Abstract
Taurine (Tau) is one of the most abundant amino acids in the brain and regulates physiological functions in the central nervous system, including anti-inflammatory effects. There is growing evidence that microglia-mediated neuro-inflammatory responses are an integral part of Parkinson’s disease (PD) onset and progression. Among the many factors regulating the inflammatory response, phosphatidylinositol-3 kinase (PI3K) is susceptible to activation by a variety of cytokines and physicochemical factors, and subsequently recruits signaling proteins containing the pleckstrin homology structural domain to further regulate protein kinase B (AKT) expression involved in the regulation of the intracellular immune response and inflammatory response. Therefore, we established a PD mouse model using paraquat (PQ) intraperitoneal injection staining to explore the mechanism of Tau action on PI3K/AKT signaling pathway. Our study showed that PD mice with Tau intervention recovered motor and non-motor functions to some extent, and the number of dopaminergic (DAc) neurons in the substantia nigra and the level of dopamine (DA) secretion in the striatum were also significantly increased compared with the PQ-dyed group, and the protein content of PI3K and PDK-1 and the phosphorylation level of AKT were reduced in parallel with the reduction in the expression of microglia and related inflammatory factors. In conclusion, our results suggest that Tau may regulate microglia-mediated inflammatory responses through inhibition of the PI3K/AKT pathway in the midbrain of PD mice, thereby reducing DAc neurons damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taurine (Tau) is one of the most abundant amino acids in the brain and regulates physiological functions in the central nervous system (CNS), such as neuromodulation, maintenance of calcium homeostasis, antioxidant and anti-inflammatory (Huxtable 1992). Previous researches have found that Tau could alleviate As2O3-induced liver inflammation by inhibiting the autophagy-CTSB-NLRP3 inflammasome pathway (Qiu et al. 2018), rescue tissues or organs damaged by regulating oxidative stress and inflammation (Abd-Elhakim et al 2020; Niu et al. 2018; Qaradakhi et al. 2020). And it is also considered a key trophic factor in the development of the CNS (Hernández-Benítez et al. 2010). Tau concentrations are particularly high in the substantia nigra (SN) and striatum (ST) and play an important role in the regulation of dopamine release and dopaminergic (DAc) neuronal activity (Ruotsalainen and Ahtee 1996), and some studies have also shown that Tau reduction is negatively correlated with exercise severity (Zhang et al. 2016). Parkinson’s disease (PD), as a central neurodegenerative disease, is characterized clinically by resting tremor, bradykinesia, myotonia, and postural gait disturbances, affecting nearly 10 million people worldwide, with nearly 60,000 new cases each year (Kalia and Lang 2015; Marras et al. 2018), affecting patients’ quality of life and increasing the economic burden on patients’ families and society, making PD one of the important public health problems (Dorsey et al. 2007). Genetic/aging factors alone do not fully explain the occurrence of PD, and epidemiological evidence suggests a strong association between the occurrence of PD and environmental chemical exposure (Hatcher et al. 2008), among which paraquat (PQ), a highly effective herbicide, is one of the potential environmental risk factors for sporadic Parkinson’s disease (Kamel 2013) and one of the recognized environmental chemicals for the preparation of animal models of PD (Dwyer et al. 2021). There is growing evidence that microglia-mediated neuro-inflammatory responses are an integral part of PD onset and progression (Huang et al. 2019), and among the many factors regulating the inflammatory response, the phosphatidylinositol-3 kinase (PI3K) family plays an important role. PI3K is susceptible to activation by a variety of cytokines and physicochemical factors, and subsequently recruits signaling proteins containing the pleckstrin homology domain to further regulate protein kinase B (AKT) expression involved in the regulation of intracellular immune response and inflammatory response (Vyas and Vohora 2017). Endogenous Tau biosynthesis is low and supplementation with exogenous Tau may be an effective way to slow down microglia-mediated neuro-inflammatory responses and promote the development of DAc neurons structure and function by exerting anti-inflammatory and antioxidant effects. Therefore, this study was conducted to establish a PD mouse model using PQ intraperitoneal injection of stained toxin and to investigate the mechanism of PI3K/AKT signaling pathway in Tau on PQ-induced neuro-inflammation in PD mice, providing new clues for the prevention and treatment of sporadic PD caused by environmental chemical exposure.
