Abstract
Docosahexaenoic acid (22:6n − 3, DHA) is known to enhance neurogenesis. However, the immediate-early effect of DHA on neurogenesis is not fully elucidated. We studied the effect of DHA supplementation (10 and 30 μM) on morphological and molecular changes at different time points of nerve growth factor (NGF, 50 ng/ml)-induced differentiation of PC12 (pheochromocytoma) cells. Cells were analyzed throughout the differentiation process (2 h, 1, 2, 3, 4, and 10 days), for neurite outgrowth (light microscopy and computer image analysis), and for mRNA levels of the immediate molecular differentiation markers Egr1, Egr3, PC3 and PC4 (quantitative real-time PCR). DHA induced significant accelerated neurite outgrowth beginning as early as 2 h post-DHA supplementation and throughout differentiation. Transcripts of the neurogenesis immediate early biomarkers Egr3 and PC3 were significantly (P < 0.05) elevated following DHA supplementation within 0.5 and 1 h post-supplementation (respectively). In conclusion, we show that DHA significantly stimulates immediate-early neurogenesis events, as is evident by both morphological and molecular markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DHA is a major polyunsaturated fatty acid (PUFA) found in the central nervous system (CNS), and the most abundant omega-3 (n − 3) PUFA in the membrane of neurons [1, 2]. DHA is essential for normal brain development in humans and animals. In the mammalian brain, the major DHA accumulation occurs late in gestation, parallel with maximal neuronal differentiation process (neurogenesis) which consists of drastic morphological and molecular changes [3, 4]. The formation of neurites (axons and dendrites) is a key feature of neurogenesis, both during development and in the adult. Several studies have shown that DHA deficiency in critical prenatal and postnatal developmental stages leads to a variety of visual, cognitive and behavioral impairments [5–8]. Furthermore, DHA levels are constantly sustained throughout adulthood, and adult DHA deficiency was found to be associated with cognitive impairment and adult neurodegenerative diseases [3, 9]. Dietary supplementation with DHA has been shown to improve mental development in humans, alleviate symptoms in peroxisomal disorders, prevent dendritic pathology in Alzheimer disease models and reduce neuronal injury in experimental brain ischemia [3, 9, 10].
The rat pheochromocytoma cell line, PC12, has been used extensively as a model system to investigate neuronal (nerve growth factor [NGF)-dependent) differentiation. PC12 cells recapitulate the program of neuronal differentiation by developing neurites after several days of NGF treatment [3, 11, 12]. NGF-induced neurogenesis in PC12 cells is characterized by alterations in the transcript levels of specific transcripts expression (mRNAs), which are biomarkers of the irreversible commitment of PC12 cells to the neuronal differentiation program. Recent studies demonstrated (using different methods, such as; expressed sequence-tag (EST) and microarray analysis) that NGF induces rapid (30–40 min) and transient expression of immediate early genes (IEGs). IEGs include genes both of the ubiquitously expressed fos and jun families as well as the neuronal specific EGR and PC families [13–16].
It has been shown that DHA uptake by NGF-differentiated PC12 cells is immediate (occurring within the first few minutes after incubation), and that DHA is rapidly incorporated to neural lipids and phospholipids [17]. In a few in vitro studies DHA has been shown to enhance neurite growth; however, as PC12 cells recapitulate the program of neuronal differentiation only after several days of NGF treatment, neurite outgrowth was studied only after several days of treatment and was not monitored immediately after NGF and/or DHA supplementation [3, 18, 19]. Additionally, the morphological changes were not studied in parallel to transcript changes.
We hypothesized that DHA can alter molecular and morphological markers in a short time scale. Thus, we studied whether DHA supplementation of NGF-induced neurogenesis will have an immediate effect on IEG transcript levels and on neurite outgrowth.
