Abstract
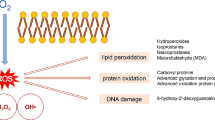
Normobaric hyperoxia (NBO) is applied for treatment of various clinical conditions related to hypoxia, but it can potentially also induce generation of reactive oxygen species, causing cellular damage. In this study, we examined the effects of 60 h NBO treatment on lipid and protein oxidative damage and activity of superoxide dismutase (Mn-SOD) in brain mitochondria of guinea pigs. Despite significant stimulation of Mn-SOD expression and activity the NBO treatment resulted in accumulation of markers of oxidative lesions, including lipid peroxidation (conjugated dienes, thiobarbituric acid reactive substances) and protein modification (bityrosines, adducts with lipid peroxidation products, oxidized thiols). When inhaled O2 was enriched with oxygen cation, O •+2 , the Mn-SOD expression and activity were stimulated to similar extend, but lipid peroxidation and protein oxidation were prevented. These results suggest that long-term NBO treatment causes oxidative stress, but enrichment of inhaled oxygen by oxygen cation can protect the brain again adverse effects of hyperoxia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxygen therapy is based on inhalation of 100% oxygen either at an atmospheric pressure (normobaric oxygen, NBO) or at pressures above atmospheric pressure (hyperbaric oxygen, HBO). HBO and NBO have been successfully applied for the treatment of various diseases and clinical conditions related to hypoxia, including carbon monoxide poisoning, traumatic brain injury and ischemic stroke [1, 2]. The beneficial effects of hyperoxia have been attributed to increased partial pressure of O2, pO2, in plasma and cerebral tissue. Increased tissue oxygenation following brain injury reduces infarction volume, prevents blood–brain barrier disruption, inflammation and edema and improves neurological function. On a molecular level, oxygen treatment leads to alterations in gene expression, inhibition of matrix metalloproteinase-9 and cyclooxygenase-2 activity and reduction of excytotoxic compounds [3, 4]. However, hyperoxia could activate apoptosis [5, 6] and increase the generation of reactive oxygen species (ROS). Although the ROS formation is believed to be a crucial factor of beneficial effects of oxygen therapy [7], excessive generation of toxic ROS may cause oxidative damage to lipids, proteins and DNA. The data on oxygen therapy-related oxidative injury are controversial. While several studies have demonstrated that oxygenation increases oxidative stress, some studies suggest that both HBO and NBO do not produce oxidative damage. In some cases oxygen treatment has even been shown to reduce oxidative stress. Several factors may account for the conflicting results, including the timing and the duration of oxygen therapy [1]. Oxidative effects have mainly been demonstrated in prolonged (greater than 24 h) inhalation of hyperbaric or normobaric oxygenation [1, 8], but could occur already during shorter periods of hyperoxia [9, 10]. Few studies have investigated the effect of antioxidant intake on hyperoxia-induced oxidative damage. Some have shown protective effect of exogenously administered antioxidants [9, 11–13], but other have failed to show prevention [10]. Recently we have reported that NBO-induced oxidative damage to rat heart and lungs is significantly decreased when inhaled O2 is enriched with oxygen cation [14, 15].
The objective of the current study was to: 1) determine whether prolonged inhalation of normobaric oxygen (NBO-O2) causes oxidative stress in brain and 2) evaluate the potential of NBO enriched with positively charged oxygen (NBO-O •+2 ) for attenuation of oxidative damage. We compare the effects of 60 h NBO treatments with and without partially positively ionized oxygen on rat brain mitochondria. To assess oxidative stress, we measured lipid and protein oxidative modifications and the activity of mitochondrial antioxidant enzyme Mn-superoxide dismutase (Mn-SOD). Our results indicate that prolonged NBO treatment is accompanied by significant increase in mitochondrial oxidative damage. We also show that inhalation of partially positively ionized oxygen attenuate NBO-related oxidative stress.
Experimental Procedure
Animals and Normobaric O2 Treatment
Presented experiments conform to the “Guide for the Care and Use of Laboratory Animals” published by The US National Institute of Health (NIH publication no. 85–23, revised 1996), and the ethical guidelines of the Jessenius Faculty of Medicine, Comenius University in Martin.