Methods
Animal dosing
80 healthy male SPF-grade C57BL/6 mice, weighing (22 ± 2) g, were selected and purchased from Spelford (Beijing) Biotechnology Co. Animals were housed in the Experimental Animal Center of Yanhu Campus of Ningxia Medical University and reviewed by the Ethics Committee of Ningxia Medical University (No. 2019-098). The rearing temperature was (25 ± 1) °C, humidity was 50–60%, 12/12 h circadian rhythm, and free access to water and food was given. After 1 week of acclimatization, the mice were divided into two parts according to the random number table method. In the first part, one group was the control group whose treatment was intraperitoneal injection of an equal volume of 0.9% saline, and the other three groups were treated with PQ for 4, 6 and 8 weeks (P-4, P-6 and P-8) by intraperitoneal injection of 15 mg/kg PQ (dissolved in saline). In the second part, the groupings are control group (control): intraperitoneal injection of equal volume of 0.9% saline; PQ treatment group (PQ): intraperitoneal injection of 15 mg/kg PQ (dissolved in saline); Taurine and PQ combination treatment group (PQ + Tau): intraperitoneal injection of 150 mg/kg Tau 1 h before 15 mg/kg PQ injection; Tau treatment group (Tau): intraperitoneal injection of 150 mg/kg Tau (dissolved in saline). 10 mice in each group were injected twice a week, and the treatment time of P-4, P-6, and P-8 were 4, 6, and 8 weeks, respectively, and the remaining groups were 8 weeks (Fig. 1).
Neurobehavioral tests
G1 performed neurobehavioral tests two days after the 8, 12, and 16 treatments, and the other groups were performed two days after the last treatment. (1) Open field test: mice were placed in the central area of an open field box (50 × 50 cm) with the bottom surface evenly divided into 20 areas of equal size (6 central areas and 14 peripheral areas). The biological activity trajectory of mice and the distance and time consumed in the central and peripheral areas were recorded within 10 min using Smart 3.0 video tracking software. (2) Tail suspension test: the mice were fixed with their tails at 2 cm from the roots on a tail suspension stand, so that the mice were hanging upside down with their heads 5 cm from the table surface, and the accumulated immobility time of each group of mice within 5 min of tail suspension was recorded. (3) Gait analysis test: the forelimbs and hindlimbs of the mice were painted with red and blue ink, and then the mice were guided to walk into the closed box along a 60 cm long and 10 cm wide runway, and a white paper of equal length and width was placed on the runway after three training sessions. Continuously measure the step length (left and right limb spacing) and standing width (front and rear limb spacing) of the mice 4–6 times. (4) Pole climbing test: a 25 mm diameter wooden 9 ball was fixed to a 50 cm long and 1 cm thick wooden pole with gauze wrapped around it to prevent slippage. The mouse was placed on the top of the wooden ball and the time it took for the mouse to come down from the ball was recorded.
Sampling and sample processing
Mice (n = 5/group) were injected intraperitoneally with 0.3% uratan (0.1 ml/10 g), into deep anesthesia, then opened the chest, cut open the right heart ear, first perfused 0.9% saline 50 ml rapidly through the left ventricle, blood washed until colorless and then slowly perfused with 4% paraformaldehyde buffer for 20 min (until the mouse liver turned white and the limbs and tail stiffened). The brain was removed by severing the head on ice, and the brain tissue was placed in 4% paraformaldehyde fixative for 4–6 h, and then removed and stored frozen in 30% sucrose solution for 24–48 h. After the brain tissue was completely sunk into the bottom of the test tube, frozen sections with a slice thickness of 20 μm were performed for immuno-histochemical staining and superoxide anion fluorescent probe staining. The remaining mice (n = 5/group) were perfused with pre-cooled 0.9% saline, then whole brains were quickly peeled off on ice and striatal and midbrain tissues were separated and placed in liquid nitrogen, and then transferred to -80℃ refrigerator for protein content determination.
Immunohistochemistry
Place frozen sections in phosphate-buffered salt solution (1 × PBS) for 5 min × 3 times; incubate 30% H2O2 solution for 10 min at room temperature to remove endogenous peroxidase; 1 × PBS for 5 min × 3 times; goat serum closed for 20 min; dropwise addition of primary antibody in a wet box incubated overnight at 4 °C,; rewarmed at 37 °C for 40 min, 1 × PBS rinse for 5 min × 3 times; secondary antibody incubated at room temperature for 20 min; 1 × PBST rinse for 5 min × 3 times; DAB color development; distilled water rinse for 30 s; hematoxylin re-staining for 1 min; 1% hydrochloric acid ethanol fractionation for 5 s; distilled water rinsing for 30 s followed by dropwise addition of neutral gum to seal the slices, observed under a light microscope and calculated by Image-pro plus 6.0 image analysis software, and the average absorbance of positive material under each field of view was measured as the relative expression of protein.
