Abstract
Doublecortin (DCX), a microtubule-associated protein, specifically expresses in neuronal precursors. This protein has been used as a marker for neuronal precursors and neurogenesis. In the present study, we observed differences in DCX immunoreactivity and its protein levels in the hippocampal dentate gyrus between adult and aged dogs. In the adult dog, DCX immunoreactive cells with well-stained processes were detected in the subgranular zone of the dentate gyrus. Numbers of DCX immunoreactive cells in the dentate gyrus of the aged dog were significantly decreased compared to those in the adult dog. DCX immunoreactive cells in both adult and aged dog did not show NeuN (a marker for mature neurons) immunoreactivity. NeuN immunoreactivity in the aged dog was poor compared to that in the adult dog. DCX protein level in the aged dentate gyrus was decreased by 80% compared to that in the adult dog. These results suggest that the reduction of DCX in the aged hippocampal dentate gyrus may be involved in some neural deficits related to the hippocampus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Two regions of brain, the subgranular zone of the hippocampal dentate gyrus and the subventricular zone of the lateral ventricle, have interested many researchers in neurogenesis because the adult mammalian central nervous system (CNS) retains a potential to generate new neurons [1–5]. It has been reported that many cells express doublecortin (DCX) in the subependymal layer, its rostral extension and olfactory bulb in the adult rat: these DCX positive cells co-label with polysialylated neural cell adhesion protein, an antigen also present on migrating neuroblasts [6].
DCX gene encodes a 40-kDa microtubule-associated protein which is specifically expressed in neuronal precursors in the developing and adult CNS. During development of the CNS, the expression of the DCX is associated with migration of neuronal precursors [6–8]. Due to its specific expression pattern, attentions have been drawn to DCX as a marker for neuronal precursors and neurogenesis [9]. DCX is expressed predominantly in migrating neurons within the developing mammalian brain [10–12] and is frequently used as a marker of neuronal migration [13]. In addition, it has been reported that DCX is expressed in differentiated neurons, suggesting a role for DCX in neuronal plasticity, axonal outgrowth, or synaptogenesis [6, 14, 15].
Dogs are widely accepted for aging study, because populations of dogs are large, and many owners insure their pets. Records of dates of their birth, death and lifespan are probably better than for any other species apart from humans [16–21]. In addition, aged dogs are good for aging study, because it has been reported that β-amyloid accumulates in the dog brain with age and is identical to the human Aβ [22, 23]. In this study, therefore, we investigated DCX distribution in the aged hippocampal dentate gyrus to take information about neurogenesis in aged brain using aged dogs.
Experimental procedures
Experimental animals
The present study used the progeny of German shepherd obtained from the SWAT, South Korea. Male dogs were used at 2–3 years of age for adult group and 10–12 years of age for aged group. The procedures for handling and caring for the animals adhered to the guidelines, which are in compliance with the current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85–23, 1985, revised 1996), and they were approved by the Institutional Animal Care and Use Committee at Hallym’s Medical Center. All of the experiments were conducted to minimize the number of animals used and the suffering caused by the procedures used in this study.
Tissue processing for histology
For the histological analysis, adult and aged dogs (n = 5 at each age) were anesthetized with ketamine and xylazine mixture and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate-buffer (PB, pH 7.4). The brains were removed and postfixed in the same fixative for 6 h. The brain tissues were cryoprotected by infiltration with 30% sucrose overnight. Thereafter the frozen tissues were serially and transversely sectioned on a cryostat (Leica, Germany) into 30-μm coronal sections, and the sections were then collected into six-well plates containing PBS.
Immunohistochemistry for DCX
Immunohistochemistry was performed under the same conditions in dogs of different ages in order to examine whether the degree of immunohistochemical staining was accurate. The sections were sequentially treated with 0.3% hydrogen peroxide (H2O2) in PBS for 30 min and 10% normal rabbit serum in 0.05 M PBS for 30 min. They were then incubated with diluted goat anti-DCX antibody (1:50, SantaCruz Biotechnology, USA) overnight at room temperature and subsequently exposed to biotinylated rabbit anti-goat IgG and streptavidin peroxidase complex (diluted 1:200, Vector, USA). They were then visualized by staining with 3,3′-diaminobenzidine in 0.1 M Tris–HCl buffer (pH 7.2) and mounted on gelatin-coated slides. The sections were mounted in Canada Balsam (Kato, Japan) following dehydration. A negative control test was carried out using pre-immune serum instead of primary antibody in order to establish the specificity of the immunostaining. The negative control resulted in the absence of immunoreactivity in any structures.