Experimental Procedure
Cell Culture and Fatty Acid Supplementation
PC12 (5,000 cells/cm2) were seeded on 60 mm dishes (Sigma Aldrich) and maintained in culture medium consisting of DMEM (Dulbecco’s modified Eagle’s medium), 10% (v/v) horse serum, 5% (v/v) fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1% (v/v) l-glutamine (Sigma–Aldrich), in a 5% CO2 humidified incubator at 37°C. Differentiation was induced by addition of 50 ng/ml NGF (Biotest, Israel), with or without DHA supplementation. DHA was conjugated with fatty acid-free bovine serum albumin (BSA, Sigma) and added to the culture medium. The final DHA concentrations (30 and 10 μM) were chosen based on previous work [20]. Morphological analyses of cells were done at 2 h and 1, 2, 3, 4 and 10 days following NGF and NGF + DHA supplementation. Morphological characterization of the cells was done using light microscopy and image analysis program (ImageJ, NIH). Molecular analyses was done at 0.5, 1, 2, 6 and 24 h following NGF and NGF + DHA supplementation.
Neurite Measurements
Visual fields of 1 cm2 were randomly selected in each dish of NGF-induced differentiated PC12 cells (3 dishes per each treatment: control, 10 and 30 μM DHA). Photographed images were generated for all fields at each time point (2 h and 1, 2, 3, 4 and 10 days) using Nikon photomicroscope (Nikon Eclipse TS100 phase microscope and Nikon collpix 4500) and transferred to a computer platform. Neurites were analyzed from photos using NIH image software ImageJ 1.35 s (http://rsb.info.nih.gov/ij). Parameters analyzed were: neuritis numbers and lengths and the number of branches per cell. The neuritis population at each time point and treatment was sub-divided into 5 groups based on neurite length (10–20, 20–40, 40–80, 80–150 and 150–400 μm).
RNA Extraction and Reverse Transcription (RT)
As significant differences in neurite outgrowth between control and DHA supplemented cells were detected 2 h following DHA supplementation, early molecular markers were analyzed using quantitative RT-PCR. For these experiments PC12 cells were grown as described above and RNA was isolated from control and DHA supplemented cells (30 μM) after 0.5, 1, 2, 6 and 24 h. Total RNA was isolated from cultures of PC12 cells using RNA isolation kit (ZYMO research, USA). RNA integrity was tested by agarose gel electrophoresis (1%) with Ethidium Bromide (Mercury, USA) staining. RNA was quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Rockland, DE, USA). One microgram of total RNA was reverse transcribed (RT) using 200U reverse transcriptase (Bioline, London, UK) with and 0.2 μg of random hexamers (Bioline, London, UK) in a final volume of 20 μl containing 2 mM of dNTP mix (Epicentre Technologies, Madison, WI, USA), 5× first-Strand buffer (Bioline, London, UK). The RT reaction was performed at 37°C for 1 h.
Quantification of mRNA by Real-Time PCR (qRT-PCR)
Transcript levels of PC3, PC4, Egr1 and Egr3 were determined by qRT-PCR using ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA) according to the instructions of the manufacturer. Gene specific primers were designed using the Primer Express Software (Applied Biosystems, Foster City, CA, USA). Primers sequences used: PC3, Forward: GACGCACTGACCGATCATTACA Reverse: GGCTGAGTCCGATCTGGCT; PC4, Forward: AGAATTGCTGCTGGCGAATC Reverse: CCAGAGCCCGGAGCATCT; Egr1, Forward: TGACCACAGAGTCC TTTTCTGACA Reverse: GAGAAGCGGCCAGTATAGGTGAT; Egr3, Forward: AGCAGTTTGCTAAATCAATTGCCT Reverse: CATTCTCTGTAGCCATCTGAG TGTAAT; 18S, Forward: AAGCAGACATTGACCTCACCAA Reverse: TGGCTATACTTC CCATCCTTCAC. The qRT-PCR primer pairs were designed across exon(s) to avoid false positive signals from potentially contaminating genomic DNA. Primer and cDNA concentrations were optimized following the guidelines of the manufacturer. For each primer pair used, melting curve analysis (ramping slowly the temperature from 60 to 95°C with continuous measurement of fluorescence) showed a single melting peak after amplification, indicating specific products. ROX was added as an internal reference in order to normalize fluctuations in fluorescence signal background. Each 20 μl reaction contained 2 μl (1–2 μg) first strand cDNA, 10 μl PCR master mix (Applied Biosystems, Foster City, CA, USA), 100–300 nM of each forward and reverse primer (according to the optimization of the primers). All reactions were performed under the following conditions: pre-incubation at 50°C for 2 min followed by denaturation at 95°C for 10 min and 40 cycles of 95°C for 15 s, annealing and elongation at 60°C for 1 min. Reactions were characterized by comparing the threshold cycle (Ct) values. For each sample, results were normalized by the transcript level of internal control gene.
