Abstract
Alzheimer’s disease is a progressive and age-related neurodegenerative disorder that is manifested by neuropathological changes and clinical symptoms. Recently, cell-based therapeutic interventions have been considered as the promising and effective strategies in this field. Herein, we investigated therapeutic effects of neural stem cell secretome on Alzheimer’s disease-like model by triggering of Wnt/β-catenin signaling pathway. In this study, mice were randomly allocated into three different groups as follows: Control, AD + Vehicle, and AD + NSCs-CM groups. To induce mouse model of AD, Aβ1-42 was injected into intracerebroventricular region. Following AD-like confirmation through thioflavin S staining and Passive avoidance test, about 5 µl mouse NSCs-CM was injected into the target areas 21 days after AD induction. For evaluation of endogenous proliferation rate (BrdU/Nestin+ cells), 50 µg/kbW BrdU was intraperitoneally injected for 5 consecutive days. To track NSC differentiation, percent of BrdU/NeuN+ cells were monitored via immunofluorescence staining. Histological Nissl staining was done to neurotoxicity and cell death in AD mice after NSCs-CM injection. Morris Water maze test was performed to assess learning and memory performance. Data showed that NSCs-CM could reverse the learning and memory deficits associated with Aβ pathology. The reduced expression of Wnt/β-catenin-related genes such as PI3K, Akt, MAPK, and ERK in AD mice was increased. Along with these changes, NSCs-CM suppressed overactivity of GSK3β activity induced by Aβ deposition. Besides, NSCs increased BrdU/Nestin+ and BrdU/NeuN+ cells in a paracrine manner, indicating proliferation and neural differentiation of NSCs. Moreover, neurotoxicity rate and cell loss were deceased after NSCs-CM injection. In summary, NSCs can regulate adult neurogenesis through modulating of Wnt/β-catenin signaling pathway and enhance the behavioral performance in the AD mice. These data present the alternative and effective approach in the management of AD and other cognitive impairments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
AD is one of the common forms of neurodegenerative disorders accounting for 70℅ of the dementia cases in the elderly populations (Alzheimer’s Association, 2016; Plassman et al., 2007). AD-related pathologies are associated with neuronal loss, synaptic deficiency, cognitive impairments, and lead to severe clinical symptoms including learning disability, progressive memory loss, psychosocial problems, and incapability to retain daily activities (Patterson, 2018; Scheltens et al., 2016; Walsh & Selkoe, 2004). Globally, over 50 million people suffer from AD and it is predicted that this number will be increased up to quadruplet by 2050 particularly in low-income countries (Esfandiary et al., 2018; Patterson, 2018). Aβ plaques and neurofibrillary tau tangles as the primary microscopic neuropathological findings are detectable in the early stages of AD. These changes can promote inflammatory response, neurotoxicity, and brain atrophy (Terry et al., 1991; Iqbal et al., 2016; Chen & Mobley, 2019). The peptide Aβ is produced by the enzymatic activity of β and γ-secretases on amyloid β-precursor protein. These enzymes are located at the extracellular and cytosolic sides of neural cell membrane, respectively (Deng et al., 2013; Wang et al., 2019). In AD patients, the aggregation of Aβ monomers, as common pathological finding, supports Aβ plaques deposition around the neurites, synaptic surface area, and blood vessels periphery (Selkoe & Hardy, 2016; Serrano-Pozo et al., 2011). In the normal condition, Tau protein stabilizes and organizes the orientation and configuration of the intracellular microtubules, leading to regulation of chemicals, nutrients, and axonal transportation (Ameri et al., 2020; Drubin & Kirschner, 1986; Weingarten et al., 1975). Upon onset of AD, tau protein is excessively phosphorylated where the accumulation of tangled clumps inside cytosol induces neurotoxicity (Binder et al., 2005; Grundke-Iqbal et al., 1986). Apart from this fact, these changes separate microtubule subunits and disrupted uniformity inhibits axonal transportation and message conduction (Ebneth et al., 1998; Hoover et al., 2010). Growing evidence shows that in AD condition, Aβ plaques binds to Wnt receptors and lead to activation of GSK-3β and Tau protein hyperphosphorylation. (Hernández et al., 2010; Hooper et al., 2008; Magdesian et al., 2008). As a correlate, the Aβ plaques and NFTs coincided with neuronal loss and failure cells communication (Alonso et al., 1997; Gouras et al., 2000). Therefore, aberrant cellular and molecular signaling pathways activity, inflamed conditions, lack of trophic factors, glial scar formation, extensive cell death, and decline of neurogenesis are the main consequences of AD and other cognitive impairments (Clevers & Nusse, 2012; Heneka et al., 2015; Lempriere, 2019).
Neurogenesis is a physiological and neural structural plasticity phenomenon that occurs in the embryonic and adult periods in rodents and humans (Eriksson et al., 1998; Ming & Song, 2011). This is a multiphase process includes proliferation, migration, differentiation of NSCs, and integration of the new-generating neurons into the pre-existing host tissue in the subgranular zone of the dentate gyrus in the hippocampus (Gage, 2019; Ming & Song, 2011). Several studies and experiments have shown that there is a close correlation between AD development and neurogenesis impairment (Moreno-Jiménez et al., 2019; Zhao et al., 2008). One of the therapeutic implications in AD patients is related to the regulation of neurogenesis and behavior of NSCs in the adult dentate gyrus of hippocampus (Baptista & Andrade, 2018; Diaz Brinton & Ming Wang, 2006; Kempermann et al., 1997). In this regarding, the crucial role of various molecular and cellular signaling pathways such as notch, sonic hedgehog, (BMP/SMAD), Wnt, and JNK have been documented in the in-vitro and in-vivo experiments (Faigle & Song, 2013; Kwak et al., 2014).
Because of fundamental role of Wnt/β-catenin signaling pathway in neural development, neurogenesis, GSK-3β inactivation, and Tauopathy suppression, this signaling axis is at the center of attention (Patapoutian & Reichardt, 2000; Wodarz & Nusse, 1998). Besides, Wnt/β-catenin signaling pathway regulates the structure and function of neurogenic niches and hippocampal neurogenesis; likewise, it seems that Wnt/β-catenin can rescue the Aβ-induced functional deficits (Rosso & Inestrosa, 2013; Wan et al., 2014). The activity of this pathway is regulated by neurotrophic factors (Chen et al., 2013; Rosso & Inestrosa, 2013). Considering the paracrine activity and potency of NSCs to release the neurotrophic factors (Lu et al., 2003), therefore, in the present study, we decided to examine the possible efficiency of NSCs-CM on Wnt/β-catenin signaling pathway, neurogenesis, and functional performance in the in-vivo setting.
