Abstract
The naturally occurring toxin rottlerin has been used by other laboratories as a specific inhibitor of protein kinase C-delta (PKC-δ) to obtain evidence that the activity-dependent distribution of glutamate transporter GLAST is regulated by PKC-δ mediated phosphorylation. Using immunofluorescence labelling for GLAST and deconvolution microscopy we have observed that d-aspartate-induced redistribution of GLAST towards the plasma membranes of cultured astrocytes was abolished by rottlerin. In brain tissue in vitro, rottlerin reduced apparent activity of (Na+, K+)-dependent ATPase (Na+, K+-ATPase) and increased oxygen consumption in accordance with its known activity as an uncoupler of oxidative phosphorylation (“metabolic poison”). Rottlerin also inhibited Na+, K+-ATPase in cultured astrocytes. As the glutamate transport critically depends on energy metabolism and on the activity of Na+, K+-ATPase in particular, we suggest that the metabolic toxicity of rottlerin and/or the decreased activity of the Na+, K+-ATPase could explain both the glutamate transport inhibition and altered GLAST distribution caused by rottlerin even without any involvement of PKC-δ-catalysed phosphorylation in the process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunocytochemical studies using rat retina in vitro have demonstrated that uptake of the non-metabolisable glutamate transport substrate d-aspartate by GLAST (glutamate/aspartate transporter, see [1, 2] for reviews and nomenclature) was reduced or abolished in the presence of protein kinase C (PKC) inhibitors, in particular when a putative PKC-δ specific inhibitor rottlerin [3] was applied [4]. These findings appeared to be consistent with the suggestion that GLAST is subject to regulation by PKC-δ [5]. However, results of subsequent experiments using astrocytes cultured from neonatal rat brain have indicated that rottlerin could inhibit the activity of GLAST by a PKC-δ-independent mechanism [6]. These studies have not identified the exact nature of the hypothetical GLAST-regulating rottlerin-sensitive system despite performing a range of tests [6]. One mechanism, through which rottlerin could interfere with glutamate transport independently of its effect on PKC-δ has not, however, been directly examined [6] namely by changing the activity of (Na+, K+)-dependent ATPase that generates ionic gradients providing the free energy to drive glutamate transport [1, 2].
Many studies have shown that glutamate transporters, including GLAST, can actively translocate l-glutamate, l-aspartate or d-aspartate into the cells in the presence of adequate transmembrane Na+ and K+ concentration gradients (for reviews and additional references see [1, 2, 7]). Moreover, GLAST transporter appears to be activity regulated, i.e. in the presence of ligands for the glutamate recognition site, especially when these are readily transportable substrates such as d-aspartate, GLAST molecules tend to change their cellular distribution by moving into plasma membrane thereby increasing the transport capacity [8] (for reviews see [5, 9, 10]). The necessary ionic gradients have to be maintained by the (Na+, K+)-dependent ATPase and, indeed, the transport of l-glutamate and l-aspartate is known to be strongly inhibited by the (Na+, K+)-dependent ATPase inhibitor ouabain [11, 12]. Furthermore, it has been claimed that there is a close functional relationship between glutamate transport and the activity of (Na+, K+)-dependent ATPase in cultured astrocytes [10] that may parallel the apparent co-localization of glutamate transporters and a subtype of (Na+, K+)-dependent ATPase in brain tissue [13]. Such a relationship could be important for the regulation of energy supply in response to changes in excitatory (glutamatergic) synaptic activity [14, 15] and for the activity of metabolic pathways that are linked to glutamatergic neurotransmission and/or to functioning glutamate transport [16–18]. Testing the effect of rottlerin on the activity of the (Na+, K+)-dependent ATPase could, therefore, not only help to elucidate the postulated PKC-δ independent mechanism of the rottlerin inhibition of glutamate transport but might also be of broader significance e.g. when considering rottlerin as a pharmacological tool in studies of brain metabolism or neuronal cell death [19, 20].
Experimental Procedures
Cultured Astrocytes
Cultured astrocytes were prepared from neocortices of Sprague-Dawley rat pups (0–3 days post natum) as described in detail in a related study [8]. Briefly, the tissue was dissociated with trypsin (0.25% in Hanks balanced salt solution) and the cells were seeded, using Dulbecco modified Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum (FBS), into 25 cm [2] flasks (usually 2–3 neonatal brains per flask) and grown until confluent (10–14 days). The cultures were subsequently passaged into 30 mm tissue culture dishes and grown for 12–14 days, again using DMEM with 10% FBS. At this time cells displayed predominantly astrocytic morphology [21]. For the purposes of immunocytochemical studies the cells were grown in the same way with a 13 mm diameter coverslip present in the culture dishes.