Statistical Analysis
The data were expressed as mean ± SE. Neurite data and mRNA expression level data were analyzed using one-way ANOVA. Results were considered significantly different if P-values were smaller than 0.05 (P < 0.05).
Results
Morphological Analysis
Effects of DHA on PC12 Differentiation: Neurite Outgrowth
In order to demonstrate the effect of DHA in NGF-induced differentiation, PC12 cells were treated with NGF (control) only or with NGF in combination with DHA supplementation (10 or 30 μM).
Neurite Outgrowth
There was significant (P < 0.05) neurite length growth in each of the groups studied (control, 10 and 30 μM DHA-supplemented) from 2 h throughout the 10 days. The DHA supplemented cells showed significantly (P < 0.05) accelerated neurite outgrowth compared to the control cells at 2 h, 3, 4 and 10 days (Fig. 1). The differential neurite growth rate was evident especially during early and late stages of neurogenesis. Neurite outgrowth in cells supplemented with the higher DHA concentration (30 μM) was further accelerated as compared to that in cells supplemented with the lower DHA concentration (10 μM); however, this difference was not statistically significant.
Effect of DHA supplementation (10 and 30 μM) on the neurite length. Neurite outgrowth was monitored for 10 days, starting 2 h post-supplementation. (The data present neurite length as average ± SE (n = 3 plates of 30–60 independent cells per each time-point and each treatment.) Treatment not sharing a symbol are significantly different (P < 0.05)
Neurites Population Distribution
The control and the DHA treated groups demonstrated visible neurite outgrowth after only 2 h, with the majority of the cell population exhibiting at that time point neurite lengths of 3–20 μm (evident in 100, 60, and 70% of the cell population for control, 10 and 30 μM DHA-supplemented cells, respectively). As early as 2 h following DHA supplementation, DHA-supplemented cells demonstrated significantly accelerated neurite outgrowths compared to control cells, with 20–30% of the cell population reaching longer neurite lengths of 20–40 μm (Fig. 2a). Following 24 h of DHA supplementation, the majority (>80%) of the DHA-supplemented (10 and 30 μM) groups showed neurite lengths ranging between 20 and 80 μm while the majority (>80%) of the control group showed neurite lengths ranging between 10 and 40 μm (Fig. 2b). Similar to the 1st day, on the 2nd day all groups reached a wider range and longer neurite lengths, with a maximum of 150 μm (Fig. 2c). On the 3rd day, only the DHA supplemented groups reached maximal neurite lengths of 400 μm, and >10% of both DHA supplemented cell populations reached neurite lengths of 80–400 μm. The cell populations retained the neurite length distribution of the 1st and 2nd days (Fig. 2d). On the 4th day, the distribution of neurite lengths was similar to that on the 3rd day. However, cells supplemented with a higher DHA dose (30 μM) showed a wider range of the neurite length distribution, where the majority of the population (>80%) was in the range of 20–150 μm, unlike the lower (10 μM) DHA supplemented cells, which retained the range of 20–80 μm (Fig. 2e). On the 10th day, also the control cells reached the maximal neurite length of 150–400 μm (Fig. 2f). These data suggested that DHA supplementation substantially enhanced NGF-induced neurite outgrowth in terms of maximal length and rate of growth. The distribution between neurite populations in the DHA-supplemented groups was not significantly different. The number of neurites per cell population and the number of branches per cell were not significantly different between the treatment groups (data not shown).