Material and Methods
Study Design and Ethical Issues
In this study, 8-week-old BALB/c male mice (n = 36), weighing 40–50 gr, were purchased from the animal house at Tabriz University of Medical Science. All mice were maintained under an air conditioned with constant temperature (23 ± 1 °C) and 12-h light–dark cycle for 2 weeks. All of the animals were accessed to chewing pellets and water during the experiment. All the experiments were conducted according to internationally accepted guidelines and approved by a local ethics committee of Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1397.239). After adaptation and acclimatization period, mice were randomly assigned into three groups, each in 12, as follows: Control (AD + vehicle) and (AD + NSCs-CM). In the AD + vehicle group, the mice were received just 5 µl PBS. In the AD + NSCs-CM group, NSCs-CM was injected into bilateral ventricles following confirmation of mice AD-like induction (Supplementary Fig. 1).
NSC Isolation
In the current study, three pregnant mice with 14-day embryo were sacrificed after anesthesia. The abdominal cavity was exposed and the embryos transferred into the culture dish containing DMEM/F-12 (Cat no: 21331-020; Gibco) plus 5% Pen-Strep solution (Cat no: 15140122; Gibco). Under operating microscope, the brains were dissected and ganglionic eminences were removed. After several washes with cold PBS, NSCs were isolated using mechanical chopping and Trypsin–EDTA enzymatic digestion for 3 min. Next, cell suspension was centrifuged at 700 rpm for 5 min and harvested single cells were cultured (50,000 cells/cm2) in DMEM/F-12 supplemented with 1% Pen-Strep, 2% B27 (Cat no: 17504044; Gibco), 20 ng/mL EGF (Gibco), 10 ng/mL (Gibco), and 2 μg/ml heparin and were incubated at 37 °C with 5% CO2. In the primary culture, these cells in 7–10 days after plating produce neurospheres with diameter approximately 200–250 µm. Following the first passage, the cells were sub-cultured and neurospheres were passaged every 5–7 days. NSCs at passages 3–5 were used to all analyses (Azari et al., 2011; Leeson et al., 2018).
Characterization of NSCs
The morphology of NSC neurospheres was assessed under a bright-field microscope for 9 days. To confirm the stemness feature of isolated cells, we performed immunofluorescence analysis of nestin (Azari, 2013; Leeson et al., 2018; Vafaei et al., 2021). NSCs at the 3 passages were detached and about 1 × 104 cells seeded at each well of eight-well chamber slide (SPL) pre-coated with laminin 1 µg/CM2. Upon 70–80% confluence, cells were fixed with 4% paraformaldehyde (PFA) for 20 min, permeabilized with Triton-X100 solution, and blocked with 1% BSA for 30 min. Cells were incubated with the PE-conjugated anti-Nestin antibody for 2 h at RT. After PBS washes, nuclei were stained with DAPI stain. Ultimately, the cells were visualized using by Olympus microscope (Model: BX41) and the percent of nestin+ cells was calculated. Three sets of experiments were performed.
ELISA
To evaluate the paracrine activity of NSCs, we performed the ELISA assay (Jahed et al., 2021; Rhee et al., 2016). Briefly, the CM was harvested and centrifuged at 300 and 2000 × g for 10 min at 4 °C, respectively. For sterilization, CM was filtered using 0.22-μm-sized filters to eliminate the cellular debris. Then, CM was transferred to the Amicon Ultra-15 centrifugal filter and centrifuged (with cut off 40 kD) at 4000 × g at 4 °C for 10 min. Finally, the concentrated CM was washed with PBS and stored in − 20 °C until use. ELISA assay was applied to determine BDNF and NGF levels according to the manufacturer’s instructions and absorbance was read at 450 nm using microplate reader (BioTek).
AD-Like Model Induction
For the induction of AD-like mouse model, Aβ1-42 was injected into the ICV space using stereotaxic surgery (Kim et al., 2016; Lee et al., 2020; Schmid et al., 2017). In brief, 1 mM Aβ1-42 was dissolved in 0.1 M PBS and aggregated by the incubation at 37 °C for 5 days. The mice in the (AD + vehicle) and (AD + NSCs-CM) groups were intraperitoneally anesthetized with the injection of the 80 mg/kg Ketamine and 10 mg/kg Xylazine along with the control of body temperature using a rectal probe and a thermocouple device throughout the experiments. The animals were placed onto stereotaxic apparatus (Stoelting, USA) and the target site (AP: − 0.8, ML: ± 1.4, and DV: − 4 mm) was localized using the stereotaxic atlas of Paxinos and Watson. Then, 100 µM Aβ1-42 (Sigma, St. Louis, MO) was injected into ICV bilaterally. After surgery, the mice were placed in heated chambers at the recovery room and returned to the home cage. Passive avoidance test was performed to assess the learning and memory impairment and AD confirmation on the 21 days following surgery.
Detection of Aβ Plaques Formation and Deposition
Thioflavin S staining was performed to determine the toxic effects of Aβ1-42 on neural cells and amyloid β sheet plaques formation (Ly et al., 2011; Rajamohamedsait & Sigurdsson, 2012). In brief, 21 days after Ad induction, 3 mice were randomly closed from the AD group, sacrificed and brain dissected and hippocampus was removed. Following fixation, tissue processing, and embedding in paraffin, the tissue sections were mounted on glass slides and dried at room temperature. Then the sections were deparaffinized, hydrated in descending alcohols washed in water, and then stained with 1% Thioflavin S for 30 min. Thereafter, the sections were dehydrated by incubating the sections in ascending alcohols, cleared in xylene, coverslipped with mounting media, and visualized under a fluorescence microscope.
Passive Avoidance Test
A passive avoidance test was done using the shuttle-box apparatus to evaluate the learning capacity and memory retention (Esfandiary et al., 2015; Karimipour et al., 2019; Xie et al., 2018). This test was scheduled into adaptation, training, and probe steps in 3 consecutive days. In the adaptation stage, the mice were habituated to a shuttle-box environment. In the training step, the mice were placed in the light chamber and one minute later, the guillotine door was opened and once the animal was entered into the dark chamber, received an electric shock (40 V, 0.5 A. 2 s). On the probe test, the mice were placed into the light chamber and allowed to enter into the dark chamber in the 5-min period. Step-through latency was considered as the memory retention or retrieval index.
Transplantation of NSCs-CM
After AD confirmation and completion of passive avoidance test, the NSCs-CM was bilaterally delivered into ICV (Nakano et al., 2020). Using stereotaxic apparatus, 5 µl NSCs-CM was injected into ICV using micro pump injection with 1 µl/min velocity rate. To prevent of backflow, the needle was kept at the site of injection for 5 additional minutes.
BrdU Administration
For labeling of endogenous NSCs, 10 mg/ml BrdU (Sigma) was dissolved in 0.9% normal saline and sterilized using 0.22-µm microfilters. Then, 50 mg/kbw BrdU was intraperitoneally injected for 7 consecutive days.
