Abstract
Dentate gyrus is a fundamental sub-region of the hippocampus which is directly engaged in higher memory and cognitive functions. This study was performed to describe the histological and immunohistochemical changes in dentate gyrus in experimental animals during postnatal development. Forty four male albino rats were classified into four equal groups: new born group aged one day, adult group aged 3–6 months, early senile group aged 18–20 months and late senile group aged 30–31 months. Specimens of hippocampus were processed and prepared for routine hematoxylin and eosin stains and immunohistochemical expressions of calretinin, glial fibrillary acidic protein and Ki67 (Kiel 67). Morphometric data were statistically analyzed. The present results showed significant reduction in thickness of granule cell layer of late senile group. Interestingly, the mean number of immature neurons was significantly increased in early senile group, while it was significantly reduced in late senile group. The number of mature granule cells showed marked reduction in both early and late senile groups. Furthermore, the number of astrocytes and optical density of glial fibrillary acidic protein revealed significant age-dependent increase. Measurable difference in number of calretinin positive interneurons was detected between adult and senile groups. However, mean number of Ki67 immune positive nuclei expressed significant age-dependent reduction. This study concluded that interneurons play a substantial role in modulating dentate gyrus neurogenesis which occurs throughout life and steadily decreases during aging. It is recommended to focus on the different stimuli and factors that potentiate neurogenesis to prevent or treat cognitive deficiencies associated with aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hippocampal neurogenesis is believed to play an important role in modulating and maintaining learning, memory, and potentially other cognitive functions (Zhao et al. 2008). The proliferative capacity of adult neurogenesis in all mammals decreases with age however the timing of this and the extent to which it occurs in the human brain remains the subject of significant debate (Capilla-Gonzalez et al. 2015). The hippocampal formation is defined as the complex of six structures; hippocampus proprius, dentate gyrus (DG), presubiculum, parasubiculum, subiculum proprium and area entorhinal cortex (EC) (Andersen et al. 2000). Dentate gyrus is a trilaminate cortical structure with a characteristic U or V shape. It is a deep region within hippocampus and is surrounded by cornu ammonis. It has two blades: suprapyramidal which is the granule cell layer of DG laid between cornu ammonis 3 (CA3) and cornu ammonis 1 (CA1) and the portion opposite this is the infrapyramidal blade. The region bridging the two blades (at the apex of V or U) is the crest (Andersen et al. 2007).
Neurogenesis in the adult DG of the hippocampus occurs constitutively throughout postnatal life, and the rate of neurogenesis within the DG can be modified under different physiological and pathophysiological conditions. Adult neurogenesis includes the process in which the division of a precursor cell occurs and the multi-step process (proliferation, differentiation, migration, targeting, and synaptic integration) which ends with the formation of a postmitotic functionally integrated new neuron (von Bohlen Und Halbach 2007). The progenitor cells lied in the subgranular zone of the DG and give rise to young neurons that can be integrated into existing neuronal circuits (Dokter and von Bohlen und Halbach 2012). Aging process is usually accompanied by cognitive decline and structural alterations in brain areas such as cortex and hippocampus which leads to morbidity and mortality (Bertoldi et al. 2017). Moreover, aging is the greatest risk factor for Alzheimer’s disease which characterized by progressive memory loss and cognitive decline. Hippocampal circuitry is essentially vulnerable to aging and neurodegenerative conditions (Chohan et al. 2011). Dentate gyrus has represented a paramount focus of scientific interest during past few decades. However, there was a paucity of researches about its cytological contributions in aging process. Therefore, this study was conducted to through more light on the histological and immunohistochemical changes in the DG in male albino rats during postnatal development and aging and correlates these changes to interneuronal modulating functions.
Materials and methods
Experimental animals
Forty four male Wistar albino rats were divided according to age into four equal groups (eleven rats each). Group I (new born group): rats aged 1 day old weighing 10–50 g. Group II (adult group): rats aged 3-6 months weighing 150-200 g. Group III (early senile group): rats aged 18–20 months weighing 250-350 g. Group IV (late senile group): rats aged 30–31 months weighing 200-300 g (Buckland et al. 2013).
The animals were obtained from the breeding animal house, Faculty of Medicine, Zagazig University. They were housed in a room illuminated for 12 h daily by daylight at room temperature, fed standard balanced diet and allowed water ad libitum (Suckow et al. 2006).
Experimental procedures: Upon reaching target age experimental animals (in accordance with guidelines of Institutional Animal Care and Use Committee (IACUC), Faculty of Medicine, Zagazig University) were anaesthetized using ether inhalation. When they became completely anesthetized, the vault of each animal cranium was rapidly removed and the cerebral cortex was exposed. Brains were carefully dissected out, divided via coronal sections into two cerebral hemispheres according to rat brain atlas (Paxinos et al. 2012) then fixed in 10% neutral formal saline. Samples were divided into two sets to be processed for both histological and immunohistochemical studies (Seidler et al. 2002).