Effect of DHA supplementation on the neurite length per neuron—sequential time monitoring. Neurite outgrowth was monitored for 10 days, starting 2 h post-supplementation. (a) 2 h, (b) 1 day, (c) 2 days, (d) 3 days, (e) 4 days and (f) 10 days. PC12 cells were treated with NGF in the absence or presence of DHA in different concentrations (10 and 30 μM). Frequency distribution of the total neurite population was monitored and analyzed (n = 30–60 independent cells per each time-point and each treatment). The data present neurite length as % of total neurites population
Molecular Analysis
Effect of DHA Supplementation on PC12 Transcript Levels of Immediate Early Genes
Significant differences were found in the length and rate of neurite growth in the cells supplemented with DHA compared to control cells. Surprisingly, these differences were detectable as early as 2 h post-DHA supplementation, indicating that immediate early transcripts, involved in neurogenesis, might be induced by DHA. As the morphological analysis of cells supplemented with low and high DHA concentrations showed a similar pattern of change, particularly during the first immediate hours post-supplementation, we studies the IEG genes transcript levels of cells supplemented with the high concentration of DHA. Two families of IEG genes were studies by qRT-PCR analysis: EGR and PC.
EGR Family
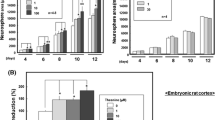
Egr1 transcripts of both control and DHA supplemented cells were significantly (P < 0.05) elevated by 60- and 70-fold (respectively), as compared to non-differentiated cells (pre-NGF supplementation). This effect was evident within 0.5 h post-NGF induction, and continued to significantly (P < 0.05) increase by 1 h post-NGF induction, reaching 80- and 90-fold increase in Egr1 transcript levels for control and DHA-supplemented cells, respectively (compared to Egr1 transcript levels in non-differentiated pre-NGF supplementation cells). However, Egr1 transcript levels were not significantly different between control and DHA supplemented cells. Egr3 transcripts were significantly (P < 0.05) elevated (by 60- and 120-fold for control and DHA-supplemented groups, respectively) compared to non-differentiated cells (pre-NGF supplement). This effect was evident within 0.5 h post-NGF induction, and continued to significantly (P < 0.05) increase by 1 h post-NGF induction: 300- and 320-fold for control and DHA-supplemented groups, respectively, compared to non-differentiated (pre-NGF supplementation) cells. Egr3 transcript levels of DHA supplemented cells were significantly (P < 0.05) elevated by twofold compared to control cells at 0.5 h. Egr3 transcript levels at 1 h post-DHA supplementation were not different from those of control cells (Fig. 3).
Levels of Egr1 (a) Egr3 (b) during NGF-induced differentiation of PC12 cells supplemented with DHA. PC12 cells were differentiated with NGF (50 ng/ml) in absence and presence of DHA (30 μM), as described in “Material and Methods”. Samples were taken at 0, 0.5 and 1 h. Total RNA was isolated and subjected to qRTPCR. Statistical analysis of qRTPCR was carried out using the (2-ΔΔCt) method, which calculates the relative change in mRNA levels normalized to an endogenous reference (18S). Results expressed as fold change (mean ± SE, 2 independent experiments, 3 repeats within each experimental group). Treatment not sharing a symbol are significantly different (P < 0.05)
PC Family
In both DHA supplemented and control cells, PC3 transcript levels were significantly (P < 0.05) elevated (by 10- and 12-fold, respectively) compared to non-differentiated (pre-NGF supplementation) cells. This effect was evident within 0.5 h, reaching a peak at 1 h post-NGF supplementation. At 1 h, PC3 transcript levels of DHA-supplemented cells were significantly (P < 0.05) elevated by twofold compared to control cells (Fig. 4). PC4 transcript levels in both DHA supplemented and control cells were significantly (P < 0.05) elevated (by twofold) compared to non-differentiated cells (pre-NGF supplement) within 0.5 h, reaching a peak at 6 h post-NGF supplementation (12- and 14-fold change for control and DHA-supplemented, respectively). Although DHA-supplemented cells showed elevated (not significant) PC4 levels at 2 and 4 h, PC4 transcript levels were not significantly different between the DHA supplemented cells and the control groups.