Double immunofluorescence staining for DCX/NeuN
To confirm the neurons containing DCX immunoreactivity, double immunofluorescence staining for goat anti-DCX (1:10)/mouse anti-NeuN (1:500, Chemicon, USA) was performed. Brain tissues were incubated in the mixture of antisera overnight at room temperature. After washing 3 times for 10 min with PBS, the sections were incubated in a mixture of both Cy3 conjugated rabbit anti-goat IgG (1:600; Jackson ImmunoResearch) and FITC-conjugated sheep anti-mouse IgG (1:200; Jackson ImmunoResearch, USA) and for 2 h at room temperature. The immunoreactions were observed under the microscope (AxioM1, Carl Zeiss, Germany) attached HBO100.
Western blot analysis
To confirm changes in DCX levels in the dentate gyrus of adult and aged dogs, 3 animals in each group were sacrificed and used for Western blot analysis. After sacrificing them and removing the hippocampus, it was serially and transversely cut into a thickness of 400 μm on a vibratome (Leica, Germany), and the dentate gyrus was then dissected with a surgical blade. The tissues were homogenized in 50 mM PBS (pH 7.4) containing EGTA (pH 8.0), 0.2% NP-40, 10 mM EDTA (pH 8.0), 15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF, 150 mM NaCl, 2 mM sodium orthvanadate, 1 mM PMSF and 1 mM DTT. After centrifugation, DCX level was determined in the supernatants using a Micro BCA protein assay kit with bovine serum albumin as the standard (Pierce Chemical, USA). Aliquots containing 20 μg of total protein were boiled in loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% SDS, 0.3% bromophenol blue and 30% glycerol. The aliquots were then loaded onto a 10% polyacrylamide gel. After electrophoresis, the gels were transferred to nitrocellulose transfer membranes (Pall Crop, East Hills, NY, USA). To reduce background staining, the membranes were incubated with 5% non-fat dry milk in PBS containing 0.1% Tween 20 for 45 min, followed by incubation with goat anti-DCX antiserum (1:100), peroxidase-conjugated rabbit anti-goat IgG (Sigma, USA) and an ECL kit (Pierce Chemical, Rockford, IL, USA).
Quantification of data and statistical analysis
All measurements were performed in order to ensure objectivity in blind conditions, by two observers for each experiment, carrying out the measures of experimental samples under the same conditions.
Fifteen sections per animal were randomly selected from the corresponding areas of the dentate gyrus in order to quantitatively analyze DCX immunoreactivity in the dentate gyrus. The corresponding areas in the dentate gyrus were measured on the monitor at a magnification of 25–50×. Images of all DCX immunoreactive structures taken from 3 layers (molecular, granule cell and polymorphic layers) of the dentate gyrus were obtained through an AxioM1 microscope (Carl Zeiss, Germany) equipped with a CCD (Charge Coupled Device) camera connected to a PC monitor. Video images were digitized into an array of 512 × 512 pixels corresponding to a tissue area of 140 × 140 μm (40× primary magnification). Each pixel resolution was 256 gray levels. The intensity of all DCX immunoreactive structures was evaluated by means of a relative optical density (ROD) value, which was obtained after transformation of mean gray values using the formula: ROD = log (256/mean gray value). The ROD was measured in the complete field, and the values of background staining were subtracted from the ROD values of all immunoreactive structures before statistically processing. The relative % of control levels was demonstrated in the graph. The results of the Western blot analysis were also scanned, and RODs were determined using Scion Image software (Scion Corp., USA).
The data shown here represent the means of experiments performed for each dentate area. Differences among the means were statistically analyzed by student t-test analysis of variance to elucidate differences between adult and aged groups.
Results
Change in DCX immunoreactivity in adult and aged dogs
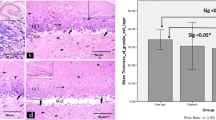
In this study, DCX immunoreactivity was detected in the dentate gyrus of adult and aged dogs (Fig. 1). DCX immunoreactivity was well found in the subgranular zone of the polymorphic layer of the dentate gyrus (Fig. 1A, B). In the adult dog, DCX immunoreactive cells in the subgranular zone had well-stained somata and processes (Fig. 1C). In the aged dog, DCX immunoreactivity was significantly decreased in the subgranular zone compared to that in the adult dog (Figs. 1B, 2). A few DCX immunoreactive cells in the aged dog were detected in this zone. Processes of these cells were poor in stain-ability compared to those in the adult dog (Fig. 1D).