Histological study
Specimens were dehydrated, embedded in paraffin wax, cut into 5 μm sections with a rotary microtome (Leica RM 2025; Nassloch, Germany). Sections were encompassing the entire anterior–posterior axis of the hippocampus (10–12 sections) (Wolf et al. 2002). At least two different series of sections were examined in each specimen (hippocampus). Sections were mounted on glass slides then stained with hematoxylin and eosin (Bancroft and Gamble 2008).
Immunohistochemical study
Immunohistochemical study was carried out applying the peroxidase labeled streptavidin Biotin technique for detection of glial fibrillary acidic protein (GFAP) as a marker for astrocytes (Larsson et al. 2004), calretinin as a marker for identification of interneurons (Gulyás et al. 1996; Huusko et al. 2015; Pelkey et al. 2017) and Kiel 67 (Ki67) for proliferating cells (Kim et al. 2009). Selected Paraffin sections (from −2.5 to −4.5 from Bregma) (Paxinos et al. 2012) were deparafinized, rehydrated and washed in phosphate buffered saline (PBS) for 3-5 min.; peroxidase activity was quenched using 3% H2O2 for 5 min and the sections were then rinsed with PBS for 15 min. Sections were blocked with 1.5% normal goat serum in PBS and were then incubated for 45 min at room temperature with the primary antibody. Sections were subsequently incubated with a second- stage biotinylated antibody (biotin- conjugated goat anti- rabbit immunoglobulin G (IgG), 1:200, 1 h, at room temperature). After rinsing in PBS, the reaction products were visualized by immersing the sections into the chromogen diamino benzidine. Finally, the sections were counterstained with hematoxylin, dehydrated, and mounted. For the negative control, the same steps were followed, but the primary antibody was replaced with PBS (Kiernan 2015; Polak and Van Noorden 2003). The primary antibodies used in the study were: (1) Monoclonal mouse anti-glial fibrillary acidic protein (anti-GFAP) delivered from Sigma Laboratories Ltd. (Catalog number MA5–12023), (2) Calretinin rabbit polyclonal antibody of Immunoglobulin G (IgG) type. Anti-calretinin antibody delivered from GeneTex Company (GeneTex, Inc. North America). (Catalog number GTX103261) and (3) Ki67 rabbit monoclonal antibody delivered from Dako Laboratories (UK) (Catalog number 275-R17). Universal kits used the avidin biotin peroxidase system produced by Novacastra Laboratories Ltd. (UK).
Morphometric study
Quantitative morphometric measurements were achieved by using the Leica Qwin 500 Image analyzer (England) computer system at Image Analysis Unit, Department of Histology, Faculty of Medicine, Zagazig University. The thickness of the dentate granule cell layer, the number of dentate mature granule cells, immature cells and astrocytes, Optical density of GFAP were quantitatively assessed by the image analysis of three different high power fields (X400). The quantification occurred at level of granule cell layer of DG. Cells were quantified from coronal sections according to rat brain atlas (Paxinos et al. 2012). Counting the numbers of calretinin positive cells and Ki67 positive cells were quantitatively assessed by the image analysis of three different high power fields (X200). The numbers of immune positive cells were counted as a proportion of the total number of cells. The thickness of dentate granule cell layer was calculated and calibration was performed before each measurement by using the semiautomatic morphometric software program (Digitizer, version 2). The thickness of the granule cell layer was measured in micrometers. All measurements were tabulated in excel sheet where the mean values of each measurement in each case are calculated.
Statistical analysis of the results was expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to compare normally distributed variables in more than two groups. Post-hoc least significant difference (LSD) test was used according to homogeneity of variances. The tests were two sided. P < 0.01 was considered statistically significant and p ≥ 0.05 was considered statistically non-significant. All data were analyzed using Statistical Package for Social Science (SPSS) 18.0 for windows (SPSS Inc., Chicago, IL, USA) & MedCalc 13 for windows (MedCalc Software bvba).
Results
Histological and morphometric results (Fig. 1)
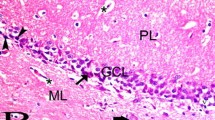
Hematoxylin and eosin (H&E) stained sections of new born group (I) revealed dentate gyrus (DG) of the hippocampus consisted of molecular layer, granule cell layer (GCL) and polymorphic cell layer. The granule cell layer (GCL) contained many compactly arranged mature granule cells with rounded pale vesicular nuclei. Many neurons with oval dark nuclei and paucity of cytoplasm were detected in subgranular zone (SGZ) (Fig. 1a). Sections of adult group (II) stained with H&E showed the dentate gyrus GCL with many compactly arranged granule cells. Few glial cells with darkly stained nuclei surrounded by lightly stained space were also detected (Fig. 1b). Sections of early senile group (III) stained with H&E showed the dentate gyrus GCL with granule cells with rounded pale vesicular nuclei and many cells with dark nuclei and scanty cytoplasm were seen in SGZ (Fig. 1c). Sections of late senile group (IV) stained with H&E revealed thin dentate gyrus GCL that contained few granule cells, edematous SGZ and numerous glial cells with darkly stained nuclei were also observed (Fig. 1d).