Western blotting analysis
50 mg of each striatal and midbrain tissues was placed in 2 ml EP tubes, 400 μl of pre-cooled tissue lysis solution and 5 mm diameter grinding beads were added, and the tissues were ground thoroughly on a high-throughput tissue lysis apparatus. After that, the ground tissue homogenate was aspirated into a 1.5 ml pre-chilled centrifuge tube and centrifuged at 12,000 r/min (5 cm radius) for 5 min, and the supernatant was taken to measure the protein concentration, and the samples were denatured and stored at − 80 °C. Each group of proteins was sampled in a volume of 50 μg, and electrophoresis was performed using 8% or 10% sodium dodecyl sulfate–polyacrylamide gels (SDS-PAGE) at a constant voltage of 120 V. The proteins were transferred to polyvinylidene difluoride (PVDF) membranes by water bath electrotransfer at 100 V for 60 min. 5% skim milk powder was closed at room temperature for 1.5 h. According to Table 1, primary antibodies were added respectively. The PVDF membranes were removed the next day, placed in 1:2500 dilution of horseradish enzyme (HRP)-labeled goat anti-rabbit IgG secondary antibody solution, incubated overnight at 4 °C, and the membranes were washed 3 times × 10 min with TBST. The ECL kit was developed, and the images were acquired by an automatic gel imaging analysis system. β-actin was used as the internal reference for the upper sample volume, and Image-J 8.0 software was used to analyze the grayscale value of the target protein expression, and the ratio of the grayscale value of the target band to the grayscale value of the internal reference was used as the relative protein expression.
Statistical analysis
Image-Pro plus 6.0 software was used for IHC image processing, and Image-J 8.0 software was used for western-blot image processing. One-way ANOVA was used for data comparison between multiple groups. SNK (q test) method was used for further two-by-two comparison between groups. Differences were considered statistically significant at p < 0.05.
Results
General behavioral changes of mice in each group
Our previous study has observed that, after 6 weeks of PQ poisoning, mice demonstrated scattered fur, reduced activity, lower response to ambient stimuli, and individual mice showed symptoms, such as posterior dorsal arch and resting tremor. No significant behavior changes were observed in the control and Tau intervention groups. The mean body weight of mice in each group was not significantly different.
Effects of PQ on non-motor and motor functions of mice
To clarify whether the model mice showed non-motor and motor symptoms related with PD, we performed neurobehavioral tests on them. The results of the open field test demonstrated that, compared with the control group, the mice in the poisoned group shifted significantly less distance and the central area dwell time decreased (Fig. 2a–c). As seen in Fig. 2e, the results of the tail suspension test revealed that the tail suspension immobility time gradually increased with the increase of the PQ-dyed time, among which the tail suspension immobility time of mice in the 6 week and 8 week PQ-dyed groups was significantly increased compared with the control group. As illustrated in Fig. 2d, f, g, compared with the control group, the gait length of mice in each group did not change significantly as the time of PQ poisoning increased, but the gait distance gradually decreased, when compared with the control group at 8 weeks of poisoning. The pole-climbing time of mice in the 6 week and 8 week poisoned groups increased significantly compared with the control group (Fig. 2h).
Changes of motor and non-motor functions in each group of mice (n = 6). a The moving trajectory map in the open field test; b The moving distance in the open field test; c The residence time in the edge/central area in the open-field test; d The schematic diagram of gait test; e The immobility time of hanging the tails; f The step length in gait test; g The step distance in gait test; h The time of climbing the pole; The data are presented as mean ± SE. a p < 0.05, compared with the control group
The effect of PQ on DAc neurons in mice
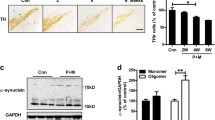
As illustrated in Fig. 3a, DAc neurons loss began to appear in the nigrostriatal area of the mice in the 4 week dose group, and the protrusion ends were curled and thinned, but most of the neurons cell bodies remained relatively intact. With the increase in the duration of the poisoning, the degree of neurons damage in the nigrostriatal area of the mice in the 6 week and 8 week groups gradually increased, which showed that the residual neurons cell bodies were reduced, the nerve fibers were sparse, and the protrusion ends were broken or disappeared, and the number of neurons was significantly reduced compared with that of the control group. In addition to the loss of neurons cytosomes, the positive expression of DAc neurons in the striatum of the mice in the dyed group was also significantly reduced in a time-dependent manner. The protein expressions of brain tyrosine hydroxylase (TH) and striatal dopamine transporter (DAT) in the mice in the toxin-treated group decreased with the increase of PQ toxicity time (Fig. 3b), and the difference was statistically significant compared with the control group. In view of the fact that the most significant changes in all the above indexes were observed in the 8 week PQ-infected group, we selected 15 mg/kg PQ intraperitoneally injected mice (8 weeks) for the follow-up test.