All procedures were carried out in accordance with the guidelines of the National Health and Medical Research Council of Australia and were approved by The University of Sydney Animal Ethics Committee.
Immunocytochemistry and Image Analysis
The procedure has been described in detail elsewhere [8]. The coverslips with astrocytes were washed in serum-free DMEM (sfDMEM) and incubated in the presence of d-aspartate and/or rottlerin dissolved in 500 μl of sfDMEM. Incubations lasted for 30 min and were carried out at 37°C in 5% CO2. Drug-free sfDMEM was used as a control. The rottlerin solution contained 0.05% dimethylsulphoxide (DMSO). Such a concentration of DMSO should have no effect on either the cell vialbility [22] or the distribution of glutamate transporters [6].
Following the exposure to d-aspartate and/or rottlerin the astrocytes were double-labelled with antibodies against glial fibrillary acidic protein (GFAP, marker for astrocytes, mouse monoclonal antibodies) and antibodies against the glutamate transporter GLAST (polyclonal antibody raised in rabbit [23]). The antibodies were diluted in phosphate buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 0.05% saponin. The coverslips were first washed in 2 ml of PBS for 5 min, then fixed with paraformaldehyde (2% in PBS) for 10 min and again washed with 2 ml of PBS for 5 min. Fixed cells were then “blocked” with the BSA-containing PBS (BSA/PBS) for 30 min and subsequently exposed to 200 μl of solutions containing the antibodies at dilutions of 1:4,000 (GLAST) and 1:400 (GFAP). The incubations (2 h) were carried out at room temperature in a humidified environment. The coverslips were then washed three times for 5 min with 2 ml of the BSA/PBS solution and exposed to the secondary antibodies.
All procedures involving the fluorescently labelled antibodies were performed in a dark environment. Goat-generated anti-mouse IgG conjugated to Alexa Fluor 488 (AF 488) was used to visualise GFAP while goat-generated anti-rabbit IgG conjugated to Alexa Fluor 594 (AF 594) was used to label the anti-GLAST antibody. Both secondary antibodies were diluted in the BSA/PBS solution; incubations were for 1 h at room temperature and washed as described above for the primary antibodies; three times for 5 min with 2 ml of BSA/PBS.
After washing, the coverslips were carefully blotted to remove excess moisture, mounted upside down, using 50% solution of glycerol in PBS, on slides and secured along the edges with nail polish, to prevent drying out.
Deconvolution Microscopy and Image Analysis
Deconvolution microscope (Axioplan 2, Zeiss) was used for image acquisition [8]. AF 488 and AF 594 were excited at 499 nm (emission at 520 nm) and 590 (emission at 618 nm), respectively. The images were optically sectioned at 0.513 μm intervals and subjected to deconvolution using an inverse filter algorithm to remove out-of-focus (background) signals. Sections from the midplane of the stacks were used for the image analyses. Each image represented a randomly selected single cell.
The images were analysed as described in detail elsewhere [8]. Mean fluorescence density (MFD) was determined in both the cytoplasm and membrane (cMFD and mMFD) and the membrane/cytoplasm ratio of fluorescent intensity (RFI = mMFD/cMFD) was used as an index of the distribution of GLAST between membrane and cytoplasm (Fig. 1).