Levels of PC3 (a) and PC4 (b) during NGF-induced differentiation of PC12 cells supplemented with DHA. PC12 cells were differentiated with NGF (50 ng/ml) in absence and presence of DHA (30 μM), as described in “Material and Methods”. Samples were taken at 0, 0.5 and 1, 2, 6 and 24 h. Total RNA was isolated and subjected to qRTPCR. Statistical analysis of qRTPCR was carried out using the (2-ΔΔCt) method, which calculates the relative change in mRNA levels normalized to an endogenous reference (18S). Results expressed as fold change (mean ± SE, 2 independent experiments, 3 repeats within each experimental group). Treatment not sharing a symbol are significantly different (P < 0.05)
Discussion
We demonstrate in this study that DHA supplementation induces immediate morphological changes of neurite outgrowth coupled with alterations in the transcript levels of early molecular markers of neuronal differentiation. These changes are evident as early as 2 h following DHA supplementation. One of the critical steps in neuronal differentiation is the outgrowth of neurites [21, 22]. In our study, DHA supplementation promoted neuronal differentiation by increasing the population of neurons with longer neurites, most notably as soon as 2 h post-DHA supplementation. This effect continued to be evident throughout the 10 days in which the experimental groups were followed. A few in vitro studies have shown the beneficial effect of DHA supplementation on neurite outgrowth for various (and different) time periods. Altogether, these and our current study studies indicate a continuous beneficial effect of DHA on neuritogenesis events [3, 23]. Generally, longer neurites bear more synaptic connections, enhancing neuronal function. The rapid DHA-induced neurite outgrowth demonstrated in our study, is in line with previous studies showing that DHA uptake and incorporation into neurons is extremely rapid, and that DHA is trafficked rapidly to the nerve endings and nerve end cones [17, 24]. DHA-supplementation with both concentrations used (10 and 30 μM) showed similar beneficial effects on neurite growth. Although the higher DHA concentration (30 μM) induced longer neurite growth than that induced by the lower concentration (10 μM), the difference between those groups was not statistically significant. This is indicative that DHA supplementation in the physiological range (10 μM) is sufficient to maximize neurite growth. The effect of DHA on neurogenesis events is highly specific as was previously shown in other studies. Other fatty acids which are abundant in the mammalian brain, such as arachidonic acid (20:4n − 6), oleic acid (18:1n − 9) and docosapentaenoic acid (22:5n − 6, an n − 6 PUFA that significantly increases to compensate the loss of DHA in n − 3 fatty acid deficiency), do not show similar effects on neurite growth and even demonstrate inhibition of neurite growth [3, 20, 25].
