP-exposure has been reported to induce blood-brain barrier (BBB) inflammation and break down [6–8], suggesting that AgNP can enter the brain. Since astrocytes cover the blood capillaries almost completely with their endfeet [9], astrocytes will be the first parenchymal brain cells that encounter the AgNP that have entered the brain from the blood. In addition, AgNP and Ag-coated AuNP were also found in brain after inhalation and transport into the brain via the olfactory bulb [10, 11]. Although AgNP have the potential to enter the brain and may affect functions of brain cells, little is known so far on the consequences of an exposure of brain cells to AgNP.
For cultured rat astrocytes we have recently reported that poly(N-vinylpyrrolidone) (PVP)-coated AgNP do not acutely compromise the cell viability although such particles are efficiently accumulated by the cells, most likely by endocytotic processes [12]. However, no information is currently available on the long term consequences of the presence of AgNP in brain cells and on the fate of such particles. To address such questions, we have loaded cultured astrocytes with AgNP by a short exposure for 4 h to a high dose of PVP-coated AgNP. Subsequently, the cells were washed and incubated for up to 7 days to monitor potential prolonged consequences of the cellular presence of AgNP on cell viability, silver content and metabolic parameters. Here we report that even the presence of large amounts of accumulated AgNP in cultured astrocytes do neither compromise cell viability nor alter the glutathione or glucose metabolism. The strong increase in the cellular level of metal-binding metallothioneins (MTs) in AgNP-treated astrocytes suggests that silver ions are released from the accumulated AgNP, at least in concentrations that are sufficient to induce MT synthesis. This upregulation of MTs may contribute to the prolonged resistance of AgNP-treated astrocytes against silver-induced toxicity.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) was from Gibco (Karlsruhe, Germany), fetal calf serum (FCS) and penicillin/streptomycin solution were obtained from Biochrom (Berlin, Germany). Bovine serum albumin and NADH were from Applichem (Darmstadt, Germany). Argon was obtained from Linde (Hamburg, Germany). Monoclonal mouse anti-MT (detects both MT1 and MT2) and polyclonal rabbit anti-glial fibrillary acidic protein (GFAP) were from Dako (Hamburg, Germany). The monoclonal mouse anti-α-tubulin, 4′,6-diamidino-2-phenylindol hydrochloride (DAPI) and paraformaldehyde were purchased from Sigma (Steinheim, Germany), the peroxidase-conjugated secondary anti-mouse-IgG, the Cy2-conjugated anti-rabbit IgG and the Cy3-conjugated anti-mouse IgG from Dianova (Hamburg, Germany). All other chemicals of the highest purity available were from either Fluka (Buchs, Switzerland), Merck (Darmstadt, Germany), Baker (Griesheim, Germany) or Riedel-de Haën (Seelze, Germany). 96-well microtiter plates and 24-well cell culture plates were obtained from Sarstedt (Nümbrecht, Germany).
Poly(N-vinylpyrrolidone) (PVP)-functionalized silver nanoparticles were synthesized by reduction of Ag+ with glucose in the presence of PVP as previously described [12, 13]. The purified spherical AgNP had an average hydrodynamic diameter of 75 ± 20 nm [12]. The given concentration of PVP-coated AgNP refers to the amount of total silver contained in the particles applied and not to the number of particles.
Cell Cultures
Astroglia-rich primary cultures derived from the whole brains of neonatal Wistar rats were prepared according to a published method [14]. Per well of 24-well dishes, 300,000 viable cells were seeded in 1 mL culture medium (90 % DMEM, 10 % FCS, 20 U/mL penicillin G and 20 μg/mL streptomycin sulphate). The cultures were maintained in a cell incubator (Sanyo, Osaka, Japan) that contained a humidified atmosphere of 10 % CO2/90 % air. The culture medium was renewed every seventh day and the cultures were used for experiments at a culture age of 14 to 23 days.
Experimental Incubation
The cultures were exposed for 4 h without (control) or with AgNP in concentrations that corresponded to 10, 30 or 100 μM silver in 500 μL culture medium. After 4 h of incubation, the medium was removed and the cells were washed once with AgNP-free culture medium. Subsequently, the cells were incubated for further 20, 68 and 164 h in 1 mL culture medium for total incubation periods of 1, 3 and 7 days, respectively. Experiments were terminated by harvesting the media and by washing the cells twice with 1 mL each of phosphate-buffered saline (PBS, 10 mM potassium phosphate buffer pH 7.4 containing 150 mM NaCl).