Inhibition of d-aspartate-induced redistribution of GLAST by rottlerin. The cultured astrocytes were labelled for GFAP (antibody dilution 1:400, incubated for 2 h, followed by a 1 h incubation with an AF 488 conjugated secondary anti-mouse antibody, green) and GLAST (dilution 1:4,000, incubated for 2 h, followed by a 1 h incubation with an AF 594 conjugated secondary anti-rabbit antibody, red). The distribution of GLAST between the membrane and cytoplasm was estimated in a group of control cells (exposed for 30 min to a solution containing neither d-aspartate nor rottlerin, example: a; mean ± SD, n = 20, in d), in cells incubated in the presence of 500 μM d-aspartate (example: b; mean ± SD, n = 11 in d), in the presence of both 500 μM d-aspartate and 50 μM rottlerin (example: c; mean ± SD, n = 9 in d) or in the presence of 50 μM rottlerin alone (mean ± SD, n = 10 in d, cf. also [39]). GLAST distribution in the presence of d-aspartate is different from control (*** P < 0.001). In the presence of rottlerin, whether with or without d-aspartate, GLAST distribution is not different from controls but it differs (P < 0.001) from that obtained with d-aspartate alone; ANOVA, Newman-Keuls test, GrahPad Prism). All cells were selected at random and the experiment was repeated three times with different batches of cells, producing the same result. Some of the results shown here have been presented as abstracts [41, 42]. Scale bar is 20 μm. For interpretation of the references to color in this figure legend, the reader is referred to the online version of this article
Activity of Na+, K+-Dependent ATPase
We have used a technique that employs uptake of Rb+ as a measure of the activity of (Na+, K+)-dependent ATPase in rat brain tissue. The methodology has been modified from that which has been previously used to study uptake of radiolabelled amino acids [11, 24]. Prisms of adult (3–6 months) rat cerebral cortex (0.1 × 0.1 × thickness of cortex) were prepared using the McIlwain tissue chopper, suspended at 25 mg/10 ml of incubation medium (phosphate buffered Krebs-Ringer [16]) and allowed 15 min for recovery in a shaking water bath at 37°C. Uptake of Rb+ was started by adding either 250 or 500 μl of the medium in which 5 mM KCl was replaced by 5 mM RbCl. Doubling the concentration of Rb+ (legends of Figs. 2, 3) doubled the value of controls (Figs. 2, 3) but would not change the activity of (Na+, K+)-dependent ATPase since, in this type of studies Rb+ acts only as a “marker” of K+ in medium [24]. Incubation was terminated 10 min later by rapid filtration (assisted by negative-pressure at ~20 psi) through Whatman No. 1 filters (2.5 cm in diameter) and washing the filters twice with 2 ml of RbCl-free medium. The filters were then extracted overnight with 1.5 ml of deionized water and the concentration of Rb+ in the extracts estimated using acetylene-flame atomic absorption spectroscopy (AAS).
Effect of rottlerin on Rb+ uptake by prisms of rat cortex. Conditions: 25 mg tissue/10 ml, 15 min preincubation/10 min incubation; 0.25 mM Rb+, 37°C, rottlerin concentrations 5 and 50 μM. The data are expressed as ng of Rb+ in the aqueous extract obtained from the tissue prisms trapped on the filters at the end of the experiment; mean ± SD of eight (control) or four (rottlerin and blank) incubations, respectively. Controls and blanks contained 0.1% of DMSO. *** different from control at P < 0.001 by ANOVA using Newman-Keuls test
Effect of metabolic inhibitors on Rb+ uptake by prisms of rat cortex. The concentration of Rb+ was 0.125 mM, For further details and full chemical names of the inhibitors FCCP and CCCP see legend of Fig. 1 and the sections on “Experimental Procedures” in the text
The methodology used to study Rb+ uptake by cultured astrocytes was also derived from previously published techniques used in amino acid uptake studies [25]. Immediately prior to the experiment, the medium was removed and the tissue culture dishes were washed twice with 1 ml of the buffer of the same composition as that used in the experiments with prisms of brain cortex. The monolayers were then covered with 1.8 ml of the buffer (with or without rottlerin), transferred to a water bath at 37°C and rotated (48 rpm) to allow for gentle stirring. After a 10-min preincubation period 0.2 ml of buffer was added in which 5 mM KCl was replaced with 5 mM RbCl. Incubation was terminated by rapid removal, using vacuum suction, of the Rb+-containing medium, washing with 1 ml of Rb+-free buffer (twice, at room temperature) and extraction, for at least 1 h, with 1.5 ml of deionized (RO) water. Taking care that all cells were detached from the tissue culture dish, the suspension was triturated with an automatic pipette and centrifuged at app. 10,000g for 15 min using a refrigerated bench centrifuge. Samples of 1 ml were taken for determination of Rb+ by AAS while the rest, including the pellet, were mixed with 200 mM NaOH, left at room temperature for 24 h and used to estimate the protein by Lowry technique. AAS was carried out as described previously [24] using SpectrAA-20plus (Varian Aust P/L, Melbourne, Victoria, Australia) and Photron Rb+ lamp (λ = 780.2 mm, slit width 0.2 mm).