MWM Performance Procedure
MWM task was conducted to explore the spatial learning and memory capacity on the 28 days after the last BrdU injection (Esfandiary et al., 2014; Karimipour et al., 2019; Nakano et al., 2016, 2020). Briefly, in the maze room, a tank (180 cm in diameter and 60 cm in high) was filled with water (22 ± 1 °C) and a platform was submerged in the tank below 2 cm the water surface. The tank virtually was divided into 4 identical quadrants and visual cues and signs were included in all directions in the maze room. In the habituation phase, the mice were placed in a random direction and allowed to swim in the pool without a platform for the 60 s. The training phase was designed into 5 blocks and 4 trials in every block for 5 consecutive days. In all trials, the mice were placed in the tank in a randomly starting position and allowed to find the hidden platform in the 60 s. After reaching the platform, they were allowed to stay there for the 30 s and then returned to the holding cage for 10 min until the next task. If the animal could not find the platform, they were gently guided to the platform and allowed to stay there for the 30 s. In all trials, escape latency (taking time to reach and find the platform) and the distance traveled by the mice were evaluated. In the probe trial (Test day), the platform was removed and the average time spent in the target quadrant was measured where the platform located in the training trials.
Tissue Sampling and Preparation
In the current study, tissue sampling was done in two distinctive time periods and the mice were sacrificed and hippocampus subjected to molecular, cellular, and histological analyses as previously described (Karimipour et al., 2019). The first tissue sampling (3 per group) was performed 1 day following the last BrdU injection for evaluation of the endogenous NSCs proliferation indicated by BrdU/Nestin double immunofluorescence (IF) staining. The second tissue sampling was performed after the MWM test to investigate the Real-time RT-PCR analysis (n = 3 per group), Nissl staining (3 per group), and neurogenesis (3 per group) indicated by BrdU/NeuN-double labeling. For histological and cellular investigations, the mice were anesthetized and perfused with normal saline and followed by 4℅ paraformaldehyde. Following decapitation and brain dissection, the right hippocampi were removed and post-fixed in 4℅ paraformaldehyde overnight at 4 °C. The next day, samples were washed in tap water and processed using Benchtop Tissue Processor and embedded in paraffin. For immunofluorescence and Nissl staining, 12 coronal serial sections were prepared using systematic uniform random sampling design. For gene expression analysis, mice were generally anesthetized and decapitated. Brains were removed, the right hemispheres dissected, and hippocampi stored at − 80 °C until use.
Double-IF Staining
To assess the potential effect of NSCs-CM on endogenous NSCs proliferation and neurogenesis, we monitored the percent of BrdU/Nestin and BrdU/NeuN double-IF staining on days 1 and 35 days after last BrdU injection. In brief, 5-µm-thick sections were mounted on the slides pre-coated with Poly-l-lysine. Then, the slides were deparaffinized, rehydrated in decreasing ethanol solutions, and incubated in Tris-buffered saline (TBS; 0.1 M Tris–HCl, pH 7.4, and 0.9% NaCl) for 10 min. For antigen retrieval, the sections were incubated in pre-heated 10 mM sodium citrate buffer for 10 min at 100 °C, and then the sections incubated in 2 N HCL at 37 °C for 30 min to promote DNA denaturation. The samples were neutralized by incubating the slides in 0.1 M boric acid (pH 8.5) solution for 10 min at RT followed by washing TBS. To reduce non-specific binding, the slides were incubated in blocking solution TBS++ (TBS + 3% goat serum + 0.3% Triton-X 100) for 30 min at RT. Then, the sections were incubated in a mixture of primary antibodies (mouse monoclonal anti-Nestin antibody, Clone: 10C2, Cat#: MA1-110, Dilution: 1:100, and mouse monoclonal anti-BrdU antibody, Clone: ZBU30, Cat#: 03-390, Dilution: 1:100). The next day, the sections were incubated in a mixture of secondary antibodies (Alexa Fluor 594 conjugated goat anti-mouse secondary antibody, Cat # A-11032, Dilution: 1:1000 and Alexa Fluor 488 conjugated goat anti-mouse secondary antibody, Cat # A28175, Dilution: 1:2000) after several washes with TBS in a dark place at RT for 1 h. For nuclear counterstaining, the slides were incubated with DAPI (Sigma-Aldrich) for 3 min. After two washes with TBS, the slides were mounted, coverslipped, and visualized with a fluorescence microscope (Zeiss, Axiophot, Germany). To examine the neurogenesis phenomenon and BrdU/NeuN double-IF staining, the same protocol was used. In this case, all of the above-mentioned antibodies were used except the primary mouse antibody anti-NeuN (Millipore, clone A60, cat#: MAB377, Dilution: 1:100), which was replaced by the Nestin Mouse Monoclonal Antibody. For quantification, ten serial coronal sections with identical interval through the entire of the dentate gyrus were selected using systematic uniform random sampling based on un-biased stereological methods and Cavalieri’s principle (Gundersen et al., 1988; Williams & Rakic, 1988). Finally, the number of BrdU/Nestin and BrdU/NeuN co-expressing cells was counted in the 100 BrdU-positive cells under fluorescence microscopy and presented as a percentage (Thuret et al., 2009).
Nissl Staining
Following deparaffinization and rehydration, the slides were washed in tap water for 5 min and then incubated in pre-warmed (37–50 °C oven) 0.1% Cresyl violet solution for 7 min. Then, the slides were rinsed quickly in distilled water and differentiated in 95% ethyl alcohol for 5 min. In the next step, the slides were dehydrated in the ascending series of alcohols, cleared in xylene, and immediately mounted. After overnight drying, the slides were visualized and examined under a light microscope (Wang et al., 2017). In this case, ten serial coronal sections were selected using systematic uniform sampling and then the mean number of dead cells in 1 mm2 was measured using Image j software.
Real-Time RT-PCR Analysis
For gene expression analysis, total RNA was isolated from the frozen samples using RNeasy mini-kit (Qiagen). In brief, cDNA synthesis was done using Revert AidTM First Strand cDNA Synthesis kit (Fermentas, K1621, K1622) according to the manufacturer’s instructions. In this study, the expression of Akt, Erk1/2, Wnt3a, β-Catenin, and GSK3β were measured (Table 1). Relative gene expression analysis was performed using Maxima™ SYBR Green/ROX qPCR Master Mix (2X) kit (Fermentas, K0221). This assay was done in triplicate.
Statistical Analysis
Using GraphPad Prism software and p < 0.05 was considered statistically significant. The escape latency and swim distance in the water maze were analyzed by two-way repeated measures ANOVA followed by Tukey’s HSD test for between-subject difference among groups and (within subjects) for effects across block interval 1–5 (“BLOCK” effect). The probe trial data for percentage of time spent in target quadrant were analyzed by multivariate ANOVA followed by Tukey’s HSD test as a post hoc analysis. Paired t test followed by Tukey’s HSD test as a post hoc analysis were performed for evaluation of training and retention trials of passive avoidance test. For other analyses, we used One-way ANOVA followed by Tukey’s HSD multiple comparison tests unless mentioned.
