Abstract
Neurogenesis occurs in dentate gyrus of adult hippocampus under the influence of various mitogenic factors. Growth factors besides instigating the proliferation of neuronal progenitor cells (NPCs) in dentate gyrus, also supports their differentiation to cholinergic neurons. In the present study, an attempt has been made to investigate the neurotrophic effect of bFGF in Kainic acid (KA) induced cognitive dysfunction in rats. Stereotaxic lesioning using (KA) was performed in hippocampal CA3 region of rat’s brain. Four-weeks post lesioning rats were assessed for impairment in learning and memory using Y maze followed by bFGF infusion in dentate gyrus region. The recovery was evaluated after bFGF infusion using neurochemical, neurobehavioural and immunohistochemical approaches and compared with lesioned group. Significant impairment in learning and memory (P < 0.01) observed in lesioned animals, four weeks post lesioning exhibited significant restoration (P < 0.001) following bFGF infusion twice at one and four week post lesion. The bFGF infused animals exhibited recovery in hippocampus cholinergic (76%)/ dopaminergic (46%) receptor binding and enhanced Choline acetyltransferase (ChAT) immunoreactivity in CA3 region. The results suggest restorative potential of bFGF in cognitive dysfunctions, possibly due to mitogenic effect on dentate gyrus neurogenic area leading to generation and migration of newer cholinergic neurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing documentation exhibits pivotal role of growth factor supporting continuous neurogenesis in hippocampal region [1–3]. This has gained worldwide attention towards therapeutic management of progressive neurodegenerative diseases, where changes in memory and other cognitive core function have been reported [4].
Cognitive dysfunction reportedly involves degeneration of cholinergic neurons and suggests severe impairment in learning and memory. Cholinergic neurons originating in basal forebrain area play an important role in functional memory and are one of the most vulnerable targets of degeneration in such conditions [5]. This is further accompanied by loss of cholinergic markers i.e. levels of acetylcholine (ACh) and choline acetyltransferses (ChAT) activity [6]. Besides this, the extent of damage to cholinergic neurons has been identified as landmark of progressive memory impairment appearing in several psychiatric and neurological disorders [7].
Continued neurogenesis in subgranular zone (SGZ) of dentate gyrus of hippocampus, which is involved in learning and memory has been documented [8, 9]. Generation of new neurons from proliferating stem /progenitor cells in the SGZ is maintained all through life in multiple species including humans [10, 11]. Further, the capability of dentate neurogenesis exhibits an intense relationship with the hippocampal function of learning and memory [12–14] and found to be under strict control of neurogenetic microenvironment of the SGZ [15, 16]. Uniquely, this was also found to be responsive to hippcampal injury in disease conditions (such as ischemia, stroke, and hypoxia) or on exposure to neurotoxins such as trimethyltin (TMT) and kainic acid (KA) where an increase in dentate gyrus neurogenesis has been observed [17–19]. In addition to this, the hippocampal injury induces transitory proliferative surge in SGZ leading to several fold increase in number of new neurons [20, 21]. One of the mechanisms supporting the endogenous neuronal sprouting is release of mitogenic factors from dying neurons [22]. It has been shown that several neurotrophic factors such as BDNF, NGF, CNTF, GDNF and others are upregulated in the hippocampus following excitotoxic injury [23–25]. Recently, others and our group have demonstrated substantial usefulness of exogenous/intrinsic neurotrophic factors in enhancing the neuronal survival, the extent of which probably depends on their affinity towards particular neuronal subtype [26–28]. It was evidenced that bFGF released from cell in response to injury has greater affinity towards cholinergic neurons and is critical for upregulation of neurogenesis in the adult dentate gyrus after kainate induced seizures and focal cerebral ischemia [29, 30]. bFGF also promotes neuronal survival and nurite extension in vitro and in vivo [31, 32].
Not only this it has also been found that bFGF supports cholinergic axonal sprouting in the injured adult brain [33]. On summerzing the scattered reports of continued neurogenesis in Subgranular zone (SGZ) and its phenotypic conversion to cholinergic subtype under the influence of bFGF, it is worthwhile to investigate the usefulness of exogenous bFGF supplement in restoring cognitive dysfunctions through dentate gyrus neurogenesis.
Therefore, in the present investigation an attempt has been made to study the neurogenic potential of bFGF in cognitive dysfunction induced by kainic acid in rats and assessed by neurobehavioural, neurochemical, and immunohistochemical parametres. The approach may suggest an alternative to fetal hippocampal cell transplantation towards long-term functional restoration in clinical cases.