Oxygen Consumption by Brain Tissue in Vitro
Respiration rate in cortical prisms was measured using an oxygen electrode (Rank Brothers, Cambridge, UK). The tissue prisms were preincubated (100 mg/10 ml of freshly oxygenated buffer) in a shaking water bath at 37°C for 15–30 min. Drugs were then added, and incubation continued for a further 5 min. An aliquot (5 ml) of the suspension was transferred into the electrode chamber (at 37°C) and the oxygen levels were recorded every 15 s for 10 min.
Preparation of Solutions
Rottlerin (1-[6-[(3-acetyl-2,4,6-trihydroxy-5-methylphenyl)methyl]-5,7-dihydroxy-2,2-dimethyl-2H-1-benzopyran-8-yl]-3-phenyl-2-propen-1-one) is listed as poorly soluble in aqueous media. In the present studies rottlerin was first dissolved at 50 mM in a small amount (usually 100 μl) of DMSO. This solution was then diluted with nine volumes of the incubation medium to 5 mM. In Rb+ uptake studies and in the oxygen consumption (respiration) assays, volumes of either 10 or 100 μl were added at the beginning of the preincubation (or 5 min prior to the transfer into the electrode chamber) to each flask containing the suspension of brain prisms thus achieving final drug concentrations of 5 and 50 μM (and DMSO concentrations 0.01 and 0.1%, respectively). In the experiments with cultured astrocytes, 20 μl of the 5 mM solution were added at the beginning of the preincubation.
Staurosporin, BIS-I, olomoucin, wortmannin, FCCP and CCCP were handled in the similar fashion to achieve the desired final concentrations. After the initial dilution from 50 mM solution in pure DMSO to 5 mM in 10% DMSO, some drugs, including rottlerin, produced apparently homogenous but opaque solutions. When added (1 in 1,000 or 1 in 100) to the incubation mixture (at 37°C) no opalescence or any other traces of solid matter were noticed and the drugs were considered fully dissolved. Controls (drug-free preparation) as well as blanks (preincubated as the other flasks but filtered immediately after the addition of Rb+) were prepared so as to match 0.1% DMSO introduced with the drugs. It was established in a separate set of experiments that 0.1 and 1% DMSO had no effect on the activity of (Na+, K+)-dependent ATPase (cf. also [26–28]).
Statistical Analyses
Statistical analysis and line fitting was performed by GraphPad Prism (San Diego, Ca USA). The same software was used to prepare the Figs. 2, 3, 4, 5.
Oxygen consumption by prisms of rat cerebral cortex in the presence of drugs. Top panel: Data are single readings taken every 15 s and expressed as % of original value at t = 0 (immediately after the transfer of tissue into the chamber). Tissue concentration was 50 mg, suspended in the volume of 5 ml. Temperature was 37°C. Bottom panel: The values are slopes of the above lines calculated by linear regression as the rate of change from the original O2 concentration per minute (Δ[O2]/min) and expressed as mean ± SD (n = 41 for control and rottlerin, 34 for FCCP). ***significantly higher than control by ANOVA using Newman-Keuls test
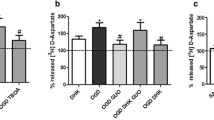
Uptake of Rb+ by astrocytes cultured from neonatal rat cortex. Conditions: 10 min preincubation followed (in experiments shown in panel B) by 5 min incubation in the presence of Rb+; temperature 37°C, drug concentrations: ouabain 100 μM, rottlerin 50 μM. The points (uptake expressed as nmol Rb+ related to the amount of protein recovered from the sample) are means of two to four values (panel A), or means ± SEM (n = 6 for controls, n = 4 for drugs). * different from controls at P < 0.05 by ANOVA using Newman-Keuls test
Sources of Materials
Antibodies against GFAP was obtained from SIGMA Chemical Company (St. Louis, Mo, USA), the AFC secondary antibodies were purchased from Bioscientific Pty Ltd. (Sydney, Australia).