Results
NSCs Characterization
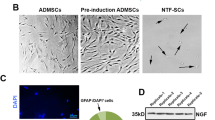
In this study, the NSCs were isolated from the ganglionic eminences of the 14-day-old mouse embryos. Bright-field microscopic observations displayed that isolated NSCs developed the cell colonies, namely neurospheres 4 to 5 days after platting. These colonies were grown and reached 200–250 µm in diameter. In this step, neurospheres were subjected to sub-culture procedure (Fig. 1A). Immunocytochemistry analysis showed near 81℅ of isolated NSCs was positive for pluripotency marker Nestin (Fig. 1B). These data showed the suitability of current protocol in isolation of mouse NSCs.
Bright-field imaging of NSC culture during 9 days in-vitro condition (A). After 5-day incubation, NSCs generated small-size spheroids and their sizes reached maximum levels after 9 days. These features showed morphological appearance which is typical to neurospheres assay. Immunofluorescence staining of Nestin-positive NSCs (B). Data exhibited the expression of Nestin marker, indicating the multipotentiality of isolated cells. Measuring levels of NGF and BDNF in NSCs-CM after 7 days at passage three, showing the production of regenerative factor in NSCs secretome (C).
NSCs Released the BDNF and NGF into the CM
To explore whether the NSCs could secrete the neurotrophic factors in the CM, we conducted the ELISA assay to measure BDNF and NGF levels in the CM (Fig. 1C). Our results showed that the NSCs release the BDNF and NGF at an average of 220 pg/ml ± 72 and 154 pg/ml ± 64, respectively (Fig. 1C).
Confirmation of Deposition of Aβ plaques
Thioflavin S staining was used to detect the formation and deposition of Aβ plaques. Thioflavin S binds to these plaques and is observed in green color under fluorescence microscopy which is indicated by yellow arrows (Fig. 2A). In the current study, the existence and overexpression of the Aβ plaques were increased in the AD group.
Thioflavin S staining evaluation and assessment of behavioral performance (A–E). Thioflavin S staining (A). Thioflavin S staining showed that the Aβ injection induced the Aβ plaques formation and deposition in the hippocampus and was observed in green color under fluorescence microscopy which herein is indicated by yellow arrows. Passive avoidance test (B). Learning and memory impairment were evident in mice following AD-like induction. Data displayed the reduction of step-through latency assessed during retention trial in the AD mice compared to control group (p < 0.001). Morris Water Maze test (C–E). According to the data, NSCs-CM reduced the escape latency over 5 days compared to the AD-Vehicle group (p < 0.05; C NSCs-CM was potential to diminish the cumulative distance to the platform in AD mice compared to the AD-Vehicle group (p < 0.05); D In addition, the time spent in target quadrants was reduced in AD mice and administration of NSCs-CM blunted these effects and closed to near-to-levels (E). Paired t test, Two-way repeated measures ANOVA, multivariate ANOVA, and Tukey post-hoc analysis. *p < 0.05; **p < 0.01; and p < 0.001
Aβ Injection Induced the Impairment of Learning and Memory Performance
Data from the passive avoidance test indicated that there are significant differences among groups in the duration time of step-through latency in the retention (Probe) trial on the 21 days after the AD-like induction model (p < 0.05; Fig. 2B). Aβ administration significantly increased the step-through latency time in the Aβ-injected groups, indicating that Aβ could affect the learning ability and memory retrieval.
NSCs-CM Enhanced the Learning and Memory Performance
This task was performed using MWM test. Our results showed that there was a significant difference in escape latency and swim distance to find the platform in the acquisition phase (Fig. 2B, C). Escape latency and swim distance significantly were reduced in the control and (AD + NSCs-CM) groups relative to the (AD + Vehicle) group during the course of the training acquisition phase (BLOCK effect; p < 0.001; Fig. 2B, C). In the probe section, the time spent in the target quadrant and the average time spent in the other non-target quadrants were measured. The statistical analysis revealed that the mean time spent in the target quadrants significantly increased in the control and (AD + NSCs-CM) groups compared to the (AD + Vehicle) group during the probe trial (p < 0.001; Fig. 2D). Taken together, this panel implied that the NSCs-CM could promote learning capacity and memory retention.
NSCs-CM Stimulated the NSCs Proliferation and Adult Neurogenesis
To investigate the proliferation and neural differentiation of endogenous NSCs in the SGZ of the dentate gyrus after NSCs-CM injection, we did the BrdU/Nestin and BrdU/NeuN double-IF staining 1 and 35 days following the last BrdU injection, respectively (Fig. 3A, B). The results from this panel showed that the number of BrdU/Nestin and BrdU/NeuN co-expressing cells was significantly decreased in AD + Vehicle mice compared to the control group (p < 0.001; Fig. 3A, B). Administration of NSCs-CM in the AD mice increased BrdU/Nestin and BrdU/NeuN-positive cells, indicating promoted proliferation and neural differentiation of endogenous NSCs as compared to the AD + Vehicle mice. Cumulatively, these findings showed that NSC secretome can regulate the proliferation and neurogenesis in the dentate gyrus of the hippocampus after AD changes in a paracrine manner.
IF imaging of double-labeled BrdU/Nestin and BrdU/NeuN cells in dentate gyrus of hippocampus in AD mice after NSCs-CM administration (A, B). NSCs-CM can increase the percent of BrdU/Nestin and BrdU/NeuN cells, showing enhanced proliferation and neural differentiation following 1 and 35 days last BrdU injection time. One-Way ANOVA and Tukey post-hoc analysis. ***p < 0.001 and ****p < 0.0001
NSCs-CM Suppressed the Neuronal Cell Death Induced by Aβ Injection
To evaluate the effects of NSCs-CM on neurotoxicity induced by Aβ, Nissl staining was performed. In normal condition, the neurons and nuclei appeared as round morphology with pale blue color, respectively (p < 0.0001; Fig. 4A). In the current study, we found that Aβ can induce neurotoxicity, cell death, and disrupted neural integrity compared to the control group (p < 0.001; Fig. 4A). In the AD + Vehicle group, profound histopathological changes including scattered and shrunken neuronal cells as well as condensed nuclei and tissue disintegration were indicated. Based on our findings, NSC-derived CM rescued these pathological alterations. In this regard, NSCs-CM significantly decreased the rate of dead neuronal cells relative to the AD + Vehicle group (p < 0.001; Fig. 4A). Despite these advantages, the neurotoxicity level was NSCs-CM group compared to the control mice (p < 0.001; Fig. 4A). These findings support a notion that NSCs-CM could promote cell survival and reorganize the integrity of neural tissue structure in the AD hippocampus.