Morphological and protein expression changes of TH in nigrostriatal and striatal DAc neurons in each group (n = 3). a Nigrostriatal and striatal tyrosine hydroxylase (TH) IHC staining (100 ×), and number of nigrostriatal and Striatal TH-positive cells; b Total protein lysates were evaluated by western blot analysis for the expression of DAc neurons markers (TH, DAT). GAPDH was used as the internal control for normalization. SN Substantia nigra, ST Striatum, TH Tyrosine hydroxylase, DAT Dopamine Transporter; Control: control group; P-4: 4-week stained group; P-6: 6-week stained group; P-8: 8-week stained group; the data are presented as mean ± SE. a: p < 0.05, compared with control group; b p < 0.05, compared with 4-week stained group; c p < 0.05, 6-week stained group
Effects of Tau on PQ-induced motor and non-motor functions in PD mice
As shown in Fig. 4a–c, the results of the open field test manifested that, compared with the PQ-treated group, the distance moved and the central area dwell time of mice in the Tau intervention group were significantly increased; compared with the control group, the distance moved and the central area dwell time of mice in the PQ-treated group were decreased. The results of tail suspension test proved that the mice in the Tau intervention group had a statistically significant decrease in tail suspension time compared with the PQ-treated group (Fig. 4d); the mice in the PQ-treated group had a statistically significant increase in tail suspension time compared with the control group. As shown in Fig. 4e, f, the gait distance of the mice in the Tau intervention group increased significantly compared with the PQ-treated group; compared with the control group, the gait distance of the mice in the PQ-treated group decreased significantly. As seen in Fig. 4g, the pole-climbing time of the mice in the Tau intervention group was significantly reduced compared with the PQ-treated group; compared with the control group, the pole-climbing time of the mice in the PQ-treated group was significantly increased.
Changes of non-motor function and motor function in each group (n = 6). a The moving trajectory map in the open field test; b The moving distance in the open field test; c The residence time in the edge/central area in the open field test; d The immobility time of hanging the tails; e The step length in gait test; f The step distance in gait test; g The time of climbing the pole; The data are presented as mean ± SE. Control: control group; PQ: PQ-treated group; PQ + Tau: Tau intervention group; Tau: Tau control group; a p < 0.05, compared with the control group; b p < 0.05, compared with the PQ-treated group
Effect of Tau on PQ-induced DAc neurons in PD mice
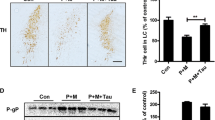
Compared with the PQ-treated group, IHC results demonstrated that TH-positive neurons in the nigrostriatal area of Tau-intervened mice were clearly stained and showed multipolar-shaped, deeply stained cytosol with densely interwoven protrusions, and the number of TH-positive cells in the nigrostriatal area and the amount of TH-positive expression in the striatal area were significantly increased (Fig. 5a). Compared with the control group, most of the neurons in the nigrostriatal area were shrunken and lightly stained, and the number of TH-positive cells was significantly reduced in the PQ-treated group, and the remaining neurons were smaller, with shorter protrusions and fewer branches. The results exposed that the relative protein expression of brain TH and striatal DAT in the Tau intervention group was significantly increased compared with the PQ stained group (Fig. 5b); the relative protein expression of brain TH and striatal DAT in the mice in the dyed group was significantly decreased compared with the control group.
Morphological and protein expression changes of TH in nigrostriatal and striatal DAc neurons in each group (n = 3). a Nigrostriatal and striatal tyrosine hydroxylase (TH) IHC staining (100 ×), and number of nigrostriatal and Striatal TH-positive cells; b Total protein lysates were evaluated by western blot analysis for the expression of DAc neurons markers (TH, DAT). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control for normalization, histogram showed the quantitative evaluation of the protein band by densitometry. SN Substantia nigra, ST Striatum, TH Tyrosine hydroxylase, DAT Dopamine Transporter; The data are presented as mean ± SE. Control: control group; PQ: PQ-treated group; PQ + Tau: Tau intervention group; Tau: Tau control group; a p < 0.05, compared with the control group; b p < 0.05, compared with the PQ-treated group
Effect of Tau on PQ-induced microglia in PD mice
IHC results found that compared with the PQ-treated group, microglia in the nigrostriatal area of Tau-intervened mice were mainly “resting”, branched, with small, elongated cells, elongated protrusions, and sparse distribution (Fig. 6a). There were fewer positive cells. In comparison with the control group, the microglia in the nigrostriatal and striatal regions of PQ-treated mice with ionized calcium-binding adaptor molecule-1 (Iba-1) positive staining were brownish-yellow in color, with enlarged cytosomes, round or oval shape, shortened and thickened protrusions, and some cells were amoeboid and increased in number, showing an obvious "activated state". As illustrated in Fig. 6b, Western blot results further showed that the relative expression of Iba-1 protein in the mice in the Tau intervention group was significantly lower compared with that in the PQ-treated group; compared with that in the control group, the relative expression of Iba-1 protein in the mice in the dyed group was significantly higher.