FCCP [370-86-5] (carbonyl-cyanide 4-(trifluoromethoxy)phenylhydrazone), wortmannine [19545-26-7] ((15,6bR, 9aS, 11R, 11bR) 11-(acetyloxy)-1,67,8,9a, 10,11,11b-octahydro-1-(methoxymethyl)-9a, 11b-dimethyl-3H-furo[4,3,2-de]indeno[4,5-h]-2-h]-benzopyrane-3,6,9-trione), olomoucin [101622-51-9] ((6-benzylamino)-2-(2-hydroxylethylamino)-9-methylpurine) and staurosporine [62996-74-1] (9S-(9a,10b,11b,13a)-2,3,10,11,12,13-hexahydro-10-methoxy-9-methyl-11-(methylamino)-epoxy-1H,9H-diindolo[1,2,3-gh:3′,2′,1′-1 m]pyrrolo[3,4-j][1,7]benzodiazonin-1-one were purchased from Tocris, (Bristol, UK); Rottlerin and bisindolylmaleimide I (BIS I) came from Calbiochem (La Jolla, CA, USA), ouabain, RbCl and all tissue culture supplies were from Sigma Chemical Co (St. Louis, MO., USA). All other chemicals were of analytical grade and were purchased from commercial suppliers.
Results
Exposure of cultured astrocytes to d-aspartate caused a major redistribution of GLAST, significantly increasing the presence of GLAST-like immunoreactivity in the cell membrane (Fig. 1a, b). Quantitative analysis of the images obtained by deconvolution microscopy indicated that the value of RFI approximately doubled in the presence of d-aspartate (Fig. 1d). Rottlerin at 50 μM strongly inhibited this effect but, when applied alone, had no influence on the GLAST distribution (Fig. 1c, d).
Uptake of Rb+ by cortical prisms remained linear for at least 12.5 min and was completely inhibited by 100 μM ouabain [24]. A 10-min incubation time was, therefore, used in all following studies of Rb+ uptake by cortical prisms. Rottlerin at 50 μM but not at 5 μM, caused about 50% inhibition of Rb+ uptake by prisms of cerebral cortex (Fig. 2). Staurosporin (1 μM), a broad spectrum inhibitor of PKC, produced no significant inhibition (<12% difference from control, P > 0.02 by Newman-Keuls test, data not shown). Other drugs that had no effect on Rb+ uptake included an alternative broad spectrum PKC inhibitor BIS-I (25 μM), cyclin-dependent kinase inhibitor olomoucin [29] (50 μM) and the inhibitor of IP3 signalling cascade Wortmannin (10 μM). However, FCCP and CCCP (both at 50 μM), two metabolic inhibitors known to act through uncoupling of mitochondrial oxidative phosphorylation, significantly inhibited Rb+ uptake (Fig. 3). Furthermore, rottlerin at 50 μM produced a significant increase in the oxygen consumption by brain slices that amounted to almost half of that caused by 50 μM FCCP (Fig. 4).
In the experiments with cultured astrocytes, the concentration of Rb+ in the incubation medium was increased to 500 μM. Under these conditions, uptake of Rb+ remained linear for at least 5-min (Fig. 5a). This time-point was, therefore, used when testing drugs. Rottlerin (50 μM) produced significant inhibition of Rb+ uptake under these condition (Fig. 5b).
Discussion
Immunocytochemical experiments in the present study confirmed that GLAST expressed by brain astrocytes can be rapidly redistributed from cytoplasmic pools towards the plasma membrane in the presence of d-aspartate [8]. These observations suggest that the redistribution depends on the activity of GLAST as a transporter since d-aspartate is known to be efficiently transported by GLAST (see [1, 2, 30, 31] for reviews). Similar conclusions have been made on the basis of observations from studies using alternative experimental approaches but the explanation of the mechanism at a molecular level has remained elusive [32, 33]. The possibility of involvement of PKC-dependent phosphorylation has been raised but the role of PKC-δ in the process has been put in doubt following a report that the PKC-δ specific inhibitor rottlerin might influence glutamate transport by an unknown PKC-δ independent process [6].