Immunohistochemistry for DCX in the dentate gyrus of adult (A and C) and aged (B and D) dogs. DCX immunoreactivity is detected in the subgranular zone (arrows) of the polymorphic layer (PL). Many DCX immunoreactive cells are detected in the adult group; in the aged group, DCX immunoreactivity is observed in a few neurons. C and D, high magnification of the boxes in A and B, respectively. GL, granule cell layer. Bar = 200 μm (A and B) and 50 μm (C and D)
Relative optical density (ROD) as % of DCX immunoreactivity in the dentate gyrus of adult and aged dogs. Differences among the means are statistically analyzed by student t-test analysis of variance (n = 5 per group; * P < 0.01, significantly different from the adult group). The bars indicate the means ±S.D
Double immunofluorescence of DCX and NeuN
Based on double immunofluorescence staining, DCX immunoreactive cells in the subgranular zone of both adult and aged dogs were not identified with co-labeling with NeuN in this region (Fig. 3). In this study, NeuN immunoreactivity in the aged dog was poor compared to that in the adult dog (Fig. 3).
Change in DCX level
In the present study, we found that results of Western blot analysis in the dentate gyrus of adult and aged dogs were similar to the pattern of immunohistochemical changes (Fig. 4). DCX protein level in the aged dog was significantly decreased by 80% compared to that in the adult dog.
Discussion
Animal models that simulate various aspects of human brain aging are an essential step in the development of interventions to manage cognitive dysfunction in the elderly. We used 10–12-year-old dogs as aged dogs in this study, because it has been reported that cortical atrophy, ventricular widening, and reduced glucose utilization are common in dogs over 8 years of age [24–26]. These changes may reflect abnormalities that are associated with neural dysfunction and ultimately death [26]. It has been studying for cognition and neuropathology in the aged dog. For example, dogs, like humans, naturally accumulate deposits of β-amyloid (Aβ) in the brain with age [27–29]. Further, dogs and humans share the same Aβ sequence [22, 23].
There are some methods to detect newly generated neurons in the CNS. Retroviral incorporation requires invasive intracranial injection, which causes parenchymal lesions and possible inflammatory reactions [30]. Bromodeoxyuridin (BrdU), which integrates in the DNA of dividing cells, is diluted after multiple subsequent divisions within the progeny of a labeled cell [31]. DCX is very useful in studying for neurogenesis because DCX is specifically expressed in neuronal precursors in the developing and adult CNS [10–12].
In the present study, we observed newly generated neurons using DCX immunohistochemistry and Western blotting in the dog dentate gyrus to identify age influence on neurogenesis using adult and aged dogs. DCX immunoreactivity and protein level in the aged dog was significantly decreased compared to those in the adult dog. This result is the first in our knowledge, and coincides with previous reports that the rate of new cell production declines steadily over time, becoming reduced at least 80% relative to young levels by about one year of age in laboratory animals [32–37]. The reduction of DCX immunoreactivity in the dentate gyrus indicates that some factors may be involved in the age-related decline of cognitive ability because the hippocampus with dentate gyrus involves in various types of learning and memory [38]. Snyder et al. [39] reported that newly generated cells in the adult hippocampus might be involved in aspects of normal hippocampal function, such as spatial learning and memory.
In this study, the morphology of DCX immunoreactive cells was migrating neuroblasts in the adult dog. Based on double immunostaining, DCX immunoreactive cells in the subgranular zone did not show NeuN (a neuronal marker) immunoreactivity. Neurogenesis in the dentate gyrus is a multi-stage process. Developmental phases include the division of precursor cells in the subgranular zone to produce newborn granule cells, and the subsequent differentiation and migration of the newborn cells within the granule cell layer [2, 4, 5].
DCX could be a candidate marker for adult neurogenesis. The mutant allele of Dcx gene impairs the migration of neuronal progenitor cells during cortical development, and leads to cortical dysplasia [40–42]. In addition, the deletion of Dcx gene in mice leads to severe postnatal lethality and disrupted lamination in the hippocampus, although cortical layering is not affected [43].