Morphology and protein expression changes of Iba-1 in nigrostriatal and striatal microglia in each group (n = 3). a Nigrostriatal and striatal ion calcium junction protein molecule-1 (Iba-1) IHC staining (400 ×), and number of nigrostriatal and Striatal TH-positive cells; b Total protein lysates were evaluated by western blot analysis for the expression of microglia markers (Iba-1). GAPDH was used as the internal control for normalization, histogram showed the quantitative evaluation of the protein band by densitometry. SN Substantia nigra, ST Striatum; Iba-1 Ionized calcium-binding adaptor molecule-1; the data are presented as mean ± SE. Control: control group; PQ: PQ-treated group; PQ + Tau: Tau intervention group; Tau: Tau control group; a p < 0.05, compared with the control group; b p < 0.05, compared with the PQ-treated group
Effect of Tau on PQ-induced inflammatory factors in PD mice
Western blot results manifested (Fig. 7) that the relative expressions of inducible nitric oxide synthase (iNOS), interleukin-1β (IL-1β), and high-mobility group box 1 (HMGB1) proteins were significantly lower in the Tau intervention group compared with the PQ-treated group. Compared with the control group, the relative expressions of brain inflammatory factors (iNOS, IL-1β and HMGB1) were significantly increased in the PQ-treated mice.
Changes of brain inflammatory factor protein expression in each group (n = 3). a Total protein lysates were evaluated by western blot analysis for the expression of inflammatory factor (iNOS, IL-1β, HMGB1). GAPDH was used as the internal control for normalization. b Histogram showed the quantitative evaluation of the protein band by densitometry. The data are presented as mean ± SE. control: control group; PQ: PQ-treated group; PQ + Tau: Tau intervention group; Tau: Tau control group; a: p < 0.05, compared with the control group; b: p < 0.05, compared with the PQ-treated group
Effect of Tau on the expression of key proteins of PI3K/AKT signaling pathway in PQ-induced PD mice
Western blot results exposed (Fig. 8) that the relative expression levels of PI3K, 3-Phosphoinositide-dependent protein kinase 1 (PDK-1), and phosphorylated protein kinase B (p-AKT) proteins in the midbrain of Tau-intervened mice were significantly lower than those in the PQ-treated group, and the differences were all statistically significant (p < 0.05). Compared with the control group, the relative expression levels of PI3K, PDK-1 and p-AKT proteins were significantly increased in the midbrain of the PQ-treated mice.
Changes in expression of PI3K/AKT pathway-related proteins in the midbrain of mice in each group (n = 3). a Total protein lysates were evaluated by western blot analysis for the expression of PI3K/AKT pathway-related proteins (p-AKT, AKT, PI3K, PDK-1). GAPDH was used as the internal control for normalization. b Histogram showed the quantitative evaluation of the protein band by densitometry. The data are presented as mean ± SE. control: control group; PQ: PQ-treated group; PQ + Tau: Tau intervention group; Tau: Tau control group; a p < 0.05, compared with the control group; b p < 0.05, compared with the PQ-treated group
Discussion
PD is the second-most common central neurodegenerative disease worldwide characterized by progressive degenerative loss of DAc neurons in the substantia nigra and reduced DA secretion in the striatum (Samii et al. 2004). PQ, as a non-selective herbicide, due to its structural similarity to the recognized DAc neurotoxin MPTP active metabolite MPP + , can highly selectively damage nigrostriatal DAc neurons through the blood–brain barrier and become one of the potential environmental risk factors for sporadic PD (Shimizu et al. 2001). The results of this study found that the body weight of C57BL/6 mice injected intraperitoneally with 15 mg/kg PQ continuously for 8 weeks was not significantly different from that of the control group, indicating that this dose of PQ staining did not produce systemic toxic effects on the mice. Clinically PD is characterized by motor symptoms mainly hypomimia swallowing difficulties, and gait staggering (Jankovic 2008), but what is often overlooked is that motor symptoms are also preceded by non-motor symptoms that are very difficult to detect, such as autonomic dysfunction, cognitive/neurobehavioral abnormalities, sleep disorders, and anosmia (Schapira et al. 2017). We therefore used different neurobehavioral tests to evaluate motor symptoms and non-motor symptoms in mice. We assessed the depressive state of mice by resting immobility time in the tail suspension test, the cognitive function of mice by moving distance and dwell time in the edge/center region in the open field test, and the motor coordination of mice by gait test and pole climbing test. We found neurobehavioral abnormalities in the mice of the PQ-treated group, which showed PD motor dysfunction, such as reduced pole-climbing time and reduced step distance. In addition to classical motor deficits, PD patients develop non-motor symptoms such as psychiatric disorders in the preclinical period (Postuma Ronald 2019). In this study, we also found that mice at 4 weeks of PQ-treated showed varying degrees of non-motor symptom changes in 2 objective behavioral tests, the open field test and the hanging tail test, suggesting that PQ induced depression and cognitive dysfunction in mice. The onset and development of these motor/non-motor symptoms may be attributed to the damage of DAc neurons. We further found by IHC that with the increase of PQ staining time, mice showed progressive degenerative loss of DAc neurons in the substantia nigra region, hypoactive nigrostriatal pathway neurological function, and significantly reduced striatal DA secretion level in a time-effective relationship. This suggests that PQ successfully induced PD pathological features in mice, providing a direct basis for the neurotoxicity of the environmental chemical PQ.