NSCs-CM Modulated the Expression of Wnt/β-Catenin Signaling Pathway Effectors in AD Brain Tissue
In this panel, we aimed to explore whether NSCs-CM could modulate the expression level of the PI3K, Akt, MAPK, ERK, Wnt, GSK-3β, and β-catenin genes in the mouse dentate gyrus after AD changes (Fig. 4B). Data showed statistically significant differences in the transcription level of the above-mentioned genes pre- and post-NSCs-CM administration in AD mice (p < 0.05). The expression of PI3K, Akt, MAPK, ERK, Wnt, and β-catenin genes were significantly down-regulated 56 days AD induction compared to the control (p < 0.05). By contrast, the promotion of AD-like condition led to overexpression of GSK-3β related to the control mice (p < 0.05; Fig. 4B). We noted that the injection of NSCs-CM reversed the expression of PI3K, Akt, MAPK, ERK, Wnt, and β-catenin genes and closed near-to-normal levels (pAD+NSCs-CM vs. AD+ Vehicle < 0.05). Expression of these genes in the AD + NSCs-CM group was less compared to the control mice (p < 0.05; Fig. 4B). In contrast to these changes, the injection of NSCs-CM reduced AD-induced overactivity of GSK-3β compared to the AD-Vehicle mice. Taken together, these results indicated that Aβ induced AD-like condition indicated by the induction of GSK-3β and suppression of PI3K, Akt, MAPK, ERK, Wnt, and β-catenin, showing that the participation of Wnt/β-catenin signaling pathway in pathogenesis of AD and NSCs secretome can reduce these changes (Fig. 4).
Nissl staining. Aβ induced neural loss and degeneration in the hippocampus and treatment with NSC secretome decreased toxicity rate and neural tissue integrity indicated by the increase of blue colored soma juxtaposed to each other (A). Measuring the levels of PI3K, Akt, MAPK, Erk, Wnt3a, β-Catenin, and GSK3β using real-time PCR analysis in AD mice after injection of NSCs-CM (B). NSCs-CM increased reduced expression of PI3K, Akt, MAPK, Erk, Wnt3a, β-Catenin in AD mice and closed to near-to-control levels. One-Way ANOVA and Tukey post-hoc analysis. ***p < 0.001 and ****p < 0.0001
Discussion
Adult neurogenesis is a finely dynamic phenomenon in the adult brain and its rate can be modulated under physiological and pathological conditions (Gould, 2007). Regulation of such a critical process is a key and integral subject in introduction and development of therapeutic approaches for neurological disorders (Kempermann et al., 1997; Song et al., 2012). A plethora of studies and experiments have indicated that the intrinsic signaling pathways combined with extrinsic modalities regulate neurogenesis by changing the balance between the quiescent and differentiation states of NSCs (de la Torre-Ubieta & Bonni, 2011; Faigle & Song, 2013; Gonçalves et al., 2016; Song et al., 2012). Among several intracellular cascades, Wnt/β-catenin axis plays a major role in the neural development and regulation of adult hippocampal neurogenesis (Faigle & Song, 2013; Patapoutian & Reichardt, 2000; Schwarz et al., 2012). It should be considered that this signaling pathway, in turn, could be promoted by extrinsic cues including exercise, growth factors, neurotrophins, environmental enrichment, and pharmacological agents (Kempermann et al., 1997; Faigle & Song, 2013; B. Y. Chen et al., 2013; Rosso & Inestrosa, 2013). In the current study, we aimed to investigate the potential effects of NSCs-CM on adult neurogenesis in the mouse model of AD-like pathology by altering the Wnt/β-catenin signaling pathway. To this end, we indicated that the intracerebroventricular injection of Aβ induced learning and memory impairment assessed by the passive avoidance platform test which is in accordance with our previous data and other experiments (Esfandiary et al., 2015; Karimipour et al., 2019; Lee et al., 2020; Yeung et al., 2020). In the next step, we isolated and expanded embryonic NSCs from 14-day-old ganglionic eminences to harvest CM. We administrated NSCs secretome into intracerebroventricular of the lateral ventricle to assess functional deficits and cognitive disabilities induced by AD. Bilateral injection of Aβ led to abortion of neurogenesis via the overexpression of GSK-3β. It was suggested that overactivity of this factor can induce neurotoxicity, neuronal cell death, and eventually led to the cognitive deficits. Besides, the promotion of inflammatory conditions after Aβ injection can increase the incidence of cognitive deficits. The reduced neurogenesis rate would be due to inflammatory cytokines in the extracellular matrix particularly in the perineuronal net (PNNs), disruption of the neurotrophin-related signaling pathway, and oxidative stress (Crapser et al., 2020; Harland et al., 2020; Pirbhoy et al., 2020; Rehman et al., 2021). In normal condition, 20% of the whole brain volume consists of PNNs which provides the unique milieu for neuronal communication and synaptic plasticity. Along with these descriptions, regulated structural remodeling and reconstruction of PNNs have been considered as alternative approaches in tissue engineering strategies (Bosiacki et al., 2019; Harris & Weinberg, 2012; Reichelt et al., 2019). Abnormal Aβ targets the presynaptic and postsynaptic areas in the PNNs, leading to the release of glutamate and neurotoxicity (Rehman et al., 2021; Yeung et al., 2020). It is believed that aggregated Tau and NFTs can induce DNA fragmentation (Lassmann et al., 1995). Moreover, Tau protein binds to presynaptic protein namely synaptogyrin-3 hence disrupts the synaptic transmission. In addition, the interaction of Tau with PSD-95 on the postsynaptic membrane leads to NMDA receptor over activation and high Ca2+ influxes, resulting in excitotoxicity, mitochondrial dysfunction, and neuronal cell death (Ittner et al., 2016; McInnes et al., 2018). According to our data, we found that the NSCs-CM reversed the Aβ-induced behavioral deficits and promoted the learning ability and memory establishment in the learning acquisition and probe trials of the MWM test.
It is well documented that there was a correlation between neurogenesis and learning and memory functions (Abrous & Wojtowicz, 2015; Stuchlik, 2014). In this regard, the regulation of neurogenesis and synaptic plasticity through molecular and cellular signaling pathways is a key and critical subject in the field of regenerative medicine (Baptista & Andrade, 2018; Berdugo-Vega et al., 2020; Faigle & Song, 2013). Real-time PCR analysis revealed that the NSCs secretome triggered Wnt/β-catenin signaling pathway via the alteration of different effectors such as PI3K, Akt, MAPK, ERK, Wnt, and β-catenin in AD mice. These changes coincided with the suppression of GSK-3β after CM injection. The close association of Wnt signaling pathway with GSK-3β has been shown in the context of NSC bioactivity. To be specific, the activation of Wnt signaling subsets such as Wnt3 and 7 promoted NSCs proliferation and differentiation via the inhibition of GSK-3β. These effects, if not completely but in part, are associated with the presence of specific factors BDNF and NGF as indicated in CM (Adachi et al., 2007). Noteworthy, the expression of different factors such as PI3K, Akt, MAPK, and ERK along with Wnt can trigger β-catenin via separate pathways which is indicated by real-time PCR analysis (Cho et al., 2018). Previous experiments have shown that stem cells can release nano-sized vesicles harboring an array of growth factors with potential to alter in situ immune system response, and increasing Aβ clearance (Guo et al., 2020). There are some limitations in the current experiment that need further investigations. It is suggested that the type and levels of different growth factors released by NSCs into the culture medium should be determined. The application of AD mouse model (transgenic AD model) is recommended for precise molecular analysis after NSCs-CM administration.