Experimental procedures
Material
Kainic acid, chloral hydrate, 5-Bromodeoxyuridine (BrdU), Atropine sulphate, haloperidol, normal goat serum (NGS), primary monoclonal anti-cholinacetyltransferase antibody, primary monoclonal anti bromodeoxyuridine (BrdU) antibody, anti-mouse IgG antibody conjugated to fluorescein isothiocyanate (FITC), anti-mouse IgG antibody conjugated to (TRITC) and anti-mouse IgG antibody linked to peroxidase was purchased from Sigma Chemical Co. (USA) Radio ligand [3H]-spiperone (specific activity 15.7 Ci/mmol), [3H]-Quinclidinyl benzilate (QNB) (specific activity 42.0 Ci/mmol) was obtained from Amersham, (UK) and GF/C glass microfibre filters were obtained from Whatman (USA). bFGF Human recombinant was procured from Invitrogen, USA. All the other chemicals used in the study were of AR grade, which were available locally.
Animals and treatment
Male albino rats of Wistar strain (200 ± 10 g, body weight) obtained from the Industrial Toxicology Research Centre animal breeding colony were used in this study. The animals were housed in plastic polypropylene cages under standard animal house conditions with a 12 h light/dark cycle and a temperature of 25 ± 2 °C, with free access to drinking water and pellet diet (Hindustan Lever Laboratory Animal Feed, Kolkata, India). The institutional animal care and ethical committee approved all procedures of animal experimentation (Ethical committee approval no. ITRC/IAEC/23/2006)
Kainic acid lesion
Unilateral lesioning of CA3 subfield was performed by intrahippocampal administration of kainic acid using stereotaxic coordinates. In brief, rats were anesthetized with chloral hydrate (300 mg/kg body wt in normal saline ip). Fully anesthetized rats were then mounted into stereotaxic apparatus (Stoelting Co. USA). The incisor bar was set at 3.7 mm below the interaural line. In each rat the dorsal surface of the skull was exposed with midline incision, and a burr hole was drilled using following coordinates; AP 3.7 mm caudal to bregma, and L 4.1 mm right lateral to mid line and V 4.5 mm [34]. A 10 μl Hamilton syringe fitted with a 25-gauge needle and filled with kainic acid solution was placed over the burr hole and lowered 4.5 mm below the surface of the dura and 0.4 μg /2 μl of KA was injected at a rate of 0.2 μl/min [35] using auto injector device attached with stereotaxic apparatus. The needle was then left in place for 5 min and slowly retracted there after. Control rats were subjected to the same protocol except that kainic acid free normal saline solution was injected.
Exposure
Animals were assigned to four groups and were exposed as mentioned below. After four weeks of kainic acid lesioning of hippocampal CA3 region, animals were infused with bFGF (0.5 μg/2 μl) twice in dentate gyrus region to investigate the mitogenic /differentiating effect of bFGF on neurogenic cells of dentate gyrus. The functional recovery was assessed using neurobehavioural, neurochemical, and immunohistochemical parameters at four-week post last bFGF infusion.
Experimental groups
-
1.
Group I-(Sham)—received 2 μI of normal saline
-
2.
Group II-(Sham +B)—received 2 μI of normal saline and infused with bFGF
-
3.
Group III-(L)—KA lesioned
-
4.
Group IV-(L + B)—KA lesioned and infused with bFGF
bFGF Infusion
bFGF was infused in dentate gyrus region of rats at one and four week post lesioning through auto injector device attached to stereotaxic apparatus following stereotaxic coordinates AP—2.4, L 1.8 mm and V 4.1 mm to bregma as discussed by Paxinos and Watson [34]. The co-ordinates were similar to those used by Krug et al. [36]. bFGF (0.5 μg/2 μl in phosphate buffer saline) was infused slowly at the rate 1 ul/min. The needle was left in place for 5 min and slowly retracted thereafter [37].
BrdU labeling
Mitogenic effect of bFGF infusion was tracked through BrdU labeling. For this, animals received multiple (three–four) injections of BrdU (50 mg/kg ip; dissolved in sterile PBS). The animals, which were assessed after one week of bFGF infusion received BrdU at 1st, 3rd and 7th day post infusion. Animals assessed after four weeks of bFGF infusion were given BrdU at 15th, 19th, 25th, and 29th day post infusion [38].
Postoperative care
Individual animals were kept in a well-ventilated room at 25 ± 2 °C under observation till they recovered from anesthesia, with pellet diet (ad libitum) and water.