Studies of the downstream effects through which DHA affects neurogenesis can shed light on normal molecular mechanisms of neuronal differentiation. To date, the molecular mechanism(s) by which DHA promotes neurite growth is mostly unknown. Several possible mechanisms of DHA-induced neurogenesis have been suggested, such as local enrichment and activation of the neuronal lipid moieties (phospholipids) and possible activity of DHA as a ligand for neural receptors (RXR), consequently activating downstream transcription factors [3, 26]. Neurogenesis in PC12 cells is known to be induced by NGF treatment. The action of NGF is transcription-dependent, and the irreversible commitment into the differentiation pathway is characterized by a sharp rise (within 30–40 min) of specific mRNAs, which are early biomarkers (IEGs) for neurogenesis [27, 28]. IEGs encode for transcriptional modulators such as ubiquitously expressed anti-mitotic genes (fos and jun families), which are the most extensively studied, and for more specific biomarkers of neuronal cell-programming such as the Egr gene family (Egr1, Egr3) and PC gene family (PC3 and PC4) [28, 29]. As we demonstrated in this study, DHA induces immediate morphological and molecular changes in PC12 cells. Egr1 and Egr3 are known to play an important role in neurite outgrowth: inhibition of Egr3 and Egr1 has been shown to effectively inhibit NGF-elicited neurite outgrowth in PC12 cells. Both genes display parallel and similar response patterns to NGF [30, 31]. Both Egr1 and Egr3 transcript levels displayed rapid (around 1 h) and significant increase following NGF stimulation, in accordance to previous studies [32]. We found that DHA supplementation induced a specific significant elevation of Egr3 transcript levels beyond those induced by NGF alone. Although both members of the EGR family, Egr1 and Egr3, share nearly identical zinc finger DNA binding domains and bind to a common Egr response element consensus sequence, the non-homologous portions of their sequences diverge considerably, indicating that they might be differentially regulated [32]. Our findings indicate that DHA might be a specific candidate regulator of Egr3. It should be noted that an additional indication of differential regulation of EGR family members was demonstrated following electroconvulsive stimulation, where both Egr-1 and Egr-3 mRNA levels were induced rapidly in dentate granule cells, yet in a sequential manner [30]. Sequential response of different family members following stimulation has been shown also for members of the Fos gene family, and represents an important mechanism underlying neuronal plasticity [30, 33]. The specific regulation of DHA found in our study might indicate time-dependent transcript changes or a specific and differential regulation pattern of Egr family members.
Another NGF-induced IEG family is the PC family. PC3 is the prototype member of a novel family of anti-proliferative genes, expressed at the onset of neuronal differentiation in PC12 cells [34]. In line with previous reports [28, 33, 34], in our study NGF induced robust transient elevation in PC3 transcripts, peaking by 1 h, PC3 encodes a putatively secreted protein that is induced by NGF with a relatively high specificity [34, 35]. We found that at its peak, the DHA supplementation further and significantly elevated PC3 transcript levels. Growing evidence indicates that PC3 plays a role in cell cycle arrest and neurogenesis, which are highly coordinated and interactive processes, governed by cell cycle genes and neural transcription factors [36]. Canzoniere et al. [36] found that over-expression of PC3 in neuronal tissues during embryonic and postnatal periods leads to increase of neuronal differentiation throughout the neural tube and in the cerebellum. Thus, our results could indicate that the supplementation of NGF-stimulated cells with DHA enhances the cell cycle arrest parallel to PC3 regulation. PC4, another member of the IEG and PC family, presents significant sequence similarity to interferon-gamma, a molecule known for its role in cellular differentiation [27]. Although PC4 function remains unknown, it is expressed during neuronal differentiation in vitro and in vivo [29]. Our results indicate that PC4 transcripts are induced by NGF; unlike Egr1, Egr3 and PC3, which all reach their peak within the first hours, PC4 reaches its peak after several hours, as was previously described [27, 34]. In contrast to PC3, DHA supplementation did not have an effect on PC4 transcript levels.
In conclusion, we show that DHA can modulate both morphological and molecular immediate-early neurogenesis events. The morphological changes, expressed by enhanced and prolonged neurite outgrowths, begin within a couple of hours post-DHA supplementation, and are coupled to a rapid and transient up-regulation of IEG gene transcripts. The up-regulation of specific IEG genes can indicate newly identified DHA-regulated genes, and suggests a possible molecular pathways by which DHA enhances neurogenesis.