Conclusion
Taken together, these data show that NSCs-derived secretome can ameliorate AD-like changes in mouse model in a paracrine manner via the engaging Wnt/β-catenin pathway which highlights the crucial role of this signaling cascade in the pathogenesis of AD. For more elucidation and targeting this signaling pathway in AD conditions and other cognitive impairments, extra reliable and complementary studies should be done.
Data Availability
The datasets presented/analyzed during the current study are available.
Abbreviations
- DAPI:
-
4,6-diamidino-2-phenylindole
- BrdU:
-
5-Bromo-20-deoxyuridine
- AD:
-
Alzheimer’s disease
- Aβ:
-
Amyloid β
- FGF-2:
-
Basic fibroblast growth factor-2
- BDNF:
-
Brain-derived neurotrophic factor
- ICV:
-
Cerebroventricular
- DMEM/F-12:
-
Dulbecco’s modified eagle medium/nutrient mixture
- ELISA:
-
Enzyme-linked immunosorbent assay
- EGF:
-
Epidermal growth factor
- GSK-3β:
-
Glycogen synthase kinase-3β
- IF:
-
Immunofluorescence
- MWM:
-
Morris water maze
- NGF:
-
Nerve growth factor
- NSCs-CM:
-
Neural stem cell condition medium
References
Abrous, D. N., & Wojtowicz, J. M. (2015). Interaction between neurogenesis and hippocampal memory system: New vistas. Cold Spring Harbor Perspectives in Biology, 7(6), a018952.
Adachi, K., Mirzadeh, Z., Sakaguchi, M., Yamashita, T., Nikolcheva, T., Gotoh, Y., et al. (2007). β-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells, 25(11), 2827–2836. https://doi.org/10.1634/stemcells.2007-0177
Alonso, A. D. C., Grundke-Iqbal, I., Barra, H. S., & Iqbal, K. (1997). Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: Sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proceedings of the National Academy of Sciences, 94(1), 298–303.
Alzheimer’s Association. (2016). 2016 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 12(4), 459–509.
Ameri, M., Shabaninejad, Z., Movahedpour, A., Sahebkar, A., Mohammadi, S., Hosseindoost, S., et al. (2020). Biosensors for detection of Tau protein as an Alzheimer’s disease marker. International Journal of Biological Macromolecules, 162, 1100–1108.
Azari, H. (2013). Isolation and enrichment of defined neural cell populations from heterogeneous neural stem cell progeny. Neural progenitor cells (pp. 95–106). Springer.
Azari, H., Sharififar, S., Rahman, M., Ansari, S., & Reynolds, B. A. (2011). Establishing embryonic mouse neural stem cell culture using the neurosphere assay. Journal of Visualized Experiments. https://doi.org/10.3791/2457
Baptista, P., & Andrade, J. P. (2018). Adult hippocampal neurogenesis: Regulation and possible functional and clinical correlates. Frontiers in Neuroanatomy, 12, 44.
Berdugo-Vega, G., Arias-Gil, G., López-Fernández, A., Artegiani, B., Wasielewska, J. M., Lee, C.-C., et al. (2020). Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nature Communications, 11(1), 1–12.
Binder, L. I., Guillozet-Bongaarts, A. L., Garcia-Sierra, F., & Berry, R. W. (2005). Tau, tangles, and Alzheimer’s disease. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease, 1739(2–3), 216–223.
Bosiacki, M., Gąssowska-Dobrowolska, M., Kojder, K., Fabiańska, M., Jeżewski, D., Gutowska, I., et al. (2019). Perineuronal nets and their role in synaptic homeostasis. International Journal of Molecular Sciences, 20(17), 4108.
Chen, B. Y., Wang, X., Wang, Z. Y., Wang, Y. Z., Chen, L. W., & Luo, Z. J. (2013). Brain-derived neurotrophic factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/β-catenin signaling pathway. Journal of Neuroscience Research, 91(1), 30–41.
Chen, X.-Q., & Mobley, W. C. (2019). Alzheimer disease pathogenesis: Insights from molecular and cellular biology studies of oligomeric Aβ and tau species. Frontiers in Neuroscience, 13, 659.
Cho, J. W., Jung, S. Y., Kim, D. Y., Chung, Y. R., Choi, H. H., Jeon, J. W., et al. (2018). PI3K-Akt-Wnt pathway is implicated in exercise-induced improvement of short-term memory in cerebral palsy rats. International Neurourology Journal, 22(Suppl 3), S156-164. https://doi.org/10.5213/inj.1836224.112
Clevers, H., & Nusse, R. (2012). Wnt/β-catenin signaling and disease. Cell, 149(6), 1192–1205.
Crapser, J. D., Spangenberg, E. E., Barahona, R. A., Arreola, M. A., Hohsfield, L. A., & Green, K. N. (2020). Microglia facilitate loss of perineuronal nets in the Alzheimer’s disease brain. EBioMedicine, 58, 102919.
de la Torre-Ubieta, L., & Bonni, A. (2011). Transcriptional regulation of neuronal polarity and morphogenesis in the mammalian brain. Neuron, 72(1), 22–40.
Deng, Y., Wang, Z., Wang, R., Zhang, X., Zhang, S., Wu, Y., et al. (2013). Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1 (BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis. European Journal of Neuroscience, 37(12), 1962–1969.
Diaz Brinton, R., & Ming Wang, J. (2006). Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer’s disease: Allopregnanolone as a proof of concept neurogenic agent. Current Alzheimer Research, 3(3), 185–190.
Drubin, D. G., & Kirschner, M. W. (1986). Tau protein function in living cells. Journal of Cell Biology, 103(6), 2739–2746.
Ebneth, A., Godemann, R., Stamer, K., Illenberger, S., Trinczek, B., Mandelkow, E.-M., et al. (1998). Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: Implications for Alzheimer’s disease. The Journal of Cell Biology, 143(3), 777–794.
Eriksson, P. S., Perfilieva, E., Björk-Eriksson, T., Alborn, A.-M., Nordborg, C., Peterson, D. A., et al. (1998). Neurogenesis in the adult human hippocampus. Nature Medicine, 4(11), 1313–1317.
Esfandiary, E., Abdolali, Z., Omranifard, V., Ghanadian, M., Bagherian-Sararoud, R., Karimipour, M., et al. (2018). Novel effects of Rosa damascena extract on patients with neurocognitive disorder and depression: A clinical trial study. International Journal of Preventive Medicine, 9, 57.
Esfandiary, E., Karimipour, M., Mardani, M., Alaei, H., Ghannadian, M., Kazemi, M., et al. (2014). Novel effects of Rosa damascena extract on memory and neurogenesis in a rat model of Alzheimer’s disease. Journal of Neuroscience Research, 92(4), 517–530.
Esfandiary, E., Karimipour, M., Mardani, M., Ghanadian, M., Alaei, H. A., Mohammadnejad, D., et al. (2015). Neuroprotective effects of Rosa damascena extract on learning and memory in a rat model of amyloid-β-induced Alzheimer’s disease. Advanced Biomedical Research, 4, 131.