Male Trik guinea pigs weighing 250–350 g were used for the experiments. The NBO treated animals were placed in a sealed metabolic chamber and exposed to 100% molecular oxygen (NBO-O2) or oxygen enriched with oxygen cation radical (NBO-O •+2 ) for 60 h as described previously [14]. The O2 concentration was monitored periodically by an oxygen analyzer (Permolyt 3, Veb Junkalor, Germany). Partial and unipolar ionization of medical O2 was induced between two electrodes with 15 kV as described previously [16] using plasma chamber Oxygen Ion 3,000 (CStronic GmBH, Austria), which is an European Union certified medical therapeutic device. The control animals were also placed in a metabolic chamber under normal atmospheric conditions.
Preparation of Tissue Homogenate and Isolation of Mitochondria
Tissue homogenates were prepared from whole cerebrum of guinea pigs as described previously [17]. Dissected tissues were homogenized in 10 volumes of ice-cold solution containing 0.32 M sucrose, 5 mM HEPES (pH 7.4), 1 mM EDTA and 0.3 mM phenylmethylsulfonyl fluoride using the Teflon-glass Potter homogenizer.
Mitochondria were isolated from homogenates by differential centrifugation according to the method of Dodd et al. [18]. The homogenate was centrifuged at 1,000×g for 10 min at 4°C. The resulting supernatant was fractionated by sucrose density gradient centrifugation at 100,000×g for 20 min. The resulting mitochondrial fraction was resuspended in 0.32 M sucrose solution and stored at −70°C for further experiments. The purity of the mitochondrial fraction was estimated by enzyme assays (succinate dehydrogenase, lactate dehydrogenase) in homogenates and isolated fractions.
Measurement of Lipid Peroxidation
Lipid peroxidation was measured according to the formation of conjugated dienes and thiobarbituric acid reactive substances (TBARS). Conjugated dienes were estimated as described previously [19] from the absorbance ratio A233nm/A215nm of mitochondria dispersed in 10 mM phosphate buffer (pH 7.1) containing 1% Lubrol.
Samples (50 μl) for determination of TBARS were mixed with 1 ml of 14% trichloroacetic acid and 1 ml of 0.67% thiobarbituric acid and were heated for 30 min at 80°C. After cooling on ice the samples were centrifuged at 2,000×g and the absorbance of supernatants were measured at 535 nm. The concentration of TBARS was calculated using a molar extinction coefficient of 1.56 × 105 M−1 cm−1.
Measurement of Total Thiol Group Content
Total thiol group content in brain mitochondria was determined spectrophotometrically as described previously [19]. Samples were incubated in medium containing 30 mM imidazole (pH 7.4), 5 mM EDTA and 0.4 mM 2,2-dithiobisnitrobenzoic acid (DTNB). After 10 min incubation at room temperature the sample absorbance at 412 nm was measured. The –SH group content was calculated using molar absorption coefficient of 13,600 M−1 cm−1 after subtraction of blank absorbance from absorbance of sample.
Fluorescence Studies on Protein Oxidation
The steady-state fluorescence measurements were performed in samples containing 0.05 mg protein per ml, 10 mM HEPES (pH 7.0), 100 mM KCl at room temperature on a PerkinElmer LS 55 spectrophotofluorimeter as described previously [19].
Emission spectra of bityrosine, a product of tyrosine oxidation, were recorded in range 380–440 nm (10 nm slit width) at excitation wavelength 325 nm (10 nm slit width).
Emission spectra (425–480 nm, 10 nm slit width) of protein conjugates with lipid peroxidation products were recorded at excitation 365 nm (10 nm slit width).
Determination of Mn-SOD and Succinate Dehydrogenase Activities
Mn-SOD activity of brain mitochondria was determined according to the inhibition of nitroblue tetrazolium (NBT) by superoxide anion produced by xanthine oxidase. The reaction mixture contained 0.05 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, 0.025 mM NBT and 0.1 mM xanthine. The absorbance change was monitored at 550 nm against a control. One unit of SOD activity is defined as the amount of enzyme causing 50% inhibition of NBT reduction [20].
Succinate dehydrogenase activity was determined according to Powell and Jackson [21] from the rate of 2,6-dichlorophenolindophenol (DCIP) reduction at 600 nm (ε = 21 mM−1cm−1). Mitochondria (0.15 mg protein per ml) were incubated at 30°C in medium containing 0.2 M KH2PO4 (pH 7.6), 0.1 M NaCN, 0.02 M phenazine methosulfate, 0.5 M succinate and 1 mM DCIP.