In fact, new neurons are continually generated in the adult hippocampus, but the important question, whether adult neurogenesis is transient or leads to the lasting presence of new neurons, has been studied [44–46]. Kempermann et al. [44] reported that BrdU-labeled neurons remained stable in number and in their relative position in the granule cell layer of the mouse dentate gyrus over at least 11 months and that the expression of doublecortin in BrdU-labeled cells peaked early after division and was not detectable after 4 weeks. They suggested that new neurons are recruited early from the pool of proliferating progenitor cells and lead to a lasting effect of adult neurogenesis.
In brief, DCX immunoreactivity and protein level in the dentate gyrus of aged dogs are significantly decreased compared to those in adult dogs. These results suggest that the reduction of DCX in aged animals may be involved with neural deficits related to the hippocampus.
References
Altman J (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124:319–336
Biebl M, Cooper CM, Winkler J, Kuhn HG (2000) Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett 291:17–20
Larsson A, Wilhelmsson U, Pekna M, Pekny M (2004) Increased cell proliferation and neurogenesis in the hippocampal dentate gyrus of old GFAP(−/−)Vim(−/−) mice. Neurochem Res 29:2069–2073
Zhu LL, Zhao T, Li HS, Zhao H, Wu LY, Ding AS, Fan WH, Fan M (2005) Neurogenesis in the adult rat brain after intermittent hypoxia. Brain Res 1055:1–6
Kanagawa T, Fukuda H, Tsubouchi H, Komoto Y, Hayashi S, Fukui O, Shimoya K, Murata Y (2006) A decrease of cell proliferation by hypothermia in the hippocampus of the neonatal rat. Brain Res 1111:36–40
Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG (2003) Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467:1–10
Bonfanti L, Theodosis DT (1994) Expression of polysialylated neural cell adhesion molecule by proliferating cells in the subependymal layer of the adult rat, in its rostral extension and in the olfactory bulb. Neuroscience 62:291–305
Jin K, Mao XO, Greenberg DA (2004) Proteomic analysis of neuronal hypoxia in vitro. Neurochem Res 29:1123–1128
Karl C, Couillard-Després S, Prang P, Munding M, Kilb W, Brigadski T, Plotz S, Mages W, Luhmann H, Winkler J, Bogdahn U, Aigner L (2005) Neuronal precursor-specific activity of a human doublecortin regulatory sequence. J Neurochem 92:264–282
Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J (1999) Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 23:247–256
Gleeson JG, Lin PT, Flanagan LA, Walsh CA (1999) Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23:257–271
Capes-Davis A, Tolhurst O, Dunn JM, Jeffrey PL (2005) Expression of doublecortin (DCX) and doublecortin-like kinase (DCLK) within the developing chick brain. Dev Dyn 232:457–467
Rao MS, Shetty AK (2004) Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci 19:234–246
Nacher J, Crespo C, McEwen BS (2001) Doublecortin expression in the adult rat telencephalon. Eur J Neurosci 14:629–644
Yang HK, Sundholm-Peters NL, Goings GE, Walker AS, Hylandm K, Szele FG (2004) Distribution of doublecortin expressing cells near the lateral ventricles in the adult mouse brain. J Neurosci Res 76:282–295
Patronek GJ, Waters DJ, Glickman LT (1997) Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol Series A – Biol Sci Med Sci 52:B171–B178
Michell AR (1999) Longevity of British breeds of dog and its relationships with sex, size, cardiovascular variables and disease. Vet Record 145:625–629
Egenvall A, Bonnett BN, Shoukri M, Olson P, Hedharmmar A, Dohoo I (2000) Age pattern of mortality in eight breeds of insured dogs in Sweden. Preventive Vet Med 46:1–14
Mattson MP (2003) Adventures in neural plasticity, aging, and neurodegenerative disorders aboard the CWC beagle. Neurochem Res 28:1631–1637
Milgram NW (2003) Cognitive experience and its effect on age-dependent cognitive decline in beagle dogs. Neurochem Res 28:1677–1682
Proschowsky HF, Rugbjerg H, Ersboll AK (2003) Mortality of purebred are mixed-breed dogs in Denmark. Preventive Vet Med 58:63–74
Selkoe DJ, Bell DS, Podisny MB, Price DL, Cork LC (1987) Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer’s disease. Science 235:873–877