Neurobehavioural
Maze learning behaviour
To assess cognitive functions (learning and memory), the maze learning behaviour was evaluated in Y-maze (Techno, India), according to Adhami et al. [39], employing foot shock motivated visual discrimination response. This instrument consisted of three identical alleys or arms (26 cm × 13 cm × 12 cm) connected at 120° angles by a triangular central chamber. Scrambled foot shock could be given through the grid floor independently in any two alleys and in the central chamber at any one time, while the third alley remained shock free and illuminated.
Animal to be tested was placed in one arm and allowed to habituate for 2 min, followed by a session of 40 trials per day for two days. Each trial consisted of delivering of foot shock (0.5 mA) for 30 s to induce the animal to escape to the illuminated arm. The direction of illumination was changed (from clockwise to anti-clockwise) on each day of testing. The number of shock received by entering another shocked arm (incorrect arm) on each day of testing was computed and the relearning index (RI) was calculated as follows:
TS is the number of shock received during test (first day), and RLS the number of shocks received during relearning (second day). The number of correct runs (C) made when the direction of the illuminated arm was reversed was calculated as follows:
RLC is the number of positive responses made during relearning (second day); TC the number of positive responses made during test (first day).
Neurochemical studies
Radio receptor assays
In order to assess changes in the regulation of cholinergic (M) and/or dopamine (DA-D2) receptors, binding assay was performed in the hippocampal synaptic membrane preparations following the method of Agrawal et al. [40]. Rats were sacrificed by cervical dislocation followed by decapitation and their brains were dissected quickly on ice pack. Hippocampal region was dissected, weighed and processed for membrane preparation. In brief, synaptic membrane was prepared by homogenizing the tissue in 19 volume of prechilled 0.32 M sucrose followed by centrifugation at 50,000 g for 10 min. The pellet was rehomogenized in 5 mM Tris–HCl (pH 7.4) in same volume and centrifuged at same speed for 10 min. This step helps in removal of endogenous neurotransmitters as well as neuronal cell lysis. The pellet was finally suspended in 40 mM Tris–HCl (pH 7.4) and stored at –20 °C till assay. The binding incubation for cholinergic (M) and/or dopamine (DA-D2) was carried out in triplicate at 37 °C for 15 min using synaptic membrane fraction 100 ul, equivalent to 250–300 ug protein, with 1 nM of 3H- QNB (specific ligand for cholinergic-muscarinic receptors) or 3H-spiperone (specific ligand for DA-D2 receptor) in 40 mM Tris–HCl buffer. Parallel assay in triplicate using high concentration (1 μM) of unlabelled atropine sulphate (cholinergic antagonist) or haloperidol (DAD2 receptor blocker) was carried out to determine non-specific binding. After 15 min incubation at 37 °C, the reaction was terminated by cooling the reaction mixture in ice, the contents were filtered through glass micro fibre filters (Whatman GF/C) under vacuum and washed twice with 5 ml cold Tris–HCl buffer. The filters were dried and radioactivity was counted in 5 ml of scintillation mixture in LKB Rack β liquid scintillation counter (Packard Instrument, Germany) having an efficiency of 50% for tritium. Specific binding was calculated by subtracting non-specific binding obtained in presence of unlabelled competitor from total binding obtained in absence of atropine sulphate or haloperidol. The results are expressed in terms of pmole of ligand bound/gm protein. Protein was estimated by the method of [41].
Immunohistochemical study
ChAT/BrdU immunohistochemistry
A separate set of animals belonging to each group were assessed for ChAT/BrdU immunoreactivity in hippocampal CA3 and dentate gyrus region following the method of Barone et al. [42]. The rats from each group were deeply anesthetized with chloral hydrate (300 mg/kg, i.p.) and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4), followed by 4% paraformaldehyde in PBS for fixation of tissue. Brains were removed and post-fixed in the same fixative for 24 h followed by transfer to 10, 20, and 30% sucrose (w/v) in PBS. Serial coronal sections of 20 μm thicknesses were cut in freezing microtome (Slee Mainz Co., Germany) [43]. Non-specific binding sites were blocked by incubating the sections in PBS containing 1.5% NGS, 0.5% BSA and 0.1% Triton X-100. These sections were then incubated for 48 h in primary antibody (anti-ChAT antibody, 1:500, anti-BrdU antibody 1:500). After removing the primary antibody, sections were washed three times with PBS and incubated in peroxidase (1:100), FITC (green) or TRITC (red) (1:200) linked secondary antibody for 2 h at room temperature followed by three washes with PBS. Colour for peroxidase was developed with DAB as chromogen. Sections were transferred onto gelatin-coated glass slides, dehydrated, mounted in DPX, cover slipped and then visualized under phase contrast microscope. The fluorescent labelled sections were mounted in anti-fade mounting medium and were visualized under fluorescent microscope using appropriate filters.