Determination of Cell Viability and Protein Content
The viability of cells was determined by quantification of the cellular and the extracellular activities of the cytosolic enzyme lactate dehydrogenase (LDH) as previously described [15]. Extracellular LDH activity is presented as percentage of the total LDH activity (sum of cellular plus extracellular LDH activity). A compromised viability of astrocytes is reflected by an increase in extracellular LDH activity. The presence of 100 μM silver in form of AgNP did not alter the amounts of detectable LDH activity in cell lysates of cultured astrocytes (data not shown), excluding the possibility that LDH released from damaged cells would not be detectable due to inactivation of LDH by AgNP. The protein content of the cultures was determined according to the Lowry method [16] using bovine serum albumin as a standard protein, after solubilisation of the cells in 400 μL of 50 mM NaOH.
Silver Measurements
The silver contents of cell lysates and media were measured as described recently [12]. Briefly, 100 μL of the lysate or media samples were incubated with 11 μL of 56 mM FeCl3 and 111 μL of a 1:1 mixture of 35 % H2O2 and 65 % HNO3 (suprapur) at 65 °C for 60 min and dried at 85 °C overnight. The dry residues were dissolved in 1 % HNO3, and the silver content of this solution was quantified by graphite furnace atomic absorption spectroscopy using a Varian (Darmstadt, Germany) AA-240Z spectrophotometer and a Varian GTA-120 graphite tube atomizer equipped with a Varian PSD-120 programmable sample dispenser. The Varian SpectrAA 5.01 software was employed to control the instruments and data analysis.
Determination of Glutathione, Lactate, Glucose and Reactive Oxygen Species
The contents of total glutathione (GSx: amount of glutathione (GSH) plus two times amount of glutathione disulfide (GSSG)) and GSSG in cell lysates were determined by the colorimetric Tietze assay in microtiter plates as described previously [17]. For all conditions used, the cellular GSSG levels were in the range of the detection limit of the assay used. The extracellular contents of glucose and lactate were determined by enzymatic assays as previously described [18]. Intracellular reactive oxygen species were detected by a modification of a recently published method [19]. Briefly, after a given incubation the cells were washed once with incubation buffer (IB: 20 mM HEPES, 145 mM NaCl, 1.8 mM CaCl2, 5.4 mM KCl, 1 mM MgCl2, 5 mM glucose, adjusted to pH 7.4) at 37 °C and incubated for 45 min with 0.5 mL IB containing 5 μg/mL dihydrorhodamine 123 and 10 μM H33342. After washing two times with IB the cells were analyzed for fluorescence.
Western Blot Analysis
For immunoblot analysis, the cells in wells of 24-well dishes were lysed in 80 μL loading buffer (62.4 mM Tris/HCl, pH 6.8, 2 % (w/v) sodium dodecyl sulphate, 10 % (w/v) glycerol, 0.01 % (w/v) bromphenolblue, 4 mM dithiothreitol) and lysates of three wells per condition were pooled. After boiling the lysates for 10 min, they were incubated for 15 min at 50 °C with 12.5 mM iodoacetamide to mask sulfhydryl groups. Per lane of a 15 % polyacrylamide gel, 25 μg lysate protein were separated and electroblotted onto a nitrocellulose membrane (GE Healthcare, Munich, Germany) as previously described [19]. After washing the membranes with TBS-T (10 mM Tris/HCl, 150 mM NaCl, 0.1 % (v/v) Tween®20, pH 7.3), the unspecific binding sites on the membrane were blocked with 5 % milk powder in TBS-T. The blocked membranes were incubated at 4 °C overnight with the monoclonal anti-MT antibody (1:500 in TBS-T) or with the anti-α-tubulin antibody (1:5000 in TBS-T) on a roller shaker (IDL, Nidderau, Germany). The membranes were washed thrice for 15 min intervals with TBS-T and subsequently incubated for 1 h at room temperature (RT) with anti-mouse-IgG peroxidase (1:20,000 in TBS-T/milk powder). After three additional washing steps, the membranes were developed with the Amersham ECL Western blotting detection kit (GE Healthcare). The signals for iodoacetamide-derivatised MTs and α-tubulin were obtained at the expected molecular masses of around 12 and 55 kDa, respectively.