References
Aveldano MI, Bazan NG (1973) Fatty acid composition and level of diacylglycerols and phosphoglycerides in brain and retina. Biochim Biophys Acta 296(1):1–9
Giusto NM, Pasquaré SJ, Salvador GA et al (2000) Lipid metabolism in vertebrate retinal rod outer segments. Prog Lipid Res 39:315–391. doi:10.1016/S0163-7827(00)00009-6
Calderon F, Kim HY (2004) Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem 90(4):979–988. doi:10.1111/j.1471-4159.2004.02520.x
Green P, Yavin E (1998) Mechanisms of docosahexaenoic acid accretion in the fetal brain. J Neurosci Res 52(2):129–136. doi:10.1002/(SICI)1097-4547(19980415)52:2<129::AID-JNR1>3.0.CO;2-C
Crawford MA, Costeloe K, Ghebremeskel K et al (1997) Are deficits of arachidonic and docosahexaenoic acids responsible for the neural and vascular complications of pre-term babies? Am J Clin Nutr 66:1032S–1041S
Nettleton JA (1993) Are n − 3 fatty acids essential nutrients for fetal and infant development? J Am Diet Assoc 93:58–64. doi:10.1016/0002-8223(93)92132-H
Das UN, Fams MD (2003) Long-chain polyunsaturated fatty acids in the growth and development of the brain and memory. Nutrition 19:62–65. doi:10.1016/S0899-9007(02)00852-3
Lucas A, Morley R, Cole TJ et al (1992) Breast milk and subsequent intelligence quotient in children born preterm. Lancet 339(8788):261–264. doi:10.1016/0140-6736(92)91329-7
Lukiw WJ, Cui JG, Marcheselli VL et al (2005) A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest 115(10):2774–2783
Akbar M, Calderon F, Wen Z et al (2005) Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci USA 102(31):10858–10863. doi:10.1073/pnas.0502903102
Greene LA, Tischler AS et al (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA 73(7):2424–2428. doi:10.1073/pnas.73.7.2424
Ulloth JE, Casiano CA, De Leon M (2003) Palmitic and stearic fatty acids induce caspase-dependent and -independent cell death in nerve growth factor differentiated PC12 cells. J Neurochem 84(4):655–668. doi:10.1046/j.1471-4159.2003.01571.x
Frade JM, Barde YA (1998) Nerve growth factor: two receptors, multiple functions. Bioassays 20(2):137–145. doi:10.1002/(SICI)1521-1878(199802)20:2<137::AID-BIES6>3.0.CO;2-Q
Greenberg ME, Greene LA, Ziff EB (1985) Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem 260:14101–14110
Leonard DG, Ziff EB, Greene LA (1987) Identification and characterization of mRNAs regulated by nerve growth factor in PC12 cells. Mol Cell Biol 7(9):3156–3167
Lee NH, Weinstock KG, Kirkness EF et al (1995) Comparative expressed-sequence-tag analysis of differential gene expression profiles in PC-12 cells before and after nerve growth factor treatment. Proc Natl Acad Sci USA 92:8303–8307. doi:10.1073/pnas.92.18.8303
Martin RE, Wickham JQ, Om A et al (2000) Uptake and incorporation of docosahexaenoic acid (dha) into neuronal cell body and neurite/nerve growth cone lipids: evidence of compartmental dha metabolism in nerve growth factor-differentiated pc12 cells. Neurochem Res 25(5):715–723. doi:10.1023/A:1007575406896
Gizang-Ginsberg E, Ziff EB (1990) Nerve growth factor regulates tyrosine hydroxylase gene transcription through a nucleoprotein complex that contains c-Fos. Genes Dev 4:477–491. doi:10.1101/gad.4.4.477
Dragunow M, Xu R, Walton M et al (2000) c-Jun promotes neurite outgrowth and survival in PC12 cells. Brain Res Mol Brain Res 83:20–33. doi:10.1016/S0169-328X(00)00191-1
Cao D, Xue R, Xu J et al (2005) Effects of docosahexaenoic acid on the survival and neurite outgrowth of rat cortical neurons in primary cultures. J Nutr Biochem 16(9):538–546. doi:10.1016/j.jnutbio.2005.02.002