Image analysis
The immunostained slides for ChAT were analyzed using Leica Qwin 500 image analysis software. The quantitative stereology was performed for each slide in triplicate with atleast 10 fields in each slide. The unbiased stereological method was employed, where a person unknown to the experimental design carried out the image analysis. The activity was expressed in terms of the % area that showed staining with DAB in respective fields.
Statistical analysis
Mean significant difference in the treatment groups was determined using one-way analysis of variance (ANOVA). The level of significance was analyzed by calculating the least significant difference. Values of P < 0.05 were considered to be statistically significant.
Result
General observation
No significant changes were observed in body weight, food and water intake and gross abnormality in behaviour between animals of lesioned and bFGF infused group, when compared to sham.
Behavioural studies
Maze learning behaviour
The results are summarized in Table 1.
A significant impairment in the learning behaviour indicated as relearning index (RI) was observed in the kainic acid lesioned rats (RI 40%, P < 0.001), when compared with sham operated animals. Similarly the memory retention as evident from frequency of positive response ΔC (when the direction of the current was reversed) was also impaired (38%, P < 0.001) in lesioned group. However the bFGF infused animals (four week post lesion) exhibited a significant restoration in learning (35%, P < 0.001) and retention (86%, P < 0.01).
Neurochemical studies
Cholinergic (Muscarinic)/dopaminergic receptor binding
The results are summarized in Fig. 1a and b.
(a) Cholinergic receptor binding in hippocampal synaptic membranes of rats (sham, sham + B, KA lesioned and KA + B groups). Significant decrease in cholinergic receptor binding in kainic acid lesioned rats is evident as compared to sham. A significant recovery in cholinergic receptor binding was observed in rats receiving bFGF infusion as compared to lesioned rats. Values represent mean ± SE of 8 rats. * One-way ANOVA, P < 0.01. Alphabet in superscripts signifies comparison to: a = versus sham; b = versus KA lesioned. (b) DA-D2 receptor binding in hippocampal synaptic membranes of rats belonging to sham, sham + B, KA lesioned and KA + B infused groups. Significant decrease in DA-D2 receptor binding in kainic acid lesioned rats is evident as compared to sham. A significant recovery of DA-D2 receptor binding was observed in rats receiving bFGF infusion as compared to lesioned rats. Values represent mean ± SE of 8 rats. One-way ANOVA, * P < 0.05, ** P < 0.01. Alphabet in superscripts signifies comparison to: a = versus sham; b = versus KA lesioned
The neurotransmitter receptor binding was carried out in hippocampal area of lesioned animals. The results revealed a significant decrease in cholinergic (58%, P < 0.01) and dopaminergic DA-D2 (39%, P < 0.01%) receptor binding in lesioned rats when compared with sham animals. The bFGF infused animals four-week post infusion exhibited significant restoration in cholinergic (76%, P < 0.01) and dopaminergic (DA-D2 46%, P < 0.05) receptor binding when compared with lesioned group. The result revealed more pronounced affect on cholinergic system over the dopaminergic receptor.
Immunohistochemical studies
Choline Acetyltransferase (ChAT) expression
The results of immunohistochemical studies in choline acetyltransferase (ChAT) expression are summarized in Fig. 2(a–f).
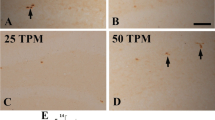
Photomicrograph of CA3/Dentate gyrus region of hippocampal sections illustrating ChAT-immunoreactive neurons and BrdU positive neurons of CA3 region KA lesioned rats (b) had shown diminished ChAT positivity as compared to sham (a). Lesioned rats receiving bFGF infusion (c) has shown high expression of ChAT immunopositive cells in comparison to lesioned rats. CA3 region has shown ChAT positive cells (d) and BrdU positive cells (e) and their colocalization (f). DG region has shown ChAT immunopositive neurons (g) and BrdU labelled neurons (h). Scale bar = 350 μm (a,b,c), 150 μm (d,e,f), 200 μm (g,h)
The neuroprotective/mitogenic affect of bFGF and functional viability of cholinergic neurons in the hippocampal CA3 was further assessed by mapping the rate limiting enzyme, ChAT for acetyl choline biosynthesis using monoclonal antibody against ChAT. In KA lesioned rats, number of surviving ChAT- ir neurons was significantly less (Fig. 2b) as compared to those in the sham group (Fig. 2a). Four weeks bFGF infused group exhibited a significant increase in ChAT- ir neurons, when compared to lesioned group (Fig. 2c).