Immunocytochemical Staining
Cells grown on coverslips that had been pre-treated without or with AgNP were washed twice with ice-cold PBS and fixed with 3.5 % (w/v) paraformaldehyde for 10 min at 4 °C. If not stated otherwise, the cells were washed thrice (5 min each) with PBS between the different steps of the staining procedure. Fixed cells were incubated with 0.1 % (w/v) glycine in PBS for 5 min at RT, directly followed by permeabilisation of the membrane with 0.3 % Triton X-100 for 10 min at RT. Incubation of the cells with anti-MT (1:100 diluted in PBS) and anti-GFAP (1:200 diluted in PBS) was carried out for 2 h at RT in a humidified atmosphere, followed by an incubation with the secondary Cy2- and Cy3-coupled antibodies (1:200 diluted in PBS) for 30 min at RT. For visualization of the nuclei, the cells were treated with DAPI (1 μg/mL in PBS) for 5 min at RT. Prior to mounting the coverslips in DPX mounting media, an ethanol gradient of 70, 90 and 100 % in 1 min intervals was applied. The fluorescent signals were documented using the Eclipse TS2000U microscope (Nikon, Düsseldorf, Germany).
Presentation of Data
The data are presented as means ± SD of values from at least three experiments that were performed on independently prepared cultures, if not stated otherwise. Analysis of significance of the differences between groups of data was performed by ANOVA followed by the Dunnetts’ post-hoc test. The significance of differences between two sets of data was analyzed by the t-test. p > 0.05 was considered as not significant.
Results
Loading of Cultured Astrocytes with AgNP
To study the long-time consequences of the cellular presence of AgNP, astrocyte cultures were loaded for 4 h with AgNP by application of different concentrations of the particles. During this loading phase, the viability of the cells was not compromised as indicated by the absence of any significant increase in the extracellular LDH activity and by the absence of any significant loss of cellular LDH or protein (Table 1). Exposure of astrocytes for 4 h to AgNP caused a concentration-dependent significant increase in the total and in the specific cellular silver contents (Table 1). While no silver was detectable for untreated astrocytes (data not shown) or cultures that had been incubated without AgNP, an exposure for 4 h to 10, 30 and 100 μM silver as AgNP increased the specific cellular silver contents to 5.5 ± 1.7, 13.8 ± 1.2 and 46.4 ± 3.6 nmol/mg, respectively (Table 1). Compared with controls (absence of AgNP), this loading with AgNP did neither alter the specific cellular contents of GSx and GSSG nor the amount of lactate released (Table 1).
After loading the astrocytes for 4 h with AgNP, the cells were washed and incubated for up to 7 days in AgNP-free culture medium. In the following paragraphs we compare data obtained for AgNP-loaded cells with those obtained for control cells that had been incubated during the loading phase without AgNP.
Cell Viability and Glucose Metabolism of AgNP-Loaded Astrocytes
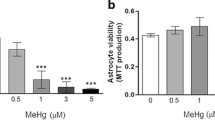
The viability of astrocytes that had been pre-incubated with AgNP in concentrations of up to 100 μM was not substantially compromised during a main incubation of up to 7 days, as indicated by the absence of any increase in extracellular LDH activity (Fig. 1a) and by the almost identical cellular protein contents per well (Fig. 1b) of AgNP-treated and control cells. For all conditions, the protein content of the cultures increased by around 25 % during the 7 days incubation in culture medium (Fig. 1b). Furthermore, the ability of AgNP-treated cells to consume glucose (Fig. 1c) and to produce and release lactate (Fig. 1d) did not differ between control cells and astrocytes that had been exposed to AgNP. For all conditions applied, the cells consumed within the 7 days of incubation about 13 mM glucose and released around 15 mM lactate (Fig. 1c, d).
Viability and metabolism of AgNP-loaded cultured astrocytes. The cells were incubated during a 4 h loading phase without (0 μM) or with 10, 30 or 100 μM silver as AgNP and further cultured in AgNP-free medium for up to 7 days. At the indicated time points, samples were collected to determine the extracellular LDH activity (a), the protein content per well (b), the extracellular glucose concentration (c), the extracellular lactate concentration (d) and the specific cellular GSx (e) and GSSG contents (f). The initial protein content was 137 ± 10 μg per well, and the initial glucose and lactate concentrations of the serum-containing culture medium were 29.0 ± 1.0 and 0.37 ± 0.0 mM, respectively. The initial GSx and GSSG contents of untreated cells (time point 0 h) were 26.4 ± 1.6 and 0.8 ± 0.5 nmol/mg protein, respectively. The values obtained for controls and AgNP-treated cells were not significantly different (p > 0.05)
Test for Glutathione Oxidation and ROS Formation in AgNP-Loaded Astrocytes
The exposure of astrocytes to AgNP during the 4 h loading phase caused neither any acute alteration in the specific cellular GSx content nor increased the ratio of GSSG to GSH (Table 1). The subsequent incubation in fresh culture medium for 20 h following the loading phase increased the cellular GSx contents by about 40 % to values of around 45 nmol/mg protein, irrespective of the absence or presence of AgNP during the loading period (Fig. 1e), most likely due to the availability of an excess of amino acid precursors for GSH synthesis. This increase in cellular GSx was transient and the specific cellular GSx contents decreased to less than 30 nmol/mg during longer incubation times (Fig. 1e). For all conditions and time points investigated, the GSx contents of the AgNP-treated cultures were not significantly different to those of control cells (loading period without AgNP) (Fig. 1e) and the specific cellular GSSG contents remained below 0.9 nmol GSx/mg (Fig. 1f). In addition, for none of the conditions an obvious increase in the levels of cellular ROS was observed (data not shown).