Western Blotting
Mitochondrial proteins were separated by 10% SDS–PAGE using a Mini-Protean electrophoresis cell (BioRad Laboratories, Hercules, USA) and then transferred into Immobilon-P transfer membranes (Millipore, Bedford, USA) using Mini Trans-Blot cell (BioRad Laboratories). Western blots were dried overnight and incubated in Tris-buffered saline (20 mM Tris/HCl and 0.05% Tween-20) containing 5% non-fat dry milk. The blots were then incubated 1 h with mouse monoclonal anti-SOD-2 (A2) antibody (Santa Cruz Biotechnology) in Tris-buffered saline (1:500). After removal of the primary antibody the blots were incubated 1 h with secondary HRP-conjugated anti-goat secondary antibody (Santa Cruz Biotechnology, 1:1,000).
The content of subunit A of SDH was detected after 2 h incubation with mouse monoclonal anti-SDHA (2E3) antibody (Santa Cruz Biotechnology) in Tris-buffered saline (1:500) followed by 1 h incubation with secondary HRP-conjugated anti-mouse secondary antibody (Santa Cruz Biotechnology, 1:1,000). Then immunoreactive proteins were visualized using the SuperSignal West Pico Chemiluminescent Substrate (Pierce) solution. Chemifluorescent signal was detected by an optical scanner Chemidoc XRS (BioRad Laboratories) and analysed using GeneTools software (SynGene).
Data Analysis
The results are presented as mean ± SEM or mean ± SD. One-way analysis of variance was first carried out to test for differences among groups. Between individual groups comparisons were made using an unpaired t-test. A value of P < 0.05 was considered to be statistically significant.
Results
Our study on prolonged NBO treatment was not designed as behavioral experiment. Nevertheless, the animals were under the inspection of the laboratory staff for the whole period of NBO treatment. From laboratory recordings it can be clearly seen that animals inhaling O2 enriched with oxygen cation were much more agile and active than animals of the other groups.
Lipid Peroxidation
The extent of LPO in mitochondria was assessed by determination of conjugated dienes and TBARS. The 60-hour inhalation of molecular oxygen (NBO-O2) was associated with significant accumulation of both markers of LPO (Table 1). In contrast, the NBO-O •+2 treatment prevented LPO, the levels of conjugated dienes and TBARS were not different from those determined in brain mitochondria of guinea pigs which inhaled atmospheric air (control group).
Protein Oxidative Lesions
To test the effect of LPO on mitochondrial proteins, we measured the fluorescence emission spectra corresponding to adduct formed from proteins and LPO end-products. As shown in Fig. 1a, NBO-O2 treatment resulted in a significant rise of protein-LPO adducts, but in NBO-O •+2 treated animals the level of adducts was even lower than in control group (P < 0.05).
Protein lesions were also assessed by the measurements of bityrosines and thiol group content. After NBO-O2 treatment the bityrosine content was significantly elevated when compared to control. After NBO-O •+2 treatment the bityrosine content was similar to that of control (Fig. 1b). In NBO-O2 treated animals the total thiol group content decreased to 73 ± 21% of control (P < 0.05) (Table 1). In NBO-O •+2 treated group thiol content also decreased, but the change was not significant when compared to control.
Activities and Protein Contents of Mn-SOD and Succinate Dehydrogenase
As shown in Fig. 2a, both, NBO-O2 and NBO-O •+2 treatments resulted in significant increase of mitochondrial Mn-SOD activity (by 33 ± 7 and 25 ± 2%, respectively). To determine whether increased activity is associated with altered expression of enzyme, Mn-SOD protein content was evaluated by Western immunoblotting. As shown in Fig. 3, there were significant increases in enzyme content in both NBO-O2 and NBO-O •+2 treated animals (by 106 ± 13 and 98 ± 12%, respectively).
Both oxygenation treatments stimulated also succinate dehydrogenase activity (Fig. 2b). After NBO-O2 treatment the activity increased by 23 ± 4% (P < 0.01) and after NBO-O •+2 treatment by 30 ± 3% (P < 0.01). However, we found significantly elevated content of SDH subunit A only in brain of NBO-O2 treated guinea pigs. In NBO-O •+2 treated animals the level of SDHA protein was similar to that in air-treated animals (Fig. 4).