There is increasing evidence that microglia-mediated chronic neuro-inflammation plays a key role in the progression of central neurodegenerative diseases (Sun et al. 2021). Autopsy analysis of PD patients revealed a large number of activated microglia in the nigrostriatal and striatal regions of the midbrain (Hirsch and Hunot 2009), and positron emission tomography imaging showed that the number of microglia activation and inflammatory mediators gradually increased as PD disease worsened (Gerhard et al. 2006). Although in vivo studies have demonstrated that taurine exerts DAc neuroprotective effects by inhibiting microglia-mediated neuro-inflammation, Che et al. showed that taurine was able to inhibit NADPH oxidase activation and the nuclear factor-κB pathway by interfering with the membrane translocation of the cytosolic subunit p47, thereby suppressing microglia M1 polarization and gene expression levels of pro-inflammatory factors (Che et al. 2018). In Wang et al.’s study, mac1 and Src Erk signaling pathways were involved in increased NADPH oxidase expression in hippocampal microglia in a Parkinson’s model mouse, and taurine treatment ameliorated learning memory impairment through a reduction in mac1 (Wang et al. 2021). The present report differs from previous ones that we found that PI3K/AKT signaling pathway is an important signaling pathway that has been identified in recent years and is involved in the regulation of immune responses pathway. In MPTP-induced PD animal models, the PI3K/AKT signaling pathway was shown to be present in microglia and to play an important role in regulating the inflammatory response (Chen et al. 2019). PI3K, as a phosphatidylinositol kinase that phosphorylates the hydroxyl group at the D-3 position of the inositol ring, is a signal transducer downstream of cell surface receptors and a signaling molecule involved in intracellular signal transduction (Jiang et al. 2015). When interacting with growth factor receptors or linker proteins with phosphorylated tyrosine residues can cause dimeric conformational changes and be activated. PI3K activation generates a second messenger PIP3 that binds to the PH region of AKT and PDK1, prompting PDK1 to phosphorylate Thr308 of AKT protein leading to AKT activation (Kilic et al. 2017). AKT is the central link in the PI3K signaling pathway and is an important target unit downstream of PI3K. Activated Akt plays an important role in regulating cell proliferation, differentiation and apoptosis by influencing the activation status of various downstream effector molecules (Zhang et al. 2011).
The results of the present study showed that the expression levels of PI3K, PDK1, and p-AKT signaling pathway-related proteins were increased in the midbrain of the mice in the toxin-infected group, and the microglia in the substantia nigra and striatum were significantly increased in an “activated state”, which paralleled the loss of neurons, and the expression of early inflammatory mediators iNOS, IL-1β, and late expression of early inflammatory mediators iNOS, IL-1β and late inflammatory factor HMGB1 was also increased in the midbrain. It is suggested that microglia-mediated inflammatory responses are involved in PQ-induced progressive damage of DAc neurons in PD mice, and the PI3K/AKT signaling pathway may play a regulatory role.
Tau is a sulfur-containing amino acid present in the free state in interstitial and intracellular fluids and has neuro-modulatory and neuroprotective functions (Wang et al. 2021). It was found that plasma taurine concentration was reduced in PD patients and negatively correlated with motor dysfunction (Zhang et al. 2016). Exogenous supplementation of Tau was able to suppress the gene expression levels of brain pro-inflammatory factors in PD mice (Che et al. 2018) and improve learning memory function in Alzheimer’s disease mice (Santa-María et al. 2007). Tau has also been shown to effectively protect against SH-SY5Y cell damage by rotenone through the inhibition of intracellular oxidative stress in an in vitro PD model (Alkholifi and Albers 2015), revealing a beneficial role of Tau in PD. Our results revealed that both motor and non-motor functions of mice were restored to some extent after 8 weeks of Tau intervention. In addition, the number of DAc neurons in the nigrostriatal area and the level of striatal DA secretion were also significantly increased in the Tau intervention group of mice compared with the PQ-treated group. It is suggested that Tau has a protective effect on PQ-induced neurotoxicity, and the mechanism may be related to the PI3K/AKT pathway and the subsequent microglia activity. After Tau intervention, the protein content of PI3K and PDK-1 and the phosphorylation level of AKT were reduced, and the reduction of microglia and related inflammatory factors expression was parallel to that of microglia. It indicates that Tau may regulate microglia-mediated inflammatory responses through inhibition of the PI3K/AKT pathway in the midbrain of PD mice, thereby reducing DAc neurons damage.