AgNP and Silver in Cells and Media
Specific cellular silver contents of about 6, 14 and 46 nmol/mg protein were established by loading astrocytes for 4 h with different concentrations of AgNP (Table 1). The cellular silver contents remained almost constant during the following 7 days incubation period (Fig. 2a). At best a low decline in cellular silver content was observed for some conditions (Fig. 2a) that was accompanied by a slight but not significant increase in extracellular silver (Fig. 2b). The sum of cellular plus extracellular silver remained almost constant throughout the total incubation period of up to 7 days (Fig. 2c).
Silver contents in AgNP-loaded astrocytes. The cells were incubated for 4 h in the absence (0 μM) or presence of 10, 30 or 100 μM silver as AgNP and further cultured in AgNP-free medium for up to 7 days. At the indicated time points, the silver contents in cells (a) and media (b) were determined. c Shows the sum of cellular plus extracellular silver contents. (*p < 0.05; **p < 0.01)
Expression of Metallothioneins in AgNP-Treated Astrocytes
MTs are metal binding proteins that are upregulated in cells to prevent metal induced toxicity [20, 21]. To test whether the expression of MTs in astrocytes is altered upon exposure of the cells to AgNP, the amount of MTs was determined by Western blot analysis and immunocytochemical staining. Untreated cells and control cultures (loading period without AgNP) that had been incubated for up to 7 days contained only low levels of MTs (Fig. 3). In contrast, the MT content in astrocytes that had been transiently exposed to AgNP increased strongly with time after removal of the AgNP (Fig. 3a). Semi-quantitative analysis of the signal intensities revealed that a short 4 h loading period with AgNP caused a delayed but significant increase in the MT signal after a prolonged incubation for 3 and 7 days (Fig. 3b). These findings were confirmed by immunocytochemical staining of cultured astrocytes for MTs. While control cultures that had been incubated in the absence of AgNP showed only a weak MT staining (Fig. 4a), the fluorescence signal for MTs was strongly increased in cultures that had been incubated for 3 days after AgNP-loading (Fig. 4d), with most of the MT-positive cells also being positive for the astrocytic marker protein GFAP (Fig. 4e).
Western Blot analysis for the presence of metallothioneins in cultured astrocytes. The cells were treated without or with 100 μM silver as AgNP for 4 h at 37 °C and further cultured in AgNP-free medium for up to 7 days. Lysates were analysed for the presence of MTs and α-tubulin. a Shows a representative Western blot. b Gives the semi-quantitative analysis of values obtained in 4 independent experiments for the ratio of signal intensities observed for MTs and α-tubulin that have been normalized on the values obtained for samples of untreated astrocytes
Immunocytochemical staining for metallothioneins in astrocyte cultures. The cells had been incubated for 4 h without (control) or with 100 μM AgNP and subsequently cultured in AgNP-free medium for 3 days. The figure shows representative pictures of the immunocytochemical staining for MTs (a, d), GFAP (b, e) and DAPI staining of the cell nuclei (c, f). The scale bar in (a) represents 50 μm and applies to all panels
Discussion
To investigate the long-time consequences of a short exposure of cultured astrocytes to AgNP, the cells were loaded for 4 h with PVP-coated AgNP. This treatment increased the specific cellular silver content of viable astrocytes in a concentration-dependent manner due to endocytotic accumulation of AgNP by astrocytes [12]. An acute exposure of astrocytes to up to 100 μM PVP-AgNP compromised neither the cell viability nor the glycolytic production of lactate by the cells, confirming the reported resistance of cultured primary astrocytes towards the acute toxicity of PVP-coated AgNP for up to 24 h [12]. However, this resistance contrasts recent data that report toxicity of secondary astrocyte cultures after a 24 h exposure to peptide-coated AgNP [22]. The most likely reason for the discrepancy in the observed toxic potential of AgNP to astrocytes is the use of AgNP with different coatings because nanoparticle toxicity strongly depends on the coating of the particles [23].