Johnstone EM, Chaney MO, Norris FH, Pascual R, Little SP (1991) Conservation of the sequence of the Alzheimer’s disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res 10:299–305
London ED, Ohata M, Takei H, French AWM, Rapoport I (1983) Regional cerebral metabolic rate for glucose in beagle dogs of different ages. Neurobiol Aging 4:121–126
Su MY, Head E, Brooks WM, Wang Z, Muggenberg BA, Adam GE, Sutherland RJ, Cotman CW, Nalcioglu O (1998) MR imaging of anatomic and vascular characteristics in a canine model of human aging. Neurobiol Aging 9:479–485
Head E, Torp R (2002) Insights into Aβ and presenilin from a canine model of human brain aging. Neurobiol Dis 9:1–10
Cummings BJ, Head E, Ruehl WW, Milgram NW, Cotman CW (1996) The canine as an animal model of human aging and dementia. Neurobiol Aging 17:259–268
Wisniewski T, Lalowski M, Bobik M, Russell M, Strosznajder J, Frangione B (1996) Amyloid beta 1–42 deposits do not lead to Alzheimer’s neuritic plaques in aged dogs. Biochem J 313:575–580
Nakamura S, Tamaoka A, Sawamura N, Kiatipattanasakul W, Nakayama H, Shoji S, Yoshikawa Y, Doi K (1997) Deposition of amyloid β protein (Aβ) subtypes [Aβ40 and Aβ42(43)] in canine senile plaques and cerebral amyloid angiopathy. Acta Neuropathol 94:323–328
Yamada M, Onodera M, Mizuno Y, Mochizuki H (2004) Neurogenesis in olfactory bulb identified by retroviral labeling in normal and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated adult mice. Neuroscience 124:173–181
Cooper-Kuhn CM, Kuhn HG (2002) Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Dev Brain Res 134:13–21
Seki T, Arai Y (1995) Age-related production of new granule cells in the adult dentate gyrus. NeuroReport 6:2479–2482
Kuhn HG, Dickinson-Anson H, Gage F (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16:2027–2033
Kempermann G, Kuhn HG, Gage FH (1998) Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci 18:3206–3212
Cameron H, KcKay RDG (1999) Restoring production of hippocampal neurons in old age. Nat Neurosci 2:894–897
Nacher J, Alonso-Llosa GA, Rosell DR, McEwan BS (2003) NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol Aging 24:273–284
Heine VM, Maslam S, Joels M, Lucassen PJ (2004) Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus. In absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging 25:361–375
Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN (2003) Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA 100:14385–14390
Snyder JS, Hong N, McDonald RJ, Wojtowicz JM (2005) A role for adult hippocampal neruogenesis in spatial long-term memory. Neuroscience 130:843–852
Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA (1998) Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell 92:63–72
Des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, Gelot A, Dupuis E, Motte J, Berwald-Netter Y, Catala M, Kahn A, Beldjord C, Chelly J (1998) A novel CNS gene required for neuronal migration and involved in X-liked subcortical laminar heterotopia and lissencephaly syndrome. Cell 92:51–61
Couillard-Després S, Winkler J, Uyanik G, Aigner L (2001) Molecular mechanisms of neuronal migration disorders, quo vadis? Curr Mol Med 1:677–688
Corbo JC, Deuel TA, Long JM, LaPorte P, Tsai E, Wynshaw-Boris A, Walsh CS (2002) Doublecortin is required in mice for lamination of the hippocampus but not the neocortex. J Neurosci 22:7548–7557
Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH (2003) Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 130:391–339
Kronenberg G, Reuter K, Steiner B et al (2003) Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol 467:455–463
Wen PH, Friedrich VL Jr, Shioi J, Robakis NK, Elder GA (2002) Presenilin-1 is expressed in neural progenitor cells in the hippocampus of adult mice. Neurosci Lett 318:53–56
Acknowledgements
The authors would like to thank Mr. Seok Han, Mr. Seung Uk Lee and Ms. Hyun Sook Kim for their technical help in this study. This work was supported by the Nano/Bio program of Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (No. M10641450002-06N4145-00200).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, I.K., Yoo, KY., Li, H. et al. Differences in Doublecortin Immunoreactivity and Protein Levels in the Hippocampal Dentate Gyrus Between Adult and Aged Dogs. Neurochem Res 32, 1604–1609 (2007). https://doi.org/10.1007/s11064-007-9366-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9366-1