Cline HT (2001) Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol 11:118–126. doi:10.1016/S0959-4388(00)00182-3
Poirazi P, Mel BW (2001) Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron 29:779–796. doi:10.1016/S0896-6273(01)00252-5
Marszalek JR, Lodish HF (2005) Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: breastmilk and fish are good for you. Annu Rev Cell Dev Biol 21:633–657. doi:10.1146/annurev.cellbio.21.122303.120624
Martin RE, Bazan NG (1992) Changing fatty acid content of growth cone lipids prior to synaptogenesis. J Neurochem 59(1):318–325. doi:10.1111/j.1471-4159.1992.tb08906.x
Ikemoto A, Kobayashi T, Watanabe S, Okuyama H (1997) Membrane fatty acid modifications of PC12 cells by arachidonate or docosahexaenoate affect neurite outgrowth but not norepinephrine release. Neurochem Res 22:671–678. doi:10.1023/A:1027393724676
de Urquiza AM, Liu S, Sjoberg M (2000) Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 290:2140–2144. doi:10.1126/science.290.5499.2140
Tirone F, Shooter EM (1989) Early gene regulation by nerve growth factor: induction of an interferon related gene. Proc Natl Acad Sci USA 86:2088–2092. doi:10.1073/pnas.86.6.2088
Roux P, Menguy I, Soubigou S (2004) Direct measurement of multiple mrnas in nerve growth factor-induced PC12 cells using electrophoretic tags to monitor biomarkers of neuronal differentiation in 96-well format. Assay Drug Dev Technol 2(6):637–646. doi:10.1089/adt.2004.2.637
Iacopetti P, Barsacchi G, Tirone F et al (1996) Expression of the PC4 gene in the developing rat nervous system. Brain Res 707:293–297. doi:10.1016/0006-8993(95)01370-9
O’Donovan KJ, Tourtellotte WG, Milbrandt J et al (1999) The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci 22:167–173. doi:10.1016/S0166-2236(98)01343-5
Levkovitz Y, O’Donovan KJ et al (2001) Blockade of NGF-induced neurite outgrowth by a dominant-negative inhibitor of the Egr family of transcription regulatory factors. J Neurosci 21(1):45–52
O’Donovan KJ, Wilkens EP et al (1998) Sequential expression of Egr-1 and Egr-3 in hippocampal granule cells following electroconvulsive stimulation. J Neurochem 70:1241–1248
Hope BT, Nye HE, Kelz MB et al (1994) Induction of a long lasting AP-I complex composed of altered fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron 13:1235–1244. doi:10.1016/0896-6273(94)90061-2
Bradbury A, Possenti R, Shooter EM et al (1991) Molecular cloning of PC3, a putatively secreted protein whose mRNA is induced by nerve growth factor and depolarization (cDNA/growth factors/brain). Proc Natl Acad Sci USA 88(8):3353–3357. doi:10.1073/pnas.88.8.3353
Tirone F (2001) The gene pc3tis21/btg2, prototype member of the pc3/btg/tob family: regulator in control of cell growth, differentiation, and DNA repair? J Cell Physiol 187(2):155–165. doi:10.1002/jcp.1062
Canzoniere D, Farioli-Vecchioli S, Conti F et al (2004) Dual control of neurogenesis by PC3 through cell cycle inhibition and induction of Math1. J Neurosci 24(13):3355–3369. doi:10.1523/JNEUROSCI.3860-03.2004
Author information
Authors and Affiliations
Corresponding author
Additional information
L. Dagai and R. Peri-Naor contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Dagai, L., Peri-Naor, R. & Birk, R.Z. Docosahexaenoic Acid Significantly Stimulates Immediate Early Response Genes and Neurite Outgrowth. Neurochem Res 34, 867–875 (2009). https://doi.org/10.1007/s11064-008-9845-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9845-z