Discussion
Although oxygenation therapy has been widely applied in the treatment of variety of conditions its mechanism is not fully understood. It has been suggested that beneficial effect of oxygenation treatment is at least partially related to ROS formation [7, 22]. On the other hand, ROS are thought to be major mediators of HBO/NBO-related tissue injury. The oxidative effects of oxygenation treatment have been investigated in variety of experimental models. Reported findings are controversial, ranging from elevation to suppression of oxidative stress. In the present study, prolonged NBO was chosen as a model for the investigation of brain oxidative stress. Our results show that 60 h inhalation of normobaric oxygen resulted in accumulation of oxidatively damaged lipids and proteins and stimulation of Mn-SOD and succinate dehydrogenase activities in brain mitochondria of guinea pigs. When inhaled O2 was enriched with oxygen cation radical, O •+2 , lipid peroxidation and protein oxidation were prevented, although the enzyme activities were similarly stimulated. Several experimental studies have demonstrated accumulation of tissue oxidative damage after HBO [13, 23–25], but only few have investigated the effects of NBO. Studies in which effects of short periods (1–2 h) of NBO were investigated show that hyperoxia does not increase oxidative stress and/or affects antioxidant activity [26, 27]. Moreover, short-term NBO treatment may even attenuate oxidative stress, since it was shown to inhibit NADPH oxidase, an important source of superoxide radicals, in experimental model of stroke [28]. On the other hand, studies which examined the effects of NBO treatments of longer durations suggest that hyperoxia produces oxidative stress and affects antioxidant defense systems. NBO treatments for 12, 36 or 48 h were associated with accumulation of fluorescent chromolipids in brain and other tissues of rats [29]. Using the protein thiol-specific spin label MAL-6, Hensley et al. [8] have shown cumulative protein oxidation in synaptosomes of aged gerbils (15–17 months) exposed to NBO for 0–48 h periods. In young gerbils (3–4 months) protein oxidative damage increased within the first 24 h of NBO, but then declined between 24 and 48 h. Authors suggest that attenuation of protein oxidative damage at prolonged treatments is related to up-regulation of antioxidant defense systems. Present and previously published studies [30] support this view showing increased content and activity of SOD in rodent brain after NBO treatment. However, our study suggests that the upregulation of Mn-SOD is unable to eliminate mitochondrial oxidative damage caused by 60 h NBO treatment. Accumulation of oxidized proteins in brain could be related to a functional impairment of proteasome, which is responsible for removal of oxidatively damaged proteins. Since proteasome activity was not measured and proteasome inhibitors were not applied in our studies we cannot exclude that increased protein oxidative damage is secondary response to NBO-induced dysfunction of this multicatalytic complex.
Besides other subcellular structures, the mitochondria are particularly sensitive to oxidative damage because of the oxygen-dependent energetic metabolism, which is a major cellular source of ROS. Production of hydrogen peroxide in heart and liver mitochondria progressively increased with the increase in the partial pressure of oxygen [31]. Although complexes I and III of the respiratory chain are thought to be the main sites of ROS production, some studies suggest that complex II (succinate dehydrogenase) may significantly contribute to ROS formation in cells [32, 33]. Succinate dehydrogenase (SDH) is the only enzyme involved in both the respiratory chain and the citric acid cycle. Thus, increased SDH activity after 60 h inhalation of normobaric oxygen might cause stimulation of both the energetic metabolism and SDH-mediated production of ROS. Although NBO-O2 treatment stimulates also the activity of main mitochondrial antioxidant enzyme, Mn-SOD, this mechanism appears to be inadequate to prevent oxidative damage. When guinea pigs inhaled O2 enriched with oxygen cation radical the activities of SOD and SDH were similarly stimulated as by the NBO-O2 treatment, nevertheless, the lipid and protein oxidation were almost completely prevented. These findings suggest that NBO-O •+2 treatment is involved with additional protection. The precise mechanism whereby NBO-O •+2 attenuates oxidative damage remains to be elucidated. One possible explanation could be that electron deficient oxygen cation preferentially interacts with generated ROS and prevents their interactions with cellular proteins and lipids. The protective mechanism may also include attenuation of ROS generation in brain. Recently, inhibition of NADPH oxidase was shown to contribute to neuroprotective effect of NBO in acute ischemic stroke [34]. Another possible explanation is that NBO-O •+2 treatment stimulates not only Mn-SOD, but also other antioxidants which were not investigated in the present study. This possibility requires further investigations in next studies. Protective effect of NBO-O •+2 treatment is also supported by Western blotting studies on SDH protein content. SDH activities expressed per relative SDHA protein content revealed opposite effects of NBO-O2 and NBO-O •+2 treatment. In NBO-O2 treated group the activity per SDHA was about 74% of that in control group, despite the fact that activity expressed per mg of total protein was increased (Fig. 2b). This data indicates that NBO-O2 treatment is associated with substantial lesion and inhibition of SDH. In contrast, after NBO-O •+2 treatment the activity per SDHA was about 135% of that in control group. An increased SDH activity, observed at unchanged SDH protein content, suggests that NBO-O •+2 treatment not only preserves but improves structural integrity and catalytical activity of this membrane located enzyme.