Faigle, R., & Song, H. (2013). Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochimica et Biophysica Acta (BBA), 1830(2), 2435–2448.
Gage, F. H. (2019). Adult neurogenesis in mammals. Science, 364(6443), 827–828.
Gonçalves, J. T., Schafer, S. T., & Gage, F. H. (2016). Adult neurogenesis in the hippocampus: From stem cells to behavior. Cell, 167(4), 897–914.
Gould, E. (2007). How widespread is adult neurogenesis in mammals? Nature Reviews Neuroscience, 8(6), 481–488.
Gouras, G. K., Tsai, J., Naslund, J., Vincent, B., Edgar, M., Checler, F., et al. (2000). Intraneuronal Aβ42 accumulation in human brain. The American Journal of Pathology, 156(1), 15–20.
Grundke-Iqbal, I., Iqbal, K., Tung, Y.-C., Quinlan, M., Wisniewski, H. M., & Binder, L. I. (1986). Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proceedings of the National Academy of Sciences, 83(13), 4913–4917.
Gundersen, H., Bendtsen, T. F., Korbo, L., Marcussen, N., Møller, A., Nielsen, K., et al. (1988). Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis, 96(1–6), 379–394.
Guo, M., Yin, Z., Chen, F., & Lei, P. (2020). Mesenchymal stem cell-derived exosome: A promising alternative in the therapy of Alzheimer’s disease. Alzheimer’s Research & Therapy, 12(1), 109. https://doi.org/10.1186/s13195-020-00670-x
Harland, M., Torres, S., Liu, J., & Wang, X. (2020). Neuronal mitochondria modulation of LPS-induced neuroinflammation. Journal of Neuroscience, 40(8), 1756–1765.
Harris, K. M., & Weinberg, R. J. (2012). Ultrastructure of synapses in the mammalian brain. Cold Spring Harbor Perspectives in Biology, 4(5), a005587.
Heneka, M. T., Carson, M. J., El Khoury, J., Landreth, G. E., Brosseron, F., Feinstein, D. L., et al. (2015). Neuroinflammation in Alzheimer’s disease. The Lancet Neurology, 14(4), 388–405.
Hernández, F., de Barreda, E. G., Fuster-Matanzo, A., Lucas, J. J., & Avila, J. (2010). GSK3: A possible link between beta amyloid peptide and tau protein. Experimental Neurology, 223(2), 322–325.
Hooper, C., Killick, R., & Lovestone, S. (2008). The GSK3 hypothesis of Alzheimer’s disease. Journal of Neurochemistry, 104(6), 1433–1439.
Hoover, B. R., Reed, M. N., Su, J., Penrod, R. D., Kotilinek, L. A., Grant, M. K., et al. (2010). Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron, 68(6), 1067–1081.
Iqbal, K., Liu, F., & Gong, C.-X. (2016). Tau and neurodegenerative disease: The story so far. Nature Reviews Neurology, 12(1), 15.
Ittner, A., Chua, S. W., Bertz, J., Volkerling, A., van der Hoven, J., Gladbach, A., et al. (2016). Site-specific phosphorylation of tau inhibits amyloid-β toxicity in Alzheimer’s mice. Science, 354(6314), 904–908.
Jahed, F. J., Rahbarghazi, R., Shafaei, H., Rezabakhsh, A., & Karimipour, M. (2021). Application of neurotrophic factor-secreting cells (astrocyte-Like cells) in the in-vitro Alzheimer’s disease-like pathology on the human neuroblastoma cells. Brain Research Bulletin, 172, 180–189.
Karimipour, M., Rahbarghazi, R., Tayefi, H., Shimia, M., Ghanadian, M., Mahmoudi, J., et al. (2019). Quercetin promotes learning and memory performance concomitantly with neural stem/progenitor cell proliferation and neurogenesis in the adult rat dentate gyrus. International Journal of Developmental Neuroscience, 74, 18–26.
Kempermann, G., Kuhn, H. G., & Gage, F. H. (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature, 386(6624), 493–495.
Kim, H. Y., Lee, D. K., Chung, B.-R., Kim, H. V., & Kim, Y. (2016). Intracerebroventricular injection of amyloid-β peptides in normal mice to acutely induce Alzheimer-like cognitive deficits. Journal of Visualized Experiments: JoVE, 109, e53308.
Kwak, Y.-D., Hendrix, B. J., & Sugaya, K. (2014). Secreted type of amyloid precursor protein induces glial differentiation by stimulating the BMP/Smad signaling pathway. Biochemical and Biophysical Research Communications, 447(3), 394–399.
Lassmann, H., Bancher, C., Breitschopf, H., Wegiel, J., Bobinski, M., Jellinger, K., et al. (1995). Cell death in Alzheimer’s disease evaluated by DNA fragmentation in situ. Acta Neuropathologica, 89(1), 35–41.
Lee, H. J., Lee, D. Y., Kim, H. L., & Yang, S. H. (2020). Scrophularia buergeriana extract improves memory impairment via inhibition of the apoptosis pathway in the mouse hippocampus. Applied Sciences, 10(22), 7987.
Leeson, H. C., Kasherman, M. A., Chan-Ling, T., Lovelace, M. D., Brownlie, J. C., Toppinen, K. M., et al. (2018). P2X7 receptors regulate phagocytosis and proliferation in adult hippocampal and SVZ neural progenitor cells: Implications for inflammation in neurogenesis. Stem Cells, 36(11), 1764–1777.
Lempriere, S. (2019). Birth of hippocampal neurons declines in Alzheimer disease. Nature Reviews Neurology, 15(5), 245–245.
Lu, P., Jones, L., Snyder, E., & Tuszynski, M. (2003). Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Experimental Neurology, 181(2), 115–129.
Ly, P. T., Cai, F., & Song, W. (2011). Detection of neuritic plaques in Alzheimer’s disease mouse model. JoVE (Journal of Visualized Experiments), 53, e2831.
Magdesian, M. H., Carvalho, M. M., Mendes, F. A., Saraiva, L. M., Juliano, M. A., Juliano, L., et al. (2008). Amyloid-β binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/β-catenin signaling. Journal of Biological Chemistry, 283(14), 9359–9368.
McInnes, J., Wierda, K., Snellinx, A., Bounti, L., Wang, Y.-C., Stancu, I.-C., et al. (2018). Synaptogyrin-3 mediates presynaptic dysfunction induced by tau. Neuron, 97(4), 823-835.e828.
Ming, G.-L., & Song, H. (2011). Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron, 70(4), 687–702.
Moreno-Jiménez, E. P., Flor-García, M., Terreros-Roncal, J., Rábano, A., Cafini, F., Pallas-Bazarra, N., et al. (2019). Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nature Medicine, 25(4), 554–560.
Nakano, M., Kubota, K., Kobayashi, E., Chikenji, T. S., Saito, Y., Konari, N., et al. (2020). Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Scientific Reports, 10(1), 1–15.
Nakano, M., Nagaishi, K., Konari, N., Saito, Y., Chikenji, T., Mizue, Y., et al. (2016). Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Scientific Reports, 6(1), 1–14.