Rottlerin is the most powerful toxic compound isolated from Kamala (Mallotus philippinensis, found chiefly in the Philippines, also in South East Asia, India and Australia [34–36]). The present findings, particularly the combination of decreased Rb+ uptake and increased oxygen consumption are consistent with rottlerin acting as an uncoupler of oxidative phosphorylation (“metabolic poison”) in brain tissue in vitro. Furthermore, it has been well established that uptake of Rb+ correlates with and, can be used as a measure of, (Na+, K+)-dependent ATPase activity in preparations containing intact metabolizing cells [12, 24]. Therefore, present data also suggest that rottlerin exerts some of its effects by inhibiting (Na+, K+)-dependent ATPase. This could explain the PKC-δ-independent effects of rottlerin on glutamate transport activity and its sequelae such as the changes in the activity-dependent redistribution of GLAST transporter [6]. Alternatively the apparent inhibition of (Na+, K+)-dependent ATPase activity (decreased Rb+ uptake) could be secondary to decreased availability of ATP caused by the metabolic toxicity of rottlerin [37]. Additional data (not shown) have, however, indicated that rottlerin can significantly inhibit (Na+, K+)-dependent ATPase activity even in cell-free homogenates prepared from rat brain astrocytes cultured by the presently described methods. In these experiments, an established technique based on liberation of inorganic phosphate (Pi) from ATP [38]; was used. About 80% of ATPase activity in this preparation was inhibited by rottlerin and the half-maximum inhibition was reached at 25.3 ± 7.1 μM inhibitor concentration (mean ± SEM, six inhibitor concentration in the range 5–200 μM, four points per each concentration). The liberation of Pi was also inhibited by (Na+, K+)-dependent ATPase inhibitor digoxin but much less so by ouabain, suggesting that (Na+, K+)-dependent ATPase in the presently used astrocyte membranes has a subunit composition different from that in astrocytes employed in similar studies elsewhere [12] (see also [8]).
Thus, we conclude that the apparent inhibition of glutamate transport by rottlerin [4, 6] can be adequately explained by perturbed energy metabolism (regardless of potential involvement of any additional rottlerin-related mechanisms [39, 40]). Uncoupling of oxidative phosphorylation would result in decreased levels of ATP and the ensuing reduction of the activity of (Na+, K+)-dependent ATPase (possibly further compounded by a direct inhibitory effect on the enzyme). As the membrane-bound (Na+, K+)-dependent ATPase represents the “Na+, K+-pump” that normally maintains the ionic gradients providing the driving force for glutamate transport, the present findings can explain why the effects of rottlerin on glutamate transport appear to be unrelated to the inhibition of PKC-δ [6]. Loss of the driving force would not only cause an apparent inhibition of glutamate transport but, since the cellular distribution of transporters, GLAST in particular, depends on the activity of glutamate transport [5, 32, 33], it would also result in the reduction of the activity dependent movement of GLAST from cytoplasm to the surface of the cells [32]. In addition, the present observations should be taken into account whenever rottlerin is employed as a tool to selectively inhibit PKC-δ in preparations derived from the central nervous system, particularly if there is a possibility that the outcome of the study could be influenced by changes in energy metabolism.
References
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105. doi:10.1016/S0301-0082(00)00067-8
Balcar VJ (2002) Molecular pharmacology of the Na+-dependent transport of acidic amino acids in the mammalian central nervous system. Biol Pharm Bull 25:291–301. doi:10.1248/bpb.25.291
Kontny E, Kurowska K, Szczepanska K, Maslinski W (2000) Rottlerin, a PKC isozyme-selective inhibitor, affects signaling events and cytokine production in human monocytes. J Leukoc Biol 67:249–258
Bull ND, Barnett NL (2002) Antagonists of protein kinase C inhibit rat retinal glutamate transport activity in situ. J Neurochem 81:472–480. doi:10.1046/j.1471-4159.2002.00819.x