Long-time consequences of the presence of large amounts of AgNP in astrocytes have not been reported so far to the best of our knowledge. The viability of AgNP-containing astrocytes was not compromised even during the prolonged incubation period of up to 7 days, as demonstrated by the absence of any increase in extracellular LDH activity and by the unaltered glucose consumption and lactate production compared with control cells. In addition, AgNP-treated astrocytes showed neither an increase in the cellular GSSG content nor an accelerated ROS production, demonstrating that AgNP-containing astrocytes do not suffer from substantial oxidative stress during prolonged incubation. This contrasts reports which describe the occurrence of oxidative stress in several AgNP-treated cell types [24–27] including secondary astrocytes that had been exposed for 24 h with peptide-coated AgNP [22]. However, also primary rat astrocytes that had been loaded with iron oxide nanoparticles did not show any compromised viability nor an increase in the cellular GSSG levels during a prolonged incubation of up to 7 d (data not shown). Primary astrocytes are known to contain a prominent antioxidative defence system [28] which may prevent the cellular accumulation of ROS to detectable amounts in PVP-AgNP-treated astrocyte cultures. The high resistance of primary astrocytes compared with other cell types against toxicity induced by metal ions and metal-containing nanoparticles appears to be a special feature of primary astrocytes as previously shown for iron and silver in low molecular weight and nanoparticle form [12, 29, 30]. Astrocytes are able to upregulate metal binding proteins such as the iron storage protein ferritin [31, 32] and MTs [20, 21, 33–35], which will help to prevent metal-induced toxicity by binding and/or storing low molecular weight metal ions in non-toxic form. In addition, the synthesis of stress proteins such as heme oxygenase 1, which has recently been reported to be upregulated in AgNP-treated astrocytes [22], may also contribute to the protection of cultured astrocytes against potential toxic consequences of the cellular presence of metal ions and metal-containing nanoparticles.
After incubation of AgNP-loaded cells in AgNP-free medium, the specific cellular silver content of the AgNP-loaded cells remained almost constant, demonstrating that cultured astrocytes do not efficiently export AgNP or AgNP-derived silver. The slight decrease in the silver contents of AgNP-treated cells and the slight increase in extracellular silver contents during the incubation is most likely caused by desorption of AgNP that had extracellularly adsorbed to the cell membranes during the loading period and accounts for around 20 % of the cellular silver content of AgNP-treated astrocytes [12].
As silver ions in high concentrations are toxic for astrocytes [12], the absence of any obvious delayed toxicity in AgNP-treated astrocytes suggests that these cells do either not liberate toxic amounts of silver ions from the accumulated PVP-AgNP and/or that AgNP-derived silver ions are stored in non-toxic form. At least some liberation of silver ions from the accumulated AgNP is highly likely due to the observed upregulation of MTs during prolonged incubation of AgNP-loaded astrocytes. MT upregulation has previously been described for astrocytes after treatment with zinc, copper, mercury or cadmium salts [20, 33, 35] as well as for cell lines of peripheral origin after exposure to AgNP [36–38]. The molecular mechanisms how AgNP-derived silver ions induce the upregulation of MTs in astrocytes remains to be elucidated. An activation of the metal regulatory transcription factor 1 (MTF-1) by zinc ions [39], which binds to metal response elements in the promoter region of the MT genes [40] could trigger MT synthesis [40, 41] in AgNP-treated astrocytes. Since silver ions have a much higher affinity to MTs than zinc ions [42], they are likely to liberate MT-bound zinc which subsequently could promote the upregulation of MT expression as described for fibroblasts where silver leads to zinc release from MTs [43]. Alternatively, an oxidative stress-induced activation of MT expression by binding of the transcription factor Nrf1 to the antioxidative response elements within the MT genes [44, 45] could be involved in the upregulation of MT synthesis in AgNP-treated astrocytes. A silver-induced liberation of zinc ions from zinc-containing proteins could lead to a zinc-induced oxidative stress, similar to that observed for zinc-treated cultured astrocytes [46]. However, for significant increases in the cellular levels of ROS and GSSG the cells had to be exposed to zinc concentrations of at least 150 μM [46]. Since no increases in cellular ROS production or in the cellular GSSG content were observed for AgNP-treated astrocytes, an involvement of MTF-1 appears to be more likely than that of Nrf1 in the observed upregulation of MTs.