In order to quantify total ChAT immunoreactivity in ipsilateral hippocampal CA3 region, image analysis (stereology) was performed in ChAT positive sections. Kainic acid lesioning caused a significant decrease (P < 0.001) in ChAT- ir area as compared to sham. It is evident from the result that number of ChAT- ir neurons is significantly upregulation (65%, P < 0.001) in bFGF infused group as compared to lesioned rats (Fig. 3).
Image analysis of photomicrographs showing ChAT immunoreactivity (% area) in hippocampus of sham, sham + B, lesioned, KA + B group of rats. The data represent mean ± SE of values obtained from the quantitative stereology performed for each slide (in triplicate, 10 microscopic fields) using Leica Qwin 500 image analysis software. * P < 0.01, ** P < 0.001. Alphabet in superscripts signifies comparison to: a = versus sham; b = versus KA lesioned
BrdU expression
To validate the extent of cell proliferation under the influence of bFGF infusion in kainic acid lesion rats BrdU labelled immunoreactive cells was assessed in DG region indicating the mitogenic effect of bFGF (Fig. 2g, h). Number of BrdU-labelled cells observed in DG (Fig. 2g) region and ChAT-ir positive cells (Fig. 2h).
Discussion
The regulation and manipulation of neurogenesis and neurodegeneration via growth factor signaling has broad implications for both the development of therapeutic strategies for repair of CNS undergoing neurodegenerative changes and for advancing our understanding of basic cellular mechanisms of regeneration from brain injury.
In the present investigation, emphasis has been laid on regenerative potential of intradentate bFGF administration on functional recovery related to cognitive dysfunction induced by kainic acid lesioning in rats and its possible correlation with neurogenesis in dentate gyrus. Hippocampus occupies a key position within the limbic system and has been associated with a variety of cognitive functions and its degeneration leads to a number of behavioural and neurochemical deficits [44, 45]. Hippocampus is composed of mainly two specific region dentate gyrus and Cornu Ammonis (CA) which is further differentiated into fields CA1, CA2, and CA3.
A Unilateral lesioning of CA3 region with Kainic acid reportedly causes degeneration of substantial pyramidal neurons mainly cholinergic subtype in this region. Intrahippocampal kainic acid lesioning further shows a uniform reduction in markers for cholinergic system as evident from decrease cholinergic receptors along with diminished ChAT immunoreactiviy of ipsilateral side suggesting substantial degeneration of cholinergic neurons. This correlates with earlier reports where the extent of cholinergic loss was associated with severity of memory impairment [46]. Consistent with the observed loss of cholinergic neurons we found a lesion induced impaired learning ability in lesioned animals as indicated by decreased Y-Maze learning four week post lesioning. A significant neurobehavioural restoration in KA lesioned animals at four week following intradentate bFGF infusion was also observed. However contrary to earlier belief that central nervous system has no neuroregeneration, recently it was discovered that even in the conditions of severe neuronal loss few areas of brain have been imparted with the unique regenerative capacity of continued neurogenesis through neuroprogenitor cell proliferation, which was suggested to be regulated by the level of neurotrophins [47–49]. Such a possibility cannot be over ruled in our experiment where we observed a significant neurobehavioural restoration in KA lesioned rats four week following intradentate bFGF infusion. This can be correlated with recent reports, where hippocampal injury inflicted by Kainic acid was shown to provoke production of new neurons in the adult DG as an immediate response under the mitogenic influence of enhanced neurotrophin release from dying neurons/reactive glia [24, 50]. Such induction however was reportedly not persistent and last for only few week post injury parallel to normalization in the levels of neurotrophic factors. To overcome, this reversal, we had selected two infusion stages of mitogenic factor (bFGF) in our experiment to cover early mitogenic phase and subsequent differentiaon stage. We were able to track significant mitogenic effect of bFGF on DG through BrdU labeling one-week post infusion where a significant number of BrdU immmunoreactve cells could be observed. Further co-localization of BrdU and ChAT Immunoreactivity post four-week infusion strongly suggests its phenotypic conversion to cholinergic phenotype. bFGF is reportedly a potent supporter of adult brain neurogenesis and modulates proliferation and differentiation of adult precursor cells as well [51, 52, 30, 53]. It was further shown to enhance the neuronal survival and to play a prominent role in the regulation of CNS responses to injury by promoting neurotrophic factor release [54–56]. bFGF treatment significantly increases sprouting of cholinergic neurons of septodentate pathway showing affinity for cholinergic neuronal fibre [54].