Robinson MB (2002) Regulated trafficking of neurotransmitter transporters: common notes but different melodies. J Neurochem 80:1–11. doi:10.1046/j.0022-3042.2001.00698.x
Susarla BTS, Robinson MB (2003) Rottlerin, an inhibitor of protein kinase C-δ (PKC-δ), inhibits astrocytic glutamate transport activity and reduces GLAST immunoreactivity by a mechanism that appears to be PKC-δ-independent. J Neurochem 86:635–645. doi:10.1046/j.1471-4159.2003.01886.x
Vandenberg RJ (1998) Molecular pharmacology and physiology of glutamate transporters in the central nervous system. Clin Exp Pharmacol Physiol 25:393–400. doi:10.1111/j.1440-1681.1998.tb02221.x
Shin JW, Nguyen KTD, Pow DV, Knight T, Buljan V, Bennett MR, Balcar VJ (2009) Distribution of glutamate transporter GLAST in membranes of cultured astrocytes in the presence of glutamate transport substrates and ATP. Neurochem Res. doi:10.1007/s11064-009-9982-z
Beart PM, O’Shea RD (2007) Transporters for l-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol 150:5–17. doi:10.1038/sj.bjp.0706949
Sheldon AL, Robinson MB (2007) The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int 51:333–355. doi:10.1016/j.neuint.2007.03.012
Balcar VJ, Johnston GAR (1972) The structural specificity of the high affinity uptake of l-glutamate and l-aspartate by rat brain slices. J Neurochem 19:2657–2666. doi:10.1111/j.1471-4159.1972.tb01325.x
Pellerin L, Magistretti PJ (1997) Glutamate uptake stimulates Na+, K+-ATPase activity in astrocytes via activation of a distinct subunit highly sensitive to ouabain. J Neurochem 69:2132–2137
Cholet N, Pellerin L, Magistretti PJ, Hamel E (2001) Similar perisynaptic glial localization for the Na+, K+-ATPase alpha 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb Cortex 12:515–525. doi:10.1093/cercor/12.5.515
Sibson NR, Dhankar A, Mason GF, Rothman DL, Behar KL, Shulman RG (1998) Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA 95:316–321. doi:10.1073/pnas.95.1.316
Voutsinos-Porsche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chaton J-Y, Magistretti PJ, Pellerin L (2003) Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron 37:275–286. doi:10.1016/S0896-6273(02)01170-4
Rae C, Lawrance ML, Dias LS, Provis T, Bubb WA, Balcar VJ (2000) Strategies for studies of neurotoxic mechanisms involving deficient transport of l-glutamate: antisense knockout in rat brain in vivo and changes in the neurotransmitter metabolism following inhibition of glutamate transport in guinea pig brain slice. Brain Res Bull 53:373–381. doi:10.1016/S0361-9230(00)00372-5
Moussa CE-H, Mitrovic AD, Vandenberg RJ, Provis T, Rae C, Bubb WA, Balcar VJ (2002) Effects of L-CCG III on metabolism and l-glutamate transport in the mammalian central nervous system. Neurochem Res 27:27–35. doi:10.1023/A:1014842303583
Moussa CE-H, Rae C, Bubb WA, Griffin JL, Deters NA, Balcar VJ (2007) Inhibitors of glutamate transport modulate distinct patterns in brain metabolism. J Neurosci Res 85:342–350. doi:10.1002/jnr.21108
Emoto Y, Manome Y, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong WW, Kamen R, Weichselbaum R, Kufe D (1995) Proteolytic activation of protein kinase C delta by an ICE-like protease in apoptotic cells. EMBO J 14:6148–6156
Zemskov EA, Jana NR, Kurosawa M, Miyazaki H, Sakamoto N, Nekooki M, Nukina N (2003) Pro-apoptotic protein kinase C delta is associated with intranuclear inclusions in a transgenic model of Huntington’s disease. J Neurochem 87:395–406. doi:10.1046/j.1471-4159.2003.02002.x
Hertz L, Juurlink BHJ, Hertz E, Fosmark H, Schousboe A (1989) Preparation of primary cultures of mouse (rat) astrocytes. In: Shahar A, de Vellis J, Vernadakis A, Haber B (eds) A dissection and tissue culture manual of the nervous system. Alan R. Liss Inc., New York, p 105–108
Danilenko M, Wang X, Studzinski GP (2001) Carnosic acid and promotion of monocytotic differentiation of HL60-G cells imitated by other agents. J Natl Cancer Inst 93:1224–1233. doi:10.1093/jnci/93.16.1224
Scott HL, Pow DV, Tanneberg AE, Dodd PR (2002) Aberrant expression of glutamate transporter EAAT1 in Alzheimer’s disease. J Neurosci 22:1–5 RC206
Nanitsos EK, Acosta GB, Saihara Y, Stanton D, Liao LP, Shin JW, Rae C, Balcar VJ (2004) Effects of glutamate transport substrates and glutamate receptors ligands on the ativity of (Na+, K+)-dependent ATPase in brain tissue in vitro. Clin Exp Pharmacol Physiol 31:762–769. doi:10.1111/j.1440-1681.2004.04090.x