Conclusion
Altogether, this study finds that Tau may inhibit microglia activation and inflammatory factor release by regulating the PI3K/AKT signaling pathway, thereby protecting against progressive damage to nigrostriatal DAc neurons by PQ and effectively ameliorating motor dysfunction and non-motor deficits in PQ-induced PD model mice. It provides a new clue for the prevention and treatment of sporadic PD induced by environmental chemicals PQ.
Availability of data and material
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Abd-Elhakim YM, Ghoneim MH, Ebraheim LLM, Imam TS (2020) Taurine and hesperidin rescues carbon tetrachloride-triggered testicular and kidney damage in rats via modulating oxidative stress and inflammation. Life Sci 254:117782. https://doi.org/10.1016/j.lfs.2020.117782
Alkholifi FK, Albers DS (2015) Attenuation of rotenone toxicity in SY5Y cells by taurine and N-acetyl cysteine alone or in combination. Brain Res 1622:409–413. https://doi.org/10.1016/j.brainres.2015.06.041
Che Y, Hou L, Sun F, Zhang C, Liu X, Piao F, Zhang D, Li H, Wang Q (2018) Taurine protects dopami-nergic neurons in a mouse Parkinson’s disease model through inhibition of microglial M1 polari-zation. Cell Death Dis 9:435. https://doi.org/10.1038/s41419-018-0468-2
Chen Y, Wu T, Li H, Li X, Li Q, Zhu X, Yu M, Kuo SH, Huang F, Wu YC (2019) nDl-3—butylphtha-lide exerts dopaminergic neuroprotection through inhibition of neuroinflammation. Front Aging Neurosci 11:44. https://doi.org/10.3389/fnagi.2019.00044
Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM (2007) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 69(2):223–224. https://doi.org/10.1212/01.wnl.0000247740.47667.03
Dwyer Z, Rudyk C, Farmer K, Beauchamp S, Shail P, Derksen A, Fortin T, Ventura K, Torres C, Ayoub K, Hayley S (2021) Characterizing the protracted neurobiological and neuroana-tomical effects of paraquat in a murine model of Parkinson’s disease. Neurobiol Aging 100:11–21. https://doi.org/10.1016/j.neurobiolaging.2020.11.013
Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ (2006) In vivo imaging of microglial activation with [11C](R)- PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis 21:404–412. https://doi.org/10.1016/j.nbd.2005.08.002
Hatcher JM, Pennell KD, Miller GW (2008) Parkinson’s disease and pesticides: a toxicological pers-pective. Trends Pharmacol Sci 29(6):322–329. https://doi.org/10.1016/j.tips.2008.03.007
Hernández-Benítez R, Pasantes-Morales H, Saldaña IT, Ramos-Mandujano G (2010) Taurine stimu-lates proliferation of mice embryonic cultured neural progenitor cells. J Neurosci Res 88:1673–1681. https://doi.org/10.1002/jnr.22328
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397. https://doi.org/10.1016/S1474-4422(09)70062-6
Huang M, Li Y, Wu K, Yan W, Tian T, Wang Y, Yang H (2019) Paraquat modulates microglia M1/M2 polarization via activation of TLR4-mediated NF-κB signaling pathway. Chem Biol Interact 310:108743. https://doi.org/10.1016/j.cbi.2019.108743
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72(1):101. https://doi.org/10.1152/physrev.1992.72.1.101
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376. https://doi.org/10.1136/jnnp.2007.131045
Jiang T, Hoekstra J, Heng X, Kang W, Ding J, Liu J, Chen S, Zhang J (2015) P2X7 receptor is critical in α-synuclein mediated microglial NADPH oxidase activation. Neurobiol Aging 36:2304–2318. https://doi.org/10.1016/j.neurobiolaging.2015.03.015
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386(9996):896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Kamel F (2013) Paths from pesticides to Parkinson’s. Science 341(6147):722–723. https://doi.org/10.1126/science.1243619
Kilic U, Caglayan AB, Beker MC, Gunal MY, Caglayan B, Yalcin E, Kelestemur T, Gundogdu RZ, Yulug B, Yılmaz B, Kerman BE, Kilic E (2017) Particular phosphorylation of PI3K/Akt on Thr308 via PDK-1 and PTEN mediates melatonin’s neuroprotective activity after focal cerebral ischemia in mice. Redox Biol 12:657–665. https://doi.org/10.1016/j.redox.2017.04.006
Marras C, Beck JC, Bower JH, Roberts E, Ritz B, Ross GW, Abbott RD, Savica R, Van Den Eeden SK, Willis AW, Tanner CM (2018) Prevalence of Parkinson’s disease across North America. NPJ Parkinson’s Dis 4:21. https://doi.org/10.1038/s41531-018-0058-0