In conclusion, the results of this study indicate that long-term normobaric hyperoxia causes mitochondrial oxidative stress in guinea pig brain. The damaging effects of treatment are considerably attenuated when inhaled O2 is enriched with oxygen cation. Further studies are needed to clarify the protective mechanisms of NBO-O •+2 treatment and to ascertain the clinical relevance of these findings.
References
Singhal AB (2007) A review of oxygen therapy in ischemic stroke. Neurol Res 29:173–183
Kumaria A, Tolias CM (2009) Normobaric hyperoxia therapy for traumatic brain injury and stroke: a review. Br J Neurosurg 26:576–584
Chen Y, Nadi NS, Chavko M et al (2009) Microarray analysis of gene expression in rat cortical neurons exposed to hyperbaric air and oxygen. Neurochem Res 34:1047–1056
Matchett GA, Martin RD, Zhang JH (2009) Hyperbaric oxygen therapy and cerebral ischemia: neuroprotective mechanisms. Neurol Res 31:114–121
Brutus NA, Hanley S, Ashraf QM et al (2009) Effect of hyperoxia on serine phosphorylation of apoptotic proteins in mitochondrial membranes of the cerebral cortex of newborn piglets. Neurochem Res 34:1219–1225
Mudduluru M, Zubrow AB, Ashraf QM et al (2010) Tyrosine phosphorylation of apoptotic proteins during hyperoxia in mitochondria of the cerebral cortex of newborn piglets Neurochem Res 35:1003–1009
Thom SR (2009) Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol 106:988–995
Henseley K, Howard BJ, Carney JM et al (1995) Membrane protein alterations in rodent erythrocytes and synaptosomes due to aging and hyperoxia. Biochim Biophys Acta 1270:203–206
Dundar K, Topal T, Ay H et al (2005) Protective effects of exogenously administrated or endogenously produced melatonin on hyperbaric oxygen-induced oxidative stress in the rat brain. Clin Exp Pharmacol Physiol 32:926–930
Bader N, Bosy-Westphal A, Koch A et al (2007) Effect of hyperbaric oxygen and vitamin C and E supplementation on biomarkers of oxidative stress in healthy men. Br J Nutr 98:826–833
Brozmanova M, Plevkova J, Bartos V et al (2008) The interaction of dietary antioxidant vitamins and oxidative stress on cough reflex in guinea-pigs after long term oxygen therapy J Physiol Pharmacol 57:45–54
Calderón Guzmán D, Trujillo Jiménez F, Hernández García E et al (2007) Assessment of antioxidant effect of 2, 5-dihydroxybenzoic acid and vitamin A in brains of rats with induced hyperoxia. Neurochem Res 32:1036–1040
Mollaoglu H, Topal T, Ozler M et al (2007) Antioxidant effects of melatonin in rats during chronic exposure to hyperbaric oxygen. J Pineal Res 42:50–54
Calkovska A, Engler I, Mokra D et al (2008) Differences in oxidative status, lung function, and pulmonary surfactant during long-term inhalation of medical oxygen and partially ionized oxygen in guinea pigs. J Physiol Pharmacol 59:173–181
Kaplan P, Tatarkova Z, Engler I et al (2009) Effects of long-term oxygenation treatments on α-ketoglutarate dehydrogenase activity and oxidative modifications in mitochondria of guinea pig heart. Eur J Med Res 14:116–120
Engler I, Atzmüeller C, Donic V et al (2009) Reactive oxygen species, especially O +•2 in cancer mechanisms. J Exp Therapeut Oncol 8(2):157–165
Babusikova E, Hatok J, Dobrota D et al (2007) Age-related oxidative modifications of proteins and lipids in rat brain. Neurochem Res 32:1351–1356