In conclusion, the persistent presence of large amounts of silver in cultured primary astrocytes after treatment with PVP-AgNP does neither compromise cell viability nor induce oxidative stress or alters cell metabolism. However, the delayed upregulation of MTs in AgNP-treated astrocytes suggests that some silver was released from the accumulated AgNP during prolonged incubation, at least in amounts that induced an upregulation of MTs. The increase in cellular MT levels will improve the capacity of the cells to scavenge AgNP-derived silver ions and is likely to contribute to the prolonged resistance of cultured astrocytes against potential toxicity of AgNP. This hypothesis should be studied by comparing the consequences of an AgNP-treatment of astrocyte cultures derived from the brains of wild type and MT1/2-deficient mice [47]. Assuming that the data obtained for cultured astrocytes reflect the properties of astrocytes in vivo, the observed efficient accumulation of AgNP by astrocytes and the upregulation of MTs to bind AgNP-derived silver ions are likely to enable astrocytes to prevent neurotoxic consequences of AgNP that have entered the brain. Such a protective function against AgNP-induced toxicity is consistent with the view that astrocytes act as sink for potentially toxic metals in brain [48–50].
References
Dastjerdi R, Montazer M (2010) A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloid Surf B 79(1):5–18
Ahamed M, AlSalhi MS, Siddiqui MKJ (2010) Silver nanoparticle applications and human health. Clin Chim Acta 411(23–24):1841–1848
Tolaymat TM, El Badawy AM, Genaidy A, Scheckel KG, Luxton TP, Suidan M (2010) An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papers. Sci Total Environ 408(5):999–1006
Stensberg MC, Wei Q, McLamore ES, Porterfield DM, Wei A, Sepúlveda MS (2011) Toxicological studies on silver nanoparticles: challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 6(5):879–898. doi:10.2217/nnm.11.78
Mahmoudi M, Azadmanesh K, Shokrgozar MA, Journeay WS, Laurent S (2011) Effect of nanoparticles on the cell life cycle. Chem Rev 111(5):3407–3432. doi:10.1021/cr1003166
Tang JL, Xiong L, Zhou GF, Wang S, Wang JY, Liu L, Li JG, Yuan FQ, Lu SF, Wan ZY, Chou LS, Xi TF (2010) Silver nanoparticles crossing through and distribution in the blood-brain barrier in vitro. J Nanosci Nanotechnol 10(10):6313–6317. doi:10.1166/jnn.2010.2625
Sharma HS, Patnaik R, Sharma A (2010) Diabetes aggravates nanoparticles induced breakdown of the blood-brain barrier permeability, brain edema formation, alterations in cerebral blood flow and neuronal injury. An experimental study using physiological and morphological investigations in the rat. J Nanosci Nanotechnol 10(12):7931–7945. doi:10.1166/jnn.2010.3616
Trickler WJ, Lantz SM, Murdock RC, Schrand AM, Robinson BL, Newport GD, Schlager JJ, Oldenburg SJ, Paule MG, Slikker W, Hussain SM, Ali SF (2010) Silver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol Sci 118(1):160–170. doi:10.1093/toxsci/kfq244
Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP (2010) The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58(9):1094–1103. doi:10.1002/glia.20990
Ji JH, Jung JH, Kim SS, Yoon J-U, Park JD, Choi BS, Chung YH, Kwon IH, Jeong J, Han BS, Shin JH, Sung JH, Song KS, Yu IJ (2007) Twenty-eight-day inhalation toxicity study of silver nanoparticles in Sprague–Dawley rats. Inhal Toxicol 19(10):857–871. doi:10.1080/08958370701432108
Win-Shwe TT, Fujimaki H (2011) Nanoparticles and neurotoxicity. Int J Mol Sci 12(9):6267–6280. doi:10.3390/ijms12096267
Luther EM, Koehler Y, Diendorf J, Epple M, Dringen R (2011) Accumulation of silver nanoparticles by cultured primary brain astrocytes. Nanotechnology 22(37):375101. doi:10.1088/0957-4484/22/37/375101
Greulich C, Diendorf J, Simon T, Eggeler G, Epple M, Köller M (2011) Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater 7(1):347–354