Patapoutian, A., & Reichardt, L. F. (2000). Roles of Wnt proteins in neural development and maintenance. Current Opinion in Neurobiology, 10(3), 392–399.
Patterson, C. (2018). The state of the art of dementia research: New frontiers. World Alzheimer Report, 2018.
Pirbhoy, P. S., Rais, M., Lovelace, J. W., Woodard, W., Razak, K. A., Binder, D. K., et al. (2020). Acute pharmacological inhibition of matrix metalloproteinase-9 activity during development restores perineuronal net formation and normalizes auditory processing in Fmr1 KO mice. Journal of neurochemistry, 155(5), 538–558.
Plassman, B. L., Langa, K. M., Fisher, G. G., Heeringa, S. G., Weir, D. R., Ofstedal, M. B., et al. (2007). Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology, 29(1–2), 125–132.
Rajamohamedsait, H. B., & Sigurdsson, E. M. (2012). Histological staining of amyloid and pre-amyloid peptides and proteins in mouse tissue. Amyloid proteins (pp. 411–424). Springer.
Rehman, I. U., Ahmad, R., Khan, I., Lee, H. J., Park, J., Ullah, R., et al. (2021). Nicotinamide ameliorates amyloid beta-induced oxidative stress-mediated neuroinflammation and neurodegeneration in adult mouse brain. Biomedicines, 9(4), 408.
Reichelt, A. C., Hare, D. J., Bussey, T. J., & Saksida, L. M. (2019). Perineuronal nets: Plasticity, protection, and therapeutic potential. Trends in Neurosciences, 42(7), 458–470.
Rhee, Y.-H., Yi, S.-H., Kim, J. Y., Chang, M.-Y., Jo, A.-Y., Kim, J., et al. (2016). Neural stem cells secrete factors facilitating brain regeneration upon constitutive Raf-Erk activation. Scientific Reports, 6(1), 1–16.
Rosso, S. B., & Inestrosa, N. C. (2013). WNT signaling in neuronal maturation and synaptogenesis. Frontiers in Cellular Neuroscience, 7, 103.
Scheltens, P., Blennow, K., Breteler, M., de Strooper, B., Frisoni, G., Salloway, S., et al. (2016). Alzheimer’s disease. Lancet (London, England), 388, 505–517.
Schmid, S., Jungwirth, B., Gehlert, V., Blobner, M., Schneider, G., Kratzer, S., et al. (2017). Intracerebroventricular injection of beta-amyloid in mice is associated with long-term cognitive impairment in the modified hole-board test. Behavioural Brain Research, 324, 15–20.
Schwarz, T. J., Ebert, B., & Lie, D. C. (2012). Stem cell maintenance in the adult mammalian hippocampus: A matter of signal integration? Developmental Neurobiology, 72(7), 1006–1015.
Selkoe, D. J., & Hardy, J. (2016). The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Molecular Medicine, 8(6), 595–608.
Serrano-Pozo, A., Frosch, M., Masliah, E., & Hyman, B. (2011). Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine, 1(1), a006189.
Song, J., Zhong, C., Bonaguidi, M. A., Sun, G. J., Hsu, D., Gu, Y., et al. (2012). Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature, 489(7414), 150–154.
Stuchlik, A. (2014). Dynamic learning and memory, synaptic plasticity and neurogenesis: An update. Frontiers in Behavioral Neuroscience, 8, 106.
Terry, R. D., Masliah, E., Salmon, D. P., Butters, N., DeTeresa, R., Hill, R., et al. (1991). Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 30(4), 572–580.
Thuret, S., Toni, N., Aigner, S., Yeo, G. W., & Gage, F. H. (2009). Hippocampus-dependent learning is associated with adult neurogenesis in MRL/MpJ mice. Hippocampus, 19(7), 658–669.
Vafaei, A., Rahbarghazi, R., Kharaziha, M., Avval, N. A., Rezabakhsh, A., & Karimipour, M. (2021). Polycaprolactone fumarate acts as an artificial neural network to promote the biological behavior of neural stem cells. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 109(2), 246–256.
Walsh, D. M., & Selkoe, D. J. (2004). Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron, 44(1), 181–193.
Wan, W., Xia, S., Kalionis, B., Liu, L., & Li, Y. (2014). The role of Wnt signaling in the development of alzheimer’s disease: a potential therapeutic target? BioMed Research International, 2014, 1–9.
Wang, P., Luo, Q., Qiao, H., Ding, H., Cao, Y., Yu, J., et al. (2017). The neuroprotective effects of carvacrol on ethanol-induced hippocampal neurons impairment via the antioxidative and antiapoptotic pathways. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2017/4079425
Wang, Z., Xu, Q., Cai, F., Liu, X., Wu, Y., & Song, W. (2019). BACE2, a conditional β-secretase, contributes to Alzheimer’s disease pathogenesis. JCI Insight. https://doi.org/10.1172/jci.insight.123431
Weingarten, M. D., Lockwood, A. H., Hwo, S.-Y., & Kirschner, M. W. (1975). A protein factor essential for microtubule assembly. Proceedings of the National Academy of Sciences, 72(5), 1858–1862.
Williams, R. W., & Rakic, P. (1988). Elimination of neurons from the rhesus monkey’s lateral geniculate nucleus during development. Journal of Comparative Neurology, 272(3), 424–436.
Wodarz, A., & Nusse, R. (1998). Mechanisms of Wnt signaling in development. Annual Review of Cell and Developmental Biology, 14(1), 59–88.
Xie, M., Zhang, G., Yin, W., Hei, X.-X., & Liu, T. (2018). Cognitive enhancing and antioxidant effects of tetrahydroxystilbene glucoside in Aβ1-42-induced neurodegeneration in mice. Journal of Integrative Neuroscience, 17(3–4), 355–365.
Yeung, J. H., Palpagama, T. H., Tate, W. P., Peppercorn, K., Waldvogel, H. J., Faull, R. L., et al. (2020). The acute effects of amyloid-beta1–42 on glutamatergic receptor and transporter expression in the mouse hippocampus. Frontiers in Neuroscience, 13, 1427.
Zhao, C., Deng, W., & Gage, F. H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell, 132(4), 645–660.
Acknowledgements
Authors wish to thank the personnel of Neuroscience Research Center for help and guidance.
Funding
This study was supported by a Grant from Tabriz University of Medical Sciences (63595).
Author information
Authors and Affiliations
Contributions
FH, RR, SSE, GB, MH, and MS performed different analyses. MK supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical Approval
All phases of this study were approved by Local Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1397.239).
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Hijroudi, F., Rahbarghazi, R., Sadigh-Eteghad, S. et al. Neural Stem Cells Secretome Increased Neurogenesis and Behavioral Performance and the Activation of Wnt/β-Catenin Signaling Pathway in Mouse Model of Alzheimer’s Disease. Neuromol Med 24, 424–436 (2022). https://doi.org/10.1007/s12017-022-08708-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-022-08708-z