Dodd PR, Hardy JA, Oakley AE (1981) A rapid method for preparing synaptosomes: comparison with alternative procedures. Brain Res 226:107–118
Kaplan P, Babusikova E, Lehotsky J et al (2003) Free radical-induced protein modification and inhibition of Ca2+-ATPase of cardiac sarcoplasmic reticulum. Mol Cell Biochem 248:41–47
Gonzales R, Auclair C, Voisin E et al (1984) Superoxide dismutase, catalase, and glutathione peroxidase in red blood cells from patients with malignant diseases. Cancer Res 44:4137–4139
Powell CS, Jackson RM (2003) Mitochondrial complex I, aconitase, and succinate dehydrogenase during hypoxia-reoxygenation: Modulation of enzyme activities by MnSOD. Am J Physiol Lung Cell Mol Physiol 285:L189–L198
Hink J, Jansen E (2001) Are superoxide and/or hydrogen peroxide responsible for some of the beneficial effects of hyperbaric oxygen therapy? Med Hypotheses 57:764–769
Ay H, Topal T, Özler M et al (2007) Persistence of hyperbaric oxygen-induced oxidative effects after exposure in rat brain cortex tissue. Life Sci 80:2025–2029
Nemoto EM, Betterman K (2007) Basic physiology of hyperbaric oxygen in brain. Neurol Res 29:116–126
Xue L, Yu Q, Zhang H et al (2008) Effect of large dose hyperbaric oxagenation therapy on prognosis and oxidative stress of acute pemanet cerebral ischemic stroke in rats. Neurol Res 30:389–393
Singhal AB, Wang X, Sumii T et al (2002) Effect of normobaric hyperoxia in a rat moedl of focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 22:861–868
Puccio AM, Hoffman LA, Bayir H et al (2009) Effect of short periods of normobaric hyperoxia on local brain tissue oxygenation and cerebrospinal fluid oxidative stress markers in severe traumatic brain injury. J Neurotrauma 26:1241–1249
Liu W, Sood R, Chen Q et al (2008) Normobaric hyperoxia inhibits NADPH oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke. J Neurochem 107:1196–1205
Ahotupa M, Mantyla E, Peltola V et al (1992) Pro-oxidant effects of normobaric hyperoxia in rat tissues. Acta Physiol Scand 145:151–157
Bigdeli MR, Rahnema M (2009) The effect of normobaric hyperoxia on superoxide dismutase activity and neurological deficits in an animal model of Huntington disease. Physiol Pharmacol 13:18–27
Boveris A, Chance B (1973) The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134:707–716
McLennan HR, Esposti MD (2000) The contribution of mitochondrial respiratory complexes to the production of reactive oxygen species. J. Bioenerg Biomembr 32:153–162
Lenaz G (2002) The mitochondrial production of reactive oxygen species: Mechanisms and implications in human pathology. IUBMB Life 52:159–164
Tang X, Liu KJ, Ramu J et al (2010) Inhibition of gp91phox contributes towards normobaric hyperoxia afforded neuroprotection in focal cerebral ischemia. Brain Res 1348:174–180
Acknowledgments
This work was partially supported by grants VEGA 1/0028/11 and VVCE APVV 0064/07 from the Ministry of Education and Science of the Slovak Republic and project “Identification of Novel Markers in Diagnostic Panel of Neurological Diseases”, code:26220220114 co-financed from EC sources and European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tatarkova, Z., Engler, I., Calkovska, A. et al. Effect of Long-Term Normobaric Hyperoxia on Oxidative Stress in Mitochondria of the Guinea Pig Brain. Neurochem Res 36, 1475–1481 (2011). https://doi.org/10.1007/s11064-011-0473-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0473-7