Hamprecht B, Löffler F (1985) Primary glial cultures as a model for studying hormone action. Method Enzymol 109:341–345
Dringen R, Kussmaul L, Hamprecht B (1998) Detoxification of exogenous hydrogen peroxide and organic hydroperoxides by cultured astroglial cells assessed by microtiter plate assay. Brain Res Prot 2(3):223–228
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Dringen R, Hamprecht B (1996) Glutathione content as an indicator for the presence of metabolic pathways of amino acids in astroglial cultures. J Neurochem 67(4):1375–1382. doi:10.1046/j.1471-4159.1996.67041375.x
Liddell JR, Zwingmann C, Schmidt MM, Thiessen A, Leibfritz D, Robinson SR, Dringen R (2009) Sustained hydrogen peroxide stress decreases lactate production by cultured astrocytes. J Neurosci Res 87(12):2696–2708. doi:10.1002/jnr.22093
Hohnholt MC, Geppert M, Dringen R (2011) Treatment with iron oxide nanoparticles induces ferritin synthesis but not oxidative stress in oligodendroglial cells. Acta Biomater 7(11):3946–3954. doi:10.1016/j.actbio.2011.06.052
Aschner M, Conklin DR, Yao CP, Allen JW, Tan KH (1998) Induction of astrocyte metallothioneins (MTs) by zinc confers resistance against the acute cytotoxic effects of methylmercury on cell swelling, Na+ uptake, and K+ release. Brain Res 813(2):254–261. doi:10.1016/s0006-8993(98)00947-0
Aschner M, Cherian MG, Klaassen CD, Palmiter RD, Erickson JC, Bush AI (1997) Metallothioneins in brain—the role in physiology and pathology. Toxicol Appl Pharmacol 142(2):229–242. doi:10.1006/taap.1996.8054
Haase A, Rott S, Mantion A, Graf P, Plendl J, Thünemann AF, Meier WP, Taubert A, Luch A, Reiser G (2012) Effects of silver nanoparticles on primary mixed neural cell cultures: uptake, oxidative stress and acute calcium responses. Toxicol Sci 126(2):457–468. doi:10.1093/toxsci/kfs003
Suresh AK, Pelletier DA, Wang W, Morrell-Falvey JL, Gu B, Doktycz MJ (2012) Cytotoxicity induced by engineered silver nanocrystallites is dependent on surface coatings and cell types. Langmuir 28(5):2727–2735. doi:10.1021/la2042058
Foldbjerg R, Olesen P, Hougaard M, Dang DA, Hoffmann HJ, Autrup H (2009) PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol Lett 190(2):156–162
Powers CM, Badireddy AR, Ryde IT, Seidler FJ, Slotkin TA (2010) Silver nanoparticles compromise neurodevelopment in PC12 Cells: critical contributions of silverion, particle size, coating, and composition. Environ Health Persp 119(1):37–44
Greulich C, Diendorf J, Gessmann J, Simon T, Habijan T, Eggeler G, Schildhauer TA, Epple M, Koller M (2011) Cell type-specific responses of peripheral blood mononuclear cells to silver nanoparticles. Acta Biomater 7(9):3505–3514. doi:10.1016/j.actbio.2011.05.030
Piao MJ, Kang KA, Lee IK, Kim HS, Kim S, Choi JY, Choi J, Hyun JW (2011) Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett 201(1):92–100. doi:10.1016/j.toxlet.2010.12.010
Hirrlinger J, Dringen R (2010) The cytosolic redox state of astrocytes: maintenance, regulation and functional implications for metabolite trafficking. Brain Res Rev 63(1–2):177–188
Tulpule K, Robinson SR, Bishop GM, Dringen R (2010) Uptake of ferrous iron by cultured rat astrocytes. J Neurosci Res 88(3):563–571. doi:10.1002/jnr.22217
Geppert M, Hohnholt MC, Thiel K, Nürnberger S, Grunwald I, Rezwan K, Dringen R (2011) Uptake of dimercaptosuccinate-coated magnetic iron oxide nanoparticles by cultured brain astrocytes. Nanotechnology 22(14):145101
Hoepken HH, Korten T, Robinson SR, Dringen R (2004) Iron accumulation, iron-mediated toxicity and altered levels of ferritin and transferrin receptor in cultured astrocytes during incubation with ferric ammonium citrate. J Neurochem 88(5):1194–1202
Dang TN, Bishop GM, Dringen R, Robinson SR (2011) The metabolism and toxicity of hemin in astrocytes. Glia 59(10):1540–1550. doi:10.1002/glia.21198
Kramer KK, Liu J, Choudhuri S, Klaassen CD (1996) Induction of metallothionein mRNA and protein in murine astrocyte cultures. Toxicol Appl Pharmacol 136(1):94–100. doi:10.1006/taap.1996.0011