Cooper B, Chebib M, Shen J, King NJC, Darvey IG, Kuchel PW, Rothstein JD, Balcar VJ (1998) Structural selectivity and molecular nature of l-glutamate transport in cultured human fibroblasts. Arch Biochem Biophys 353:356–364. doi:10.1006/abbi.1998.0626
Gonçalves de Moraes VL (1990) Dimethyl sulphoxide: a possible effect on the interconversion of phosphorylated forms of Na+, K+-ATPase. Biochim Biophys Acta Biomembr 1026:135–140. doi:10.1016/0005-2736(90)90055-S
McConnel EJ, Wagoner MJ, Keenan CE, Raess BU (1999) Inhibition of calmodulin-stimulated (Ca2+, Mg2+)-ATPase activity by dimethyl sulphoxide. Biochem Pharmacol 57:139–144. doi:10.1016/S0006-2952(98)00259-7
Delpire E, Gullans SR (1994) Cell volume and K+ transport during differentiation of mouse erythroleukemia cells. Am J Physiol 266((2 Pt1)):C515–C523
Veselý J, Havlíček L, Strnad M, Blow JJ, Donella-Deana A, Pinna L, Letham DS, Kato J, Detivaud L, Leclerc S, Meijer L (1994) Inhibition of cyclin-dependent kinases by purine analogues. Eur J Biochem 224:771–786. doi:10.1111/j.1432-1033.1994.00771.x
Bridges RJ, Kavanaugh MP, Chamberlain AR (1999) A pharmacological review of competitive inhibitors and substrates of high-affinity sodium-dependent glutamate transport in the central nervous system. Curr Pharm Des 5:363–379
Balcar VJ, Takamoto A, Yoneda Y (2001) Neurochemistry of l-glutamate transport in the CNS: thirty years of progress. Collect Czech Chem Commun 66:1315–1340. doi:10.1135/cccc20011315
Duan S, Anderson CM, Stein BA, Swanson RA (1999) Glutamate induces rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J Neurosci 19:10193–10200
Poitry-Yamate CL, Vutskits L, Rauen T (2002) Neuronal-induced and glutamate-dependent activation of glial glutamate transporter function. J Neurochem 82:987–997. doi:10.1046/j.1471-4159.2002.01075.x
Telle H (1906) Kamala and rottlerin. Arch Pharm 244:441–448. doi:10.1002/ardp.19062440606
Dutt S (1925) Constitution of Indian kamala. J Chem Soc 127:2044–2052
McGookin A, Reed FP, Robertson A (1937) Rottlerin I. J Chem Soc 748–755. doi:10.1039/jr9370000748
Soltoff SP (2001) Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase C delta tyrosine phosphorylation. J Biol Chem 276:37986–37992
Turner N, Haga KL, Hulbert AJ, Else PL (2005) Relationship between body size, (Na+, K+)-ATPase activity and membrane lipid composition in mammal and bird kidney. Am J Physiol Regul Integr Comp Physiol 288:R301–R310. doi:10.1152/ajpregu.00297.2004
Susarla BT, Seal RP, Zelenaia O, Watson DJ, Wolf JH, Amara SG, Robinson MB (2004) Differential regulation of GLAST immunoreactivity and activity by protein kinase C; evidence for modification of amino and carboxyl termini. J Neurochem 91:1151–1193. doi:10.1111/j.1471-4159.2004.02791.x
Davies SP, Reddy H, Caivano M, Cohen P (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351:95–105. doi:10.1042/0264-6021:3510095
Balcar VJ, Nguyen KTD, Shin JW, Rae C, Nanitsos EK, Acosta GB, Pow DV, Bennett MR (2004) Rottlerin inhibits (Na+, K+)-ATPase activity but does not cause redistribution of glutamate transporter GLAST in cultured astrocytes. Abstracts of 44th annual meeting of the american society for cell biology, Washington DC, p 494a. Abstr. No. 1893
Nguyen KTD, Shin JW, Knight T, Clarke R, Else P, Balcar VJ (2005) Effects of PKC-delta inhibitor rottlerin on (Na+, K+)-ATPase activity in rat brain tissue in vitro. Abstracts of 45th annual meeting of the american society for cell biology, San Francisco, p 154a. Abstr. No. 560
Acknowledgments
The work was supported by grants from The Rebecca L. Cooper Medical Research Foundation (VJB), Australian Health Management Group (VJB) and The University of Sydney Sesquicentennial Research Grant Scheme (VJB). Special thanks are due to Dr. Toby Knight who helped with the deconvolution microscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, K.T.D., Shin, JW., Rae, C. et al. Rottlerin Inhibits (Na+, K+)-ATPase Activity in Brain Tissue and Alters d-Aspartate Dependent Redistribution of Glutamate Transporter GLAST in Cultured Astrocytes. Neurochem Res 34, 1767–1774 (2009). https://doi.org/10.1007/s11064-009-9996-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-9996-6