In addition to impaired cholinergic receptor, the lesioned animals also exhibited significant decrease in dopamine receptor binding as well. This effect could be of significance in maintaining the synaptic plasticity of this region [57, 58]. Further a restoration in dopamine receptor binding in hippocampal region was observed in bFGF infused animals, which can be correlated with cholinergic recovery. The hippocampus receives rich dopaminergic input particularly from the ventral tegmental area, and expresses DA receptors/enzymatic machinery associated with dopaminergic target cells [59]. A functional role of the hippocampal dopaminergic system has been substantiated in cognitive functions such as positive reinforcement learning, visual discrimination and passive avoidance behaviour [60]. Our observation on upregulation of DAD2 receptor as part of bFGF infusion in hippocampal region further strengthen the possibility of mitogenic effect of bFGF on cholinergic and dopaminergic input which is lost as part of kainic acid lesion.
Our results suggests the possible mitogenic effect of bFGF infusion on dentate gyrus (DG) region may potentiate the ability of continuous dividing and regenerating cells (progenitor cells) of DG (subgranular cells) and their transformation to cholinergic type (phenotypic). A possibility of migration of the new cells from lesioned CA3 region is also foreseen, as a possible repair mechanism. The study further implicates the usefulness of mitogenic influence of bFGF towards recovery in potentiating the learning ability at early stage of dementia avoiding cell replacement therapy.
References
Kempermann G, Wiskott L, Gage FH (2004) Functional signicance of adult neurogenesis. Curr Opin Neurobiol 14:186–191
Abrous DN, Koehl M, Moal ML (2005) Adult neurogenesis: from precursors to network and physiology. Physiol Rev 85:523–569
Doetsch F, Hen R (2005) Young and excitable: the function of new neurons in the adult mammalian brain. Curr Opin Neurobiol 15:121–128
Rosato SM, Cattaneo A, Cherubini E (2006) Nicotine-induced enhancement of synaptic plasticity at CA3–CA1 synapses requires GABAergic interneurons in adult anti-NGF mice. J Physiol 576:361–377
Row BW, Kheirandish L, Cheng Y, Rowell PP, Gozal D (2007) Impaired spatial working memory and altered choline acetyltransferase (ChAT) immunoreactivity and nicotinic receptor binding in rats exposed to intermittent hypoxia during sleep. Behav Brain Res 177:308–314
Auls DS, Kornecook TJ, Bastianetto S, Quirion R (2002) Alzheimer’s disease and the basal forebrain cholinergic system: relation to β amyloid peptides, cognition, and treatment strategies. Prog Neurobiol 68:209–245
Kar S, Slowikowski SP, Westaway D, Mount HT (2004) Interactions between beta-amyloid and central cholinergic neurons: implications for Alzheimer’s disease. J Psychiatry Neurosci 29:427–441
Chun SK, Sun W, Park JJ, Jung MW (2006) Enhanced proliferation of progenitor cells following long-term potentiation induction in the rat dentate gyrus. Neurobiol Learn Mem 86:322–329
Shimazu K, Zhao M, Sakata K, Akbarian S, Bates B, Jaenisch R (2006) NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn Mem 13:307–315
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4:1313–1317
Gould E, Gross CG (2002) Neurogenesis in adult mammals: some progress and problems. J Neurosci 22:619–623
Gross CG (2000) Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci 1:67–73
Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, Kim SH, Langdon RB, Sisodia SS, Tsien JZ (2001) Deficient neurogenesis in forebrain specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron 32:911–926
Monje ML, Toda H, Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science 302:1760–1765
Monje ML, Mizumatsum S, Fike JR, Palmer TD (2002) Irradiation induces neural precursor-cell dysfunction. Nat Med 8:955–962
Monje ML, Palmer T (2003) Radiation injury and neurogenesis. Curr Opin Neurol 16:129–134
Gray WP, Sundstrom LE (1998) Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res 790:52–59
Choi YS, Lee MY, Sung KW, Jeong SW, Choi JS, Park HJ, Kim ON, Lee SB, Kim SY (2003) Regional differences in enhanced neurogenesis in the dentate gyrus of adult rats after transient forebrain ischemia. Mol Cells 16:232–238
Felling RJ, Levison SW (2003) Enhanced neurogenesis following stroke. J Neurosci Res 73:277–283
Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH (1997) Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 17:3727–3738
Nakagawa E, Aimi Y, Yasuhara O, Tooyama I, Shimada M, Mc Geer PL, Kimura H (2000) Enhancement of progenitor cell division in the dentate gyrus triggered by initial limbic seizures in rat models of epilepsy. Epilepsia 41:10–18
Dinocourt CL, Gallagher SE, Thompson SM (2006) Injury-induced axonal sprouting in the hippocampus is initiated by activation of trkB receptors. Euro J Neurosci 24:1857–1866
Ikeda T, Koo H, Xia YX, Ikenoue T, Choi BH (2002) Bimodal upregulation of glial cell line-derived neurotrophic factor (GDNF) in the neonatal rat brain following ischemic/hypoxic injury. Int J Dev Neurosci 20:555–562
Shetty AK, Zaman V, Shetty GA (2003) Hippocampal neurotrophin levels in a kainate model of temporal lobe epilepsy: a lack of correlation between brain-derived neurotrophic factor content and progression of aberrant dentate mossy fiber sprouting. J Neurochem 87:147–159
Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, Potts MB, Fike JR (2006) Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol 202:189–199
Chaturvedi RK, Shukla S, Seth K, Agrawal AK (2005) Nerve growth factor increases survival of dopaminergic graft, rescue nigral dopaminergic neurons and restores functional deficits in rat model of Parkinson’s disease. Neurosci Lett 398:44–49
Sun Y, Jin K, Childs JT, Xie L, Mao XO (2006) Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev Biol 289:329–335
Youssoufian M, Walmsley B (2007) Brain-derived neurotrophic factor modulates cell excitability in the mouse medial nucleus of the trapezoid body. Eur J Neurosci 25:1647–1652
Yoshimura S, Takagi Y, Harada J, Teramoto TS, Thomas S, Waeber C, Bakowska JC, Xandra O, Breakefield, Moskowitz MA (2001) FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. PNAS 98:5874–5879
Jin K, LaFevre- BM, Sun Y, Chen S, Gafni J, Crippen D, Logvinova A, Ross CA, Greenberg DA, Ellerby LM (2005) FGF-2 promotes neurogenesis and neuroprotection and prolongs survival in a transgenic mouse model of Huntington’s disease. Proc Natl Acad Sci 102:17889–17890
Spencer B, Agarwala S, Gentry L, Brandt CR (2001) HSV-1 vector-delivered FGF2 to the retina is neuroprotective but does not preserve functional responses. Mol Ther 3:746–756
Machon O, Backman M, Krauss S, Kozmik Z (2005) the cellular fate of cortical progenitors is not maintained in neurosphere cultures. Mol Cell Neurosci 30:388–397
Fagan AM, Suhr ST, Lucidi-Phillipi AA, Peterson DA, Holtzman DM, Gage FH (1997) Endogenous FGF-2 is important for cholinergic sprouting in the denervated hippocampus. J Neurosci 1:2499–2511
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates, 4th edn. Academic Press, California, USA
Zaman V, Shetty AK (2001) Fetal hippocampal ca3 cell grafts transplanted to lesioned ca3 region of the adult hippocampus exhibit long-term survival in a rat model of temporal lobe epilepsy. Neurobio Dis 8:942–952
Manfred Krug, Rudolf Brödemann, Renate Matthies, Heinz Rüthrich, Maria Wagner (2001) Activation of the dentate gyrus by stimulation of the contralateral perforant pathway: evoked potentials and long-term potentiation after ipsi- and contralateral induction. Hippocampus 11:157–167
Vorobyova V, Schibaev N, Kovalev G, Alzheimerd C (2005) Effects of neurotransmitter agonists on electrocortical activity in the rat kainate model of temporal lobe epilepsy and the modulatory action of basic fibroblast growth factor. Brain Res 1051:123–136
Yoshimura S, Teramoto T, Whalen MJ, Irizarry MC, Takagi Y, Jun Harada JQ, Waeber C, Xandra OB, Moskowitz MA (2003) FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J Clin Invest 112:1202–1210
Adhami VM, Husain R, Husain R, Seth PK (1996) Influence of iron deficiencies and lead treatment on behaviour and cerebellar and hippocampal polyamine levels in neonatal rats. Neurochem Res 21:915–922
Agrawal AK, Squib RE, Bondy SC (1981) Effect of acrylamide treatment upon dopamine receptor binding. Toxicol Appl Pharmacol 58:89–99
Lowry OH, Rosenburg NJ, Farr AL, Randall RJ (1951) Protein measurement by Folin phenol reagent. J Biol Chem 193:165–175
Barone S, Tandon P, McGinty JF, Tilson HA (1991) The effect of NGF and fetal cell transplantation on spatial learning after intra-dentate administration of colchicines. Exp Neurol 114:351–363