West AK, Hidalgo J, Eddins D, Levin ED, Aschner M (2008) Metallothionein in the central nervous system: roles in protection, regeneration and cognition. Neurotoxicology 29(3):489–503. doi:10.1016/j.neuro.2007.12.006
Hidalgo J, Garcia A, Oliva AM, Giralt M, Gasull T, Gonzalez B, Milnerowicz H, Wood A, Bremner I (1994) Effect of zinc, copper and glucocorticoids on metallothionein levels of cultured neurons and astrocytes from rat brain. Chem Biol Interact 93(3):197–219
Miura N, Shinohara Y (2009) Cytotoxic effect and apoptosis induction by silver nanoparticles in HeLa cells. Biochem Biophys Res Commun 390(3):733–737. doi:10.1016/j.bbrc.2009.10.039
Kang SJ, I Ryoo, Lee YJ, Kwak M-K (2012) Role of the Nrf2-heme oxygenase-1 pathway in silver nanoparticle-mediated cytotoxicity. Toxicol Appl Pharmacol 258(1):89–98. doi:10.1016/j.taap.2011.10.011
AshaRani P, Hande MP, Valiyaveettil S (2009) Anti-proliferative activity of silver nanoparticles. BMC Cell Biol 10(1):65
Heuchel R, Radtke F, Georgiev O, Stark G, Aguet M, Schaffner W (1994) The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J 13(12):2870–2875
Andrews GK (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol 59(1):95–104. doi:10.1016/s0006-2952(99)00301-9
Vašák M, Meloni G (2011) Chemistry and biology of mammalian metallothioneins. J Biol Inorg Chem 16(7):1067–1078. doi:10.1007/s00775-011-0799-2
Floriańczyk B (2007) Metallothioneins and its role in metal regulation, binding of reactive oxygen species, apoptosis and cell differentiation. J Pre-Clin Clin Res 1(1):016–018
Cortese-Krott MM, Münchow M, Pirev E, Heβner F, Bozkurt A, Uciechowski P, Pallua N, Kröncke K-D, Suschek CV (2009) Silver ions induce oxidative stress and intracellular zinc release in human skin fibroblasts. Free Radic Biol Med 47(11):1570–1577. doi:10.1016/j.freeradbiomed.2009.08.023
Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, Yamamoto M (2008) Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem 283(48):33554–33562. doi:10.1074/jbc.M804597200
Reisman SA, Aleksunes LM, Klaassen CD (2009) Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2-independent processes. Biochem Pharmacol 77(7):1273–1282
Bishop GM, Dringen R, Robinson SR (2007) Zinc stimulates the production of toxic reactive oxygen species (ROS) and inhibits glutathione reductase in astrocytes. Free Rad Biol Med 42(8):1222–1230
Yao CP, Allen JW, Mutkus LA, Xu SB, Tan KH, Aschner M (2000) Foreign metallothionein-I expression by transient transfection in MT-I and MT-II null astrocytes confers increased protection against acute methylmercury cytotoxicity. Brain Res 855(1):32–38. doi:10.1016/s0006-8993(99)02211-8
Hidalgo J, Aschner M, Zatta P, Vašák M (2001) Roles of the metallothionein family of proteins in the central nervous system. Brain Res Bull 55(2):133–145. doi:10.1016/s0361-9230(01)00452-x
Tiffany-Castiglioni E, Qian Y (2001) Astroglia as metal depots: molecular mechanisms for metal accumulation, storage and release. Neurotoxicology 22(5):577–592
Dringen R, Bishop G, Koeppe M, Dang T, Robinson S (2007) The pivotal role of astrocytes in the metabolism of iron in the brain. Neurochem Res 32(11):1884–1890. doi:10.1007/s11064-007-9375-0
Acknowledgments
E.M. Luther is a member of the Ph.D. graduate school nanoToxCom at the University of Bremen and would like to thank the Hans-Böckler Stiftung for her Ph.D. fellowship. M. Epple thanks the Deutsche Forschungsgemeinschaft for funding within the Priority Program SPP 1313 BioNanoResponses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luther, E.M., Schmidt, M.M., Diendorf, J. et al. Upregulation of Metallothioneins After Exposure of Cultured Primary Astrocytes to Silver Nanoparticles. Neurochem Res 37, 1639–1648 (2012). https://doi.org/10.1007/s11064-012-0767-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0767-4