Niu X, Zheng S, Liu H, Li S (2018) Protective effects of taurine against inflammation, apoptosis, and oxidative stress in brain injury. Mol Med Rep 18(5):4516–4522. https://doi.org/10.3892/mmr.2018.9465
Postuma Ronald B (2019) Prodromal Parkinson disease: do we miss the signs? Nat Rev Neurol 15(43):7–438. https://doi.org/10.1038/s41582-019-0215-z
Qaradakhi T, Gadanec LK, McSweeney KR, Abraham JR, Apostolopoulos V, Zulli A (2020) The anti-inflammatory effect of taurine on cardiovascular disease. Nutrients 12(9):2847. https://doi.org/10.3390/nu12092847
Qiu T, Pei P, Yao X, Jiang L, Wei S, Wang Z, Bai J, Yang G, Gao N, Yang L, Qi S, Yan R, Liu X, Sun X (2018) Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis 9(10):946. https://doi.org/10.1038/s41419-018-1004-0
Ruotsalainen M, Ahtee L (1996) Intrastriatal taurine increases striatal extracellular dopamine in a tetro-dotoxin-sensitive manner in rats. Neurosci Lett 212(3):175–178. https://doi.org/10.1016/0304-3940(96)12821-4
Samii A, Nutt JG, Ransom BR (2004) Parkinson’s disease. Lancet 363(9423):1783–1793. https://doi.org/10.1016/S0140-6736(04)16305-8
Santa-María I, Hernández F, Moreno FJ, Avila J (2007) Taurine, an inducer for tau polymerization and a weak inhibitor for amyloid-beta-peptide aggregation. Neurosci Lett 429:91–94. https://doi.org/10.1016/j.neulet.2007.09.068
Schapira AHV, Chaudhuri KR, Jenner P (2017) Non-motor features of Parkinson disease. Nat Rev Neurosci 18(7):435–450. https://doi.org/10.1038/nrn.2017.62
Shimizu K, Ohtaki K, Matsubara K, Aoyama K, Uezono T, Saito O, Suno M, Ogawa K, Hayase N, Kimura K, Shiono H (2001) Carrier-mediated processes in blood–brain barrier penetration and neural uptake of paraquat. Brain Res 906(1–2):135–142. https://doi.org/10.1016/s0006-8993(01)02577-x
Sun J, Tian T, Wang Y, Yan W, Zhang B, Wang K, Yang H, Huang M (2021) Paraquat-activated BV-2 microglia induces neuroinflammatory responses in the neuron model through NF-κB signaling pathway. Toxicol in Vitro 72:105076. https://doi.org/10.1016/j.tiv.2021.105076
Vyas P, Vohora D (2017) Phosphoinositide-3-kinases as the novel therapeutic targets for the inflam-matory diseases: current and future perspectives. Curr Drug Targets 18:1622–1640. https://doi.org/10.2174/1389450117666161013115225
Wang K, Shi Y, Liu W, Liu S, Sun MZ (2021) Taurine improves neuron injuries and cognitive impair-ment in a mouse Parkinson’s disease model through inhibition of microglial activation. Neurotoxi-Cology 83:129–136. https://doi.org/10.1016/j.neuro.2021.01.002
Zhang D, Hu X, Qian L, Chen SH, Zhou H, Wilson B, Miller DS, Hong JS (2011) Microglial MAC1 receptor and PI3K are essential in mediating β-amyloid peptide-induced microglial activation and subsequent neurotoxicity. J Neuroin-Flamm 8:3. https://doi.org/10.1186/1742-2094-8-3
Zhang L, Yuan Y, Tong Q, Jiang S, Xu Q, Ding J, Zhang L, Zhang R, Zhang K (2016) Reduced plasma taurine level in Parkinson’s disease: asso-ciation with motor severity and levodopa treatment. Int J Neurosci 126(7):630–636. https://doi.org/10.3109/00207454.2015.1051046
Acknowledgements
The present study was supported by the Ningxia Natural Science Foundation (2020AAC02018), National Natural Science Foundation of China (81560538).
Author information
Authors and Affiliations
Contributions
Study conception and design: KW, MH, BZ; manuscript writing and preparation of figures: TT, KW, BZ; reference search and formatting: BZ, GS; manuscript review: CZ, GL. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All animals were housed in the Experimental Animal Center of Yanhu Campus of Ningxia Medical University and reviewed by the Ethics Committee of Ningxia Medical University (No. 2019-098).
Additional information
Handling editor: S. W. Schaffer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, K., Zhang, B., Tian, T. et al. Taurine protects dopaminergic neurons in paraquat-induced Parkinson’s disease mouse model through PI3K/Akt signaling pathways. Amino Acids 54, 1–11 (2022). https://doi.org/10.1007/s00726-021-03104-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-021-03104-6