Agrawal AK, Shukla S, Chaturvedi RK, Seth K, Srivastava N, Ahmad A, Seth PK (2004) Olfactory ensheathing cell transplantation restores functional deficits in rat model of Parkinson’s disease: a cotransplantation approach with fetal ventral mesencephalic cells. Neurobiol Dis 16:516–526
Roy TS, Seidler FJ, Slotkin TA (2002) Prenatal nicotine exposure evokes alterations of cell structure in hippocampus and somatosensory cortex. J Pharmacol Exp Ther 300:124–133
Roy TS, Sharma V, Seidler FJ, Slotkin TA (2005) Quantitative morphological assessment reveals neuronal and glial deficits in hippocampus after a brief subtoxic exposure to chlorpyrifos in neonatal rats. Brain Res Dev Brain Res 155:71–80
Bellucci A, Luccarini I, Scali C, Costanza P, Giovannini MG, Casamenti PF (2006) Cholinergic dysfunction, neuronal damage and axonal loss in TgCRND8 mice. Neuro Dis 23:260–272
Bauer S, Patterson PH (2006) Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci 26:12089–12099
Leker RR, Soldner F, Velasco I, Gavin DK, Androutsellis-TA, McKay RD (2007) Long-lasting regeneration after ischemia in the cerebral cortex. Stroke 38:153–161
Broughton SK, Chen H, Riddle A, Kuhn SE, Nagalla S, Roberts Jr, Back SA (2007) Large-scale generation of highly enriched neural stem-cell-derived oligodendroglial cultures: maturation-dependent differences in insulin- like growth factor-mediated signal transduction. J Neurochem 100:628–638
Shetty AK, Rao MS, Hattiangady B, Zaman V, Shetty GA (2004) Hippocampal neurotrophin levels after injury: relationship to the age of the hippocampus at the time of injury. J Neurosci Res 78:520–532
Ford-Perriss M, Abud H, Murphy M (2001) Fibroblast growth factors in the developing central nervous system. Clin Exp Pharmacol Physiol 28:493–503
Tureyen K, Vemuganti R, Bowen KK, Sailor KA, Dempsey RJ (2005) EGF and FGF-2 infusion increases post-ischemic neural progenitor cell proliferation in the adult rat brain. Neurosurgery 57:1254–1263
Maric D, Fiorio PA, Chang YH, Barker JL (2007) Self-renewing and differentiating properties of cortical neural stem cells are selectively regulated by basic fibroblast growth factor (FGF) signaling via specific FGF receptors. J Neurosci 27:1836–1852
Ramirez JJ, Finklestein SP, Keller J, Abrams W, George MN, Parakh T (1999) Basic fibroblast growth factor enhances axonal sprouting after cortical injury in rats. Neuroreport 10:1201–1204
Smith C, Berry M, Clarke WE, Logan A (2001) Differential expression of fibroblast growth factor-2 and fibroblast growth factor receptor 1 in a scarring and nonscarring model of CNS injury in the rat. Eur J Neurosci 13:443–456
Hagood SK, McGinn MJ, Sun D, Colello RJ (2006) characterizes the mitogenic effect of basic fibroblast growth factor in the adult rat striatum. J Neurotrauma 23:205–215
Otmakhova NA, Lisman JE (1998) D1/D5 dopamine receptors inhibit depotentiation at ca1 synapses via camp-dependent mechanism. J Neurosci 18:1270–1279
Laplante F, Sibley DR, Quirion R (2004) Reduction in acetylcholine release in the hippocampus of dopamine D5 receptor-deficient mice. Neuropsychopharm 29:1620–1627
Otmakhova NA, Lisman JE (1996) D1/D5 dopamine receptor activation increases the magnitude of early long- term potentiation at CA1 hippocampal synapses. J Neurosci 16:7478–7486
Lisman JE, Otmakhova NA (2001) Storage, recall and novelty detection of sequences by the hippocampus; elaborating on the Socratic model to account for normal and aberrant effects of dopamine. Hippocampus 11:551–568
Acknowledgments
We are grateful to Dr. C.M. Gupta, Director, and ITRC for his continuous support during this study. Nishi Srivastava is recipient of Senior Research Fellowship from ICMR, New Delhi. K. Seth is a recipient of WOS (Women Scientist Award) from Department of Science and Technology (DST), New Delhi. Technical assistance of Mr. Kailash Chandra is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srivastava, N., Seth, K., Srivastava, N. et al. Functional Restoration Using Basic Fibroblast Growth Factor (bFGF) Infusion in Kainic Acid Induced Cognitive Dysfunction in Rat: Neurobehavioural and Neurochemical Studies. Neurochem Res 33, 1169–1177 (2008). https://doi.org/10.1007/s11064-007-9478-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9478-7