A higher magnification of the boxed area in inset showing GCL (arrow) containing many mature granule cells with rounded pale vesicular nuclei (new born and adult). Multiple neurons (thick arrows) with oval dark nuclei and paucity of cytoplasm in SGZ (a & b). Few granule cells in senile and late senile groups (c & d). Numerous neurons with oval dark nuclei and scanty cytoplasm (c) are also noticed, however, few ones are observed in late senile group (d). Glial cells with darkly stained nuclei surrounded by lightly stained space (arrow head) are few in adult (b) and numerous in senile groups (c & d) [H&E, Scale Bar = 50 μm] and the thickness of granule cell layer in the different studied groups
Morphometric results
The mean values of the granule cell layer thickness were 24.3982 ± 2.54132 μm in the new born group, 29.1889 ± 6.64713 μm in the adult group, 34.0255 ± 2.73913 μm in the early senile group and 30.5227 ± 6.44952 μm in the late senile group. Statistical analysis of these results revealed that there was statistically significant decrease in the thickness of granule cell layer (GCL) of the late senile group (IV) compared to the new born group (I) as P value <0.001. On the other hand, results showed no significant difference between group (I) and group (II) and non-significant difference between group (II) and group (III). Post-hoc test comparisons using LSD (least significant difference) test indicated that the mean of GCL thickness in new born and adult groups was significantly different from late senile group. However, the mean of GCL thickness in early senile group was significantly different from the mean of new born and late senile groups.
Immunohistochemical and morphometric results
Immunohistochemical and morphometric results for GFAP (Fig. 2)
Immuno peroxidase technique for GFAP showed astrocytes with positive reaction. Astrocytes appeared as small bodies with few short thin processes in new born group (I) (Fig. 2a). They appeared larger in size with multiple thin processes in adult group (II) (Fig. 2b). In early senile group (III) multiple strong positive astrocytes appeared larger in size with many thick processes (Fig. 2c). Similar findings were detected in late senile group (IV) with dilated perivascular space (Fig. 2d). Morphometric results: mean values of the optical density of GFAP were 2.4511 ± 0 .00935 in the new born group; 2.4400 ± 0.01549 in the adult group; 2.4745 ± 0.01864 in the early senile group and 2.4601 ± 0.00806 in the late senile group. Statistical analysis of these results revealed that there was statistically significant increase in the mean of optical density of GFAP towards the late senile group (IV) compared to the new born group (I) as P value <0.001. While there was no statistically significant difference between new born group (I) and late senile group (IV). Post-hoc test comparisons using LSD test indicated that the mean number of optical density of GFAP of new born group was statistically significant different from early senile group. Also the adult group its mean was statistically significant different from older age groups. While early senile group was significantly different from all age groups.
Immuno-reactivity of GFAP (arrow heads) showing astrocytes with small bodies and few short thin processes in new born group (a). Astrocytes appear larger in size with multiple thin processes in adult group (b). Multiple astrocytes appear larger in size with multiple thick processes in early& late senile groups (c & d). [Immuno peroxidase technique for GFAP, Scale Bar = 50 μm] and mean values of Optical density of GFAP for astrocytes
Immunohistochemical and morphometric results for calretinin (Fig. 3)
Immunohistochemical staining for calretinin showed strong positive immune reaction in numerous interneurons of newborn group (I) (Fig. 3a). Strong positive immune reaction was expressed in many interneurons with large nerve cell body and prominent dendrite in adult group (II) (Fig. 3b). However, few immuno-positive interneurons were noticed in early senile group (III) (Fig. 3c). Few positive immune reactions for calretinin appeared in interneurons in late senile group (IV). Some of interneurons appeared with cytoplasmic reaction, while others appeared with cytoplasmic and nuclear reaction (Fig. 3d). Morphometric results: mean number of immune positive interneurons was 7.1618 ± 1.75149 in the new born group; 2.6764 ± 0.61331 in the adult group; 1.9169 ± 0.23464 in the early senile group and 1.1645 ± 0.10053 in the late senile group. Statistical analysis of these results revealed that there was statistically significant decrease in the mean of the number of immune positive cells of calretinin as a proportion of the total number of cells in adult, early senile and late senile groups (II, III, IV) compared to the new born group (I) as P value <0.001. However, non-significant difference was detected between early and late senile groups. Post-hoc test comparisons using LSD test indicated that the mean of the number of immune positive cells of calretinin as a proportion of the total number of cells in each age group was statistically significant different from other age group one.
Immuno-reactivity of calretinin (arrow heads) is strong positive in (a) newborn group and (b) adult group, few positive in (c) early senile group and (d) late senile group. [Immuno peroxidase technique for calretinin, Scale Bar = 50 μm] and mean number of immuno-positive interneurons for calretinin in the different studied groups
Immunohistochemical and morphometric results for Ki67 (Fig. 4)
Immunohistochemical staining for Ki67 showed positive immune reaction in numerous nuclei of DG of new born group (I) (Fig. 4a). Adult group (II) showed positive immune reaction for Ki67 in many nuclei of DG (Fig. 4b). Early senile group (III) showed positive immune reaction for Ki67 in few nuclei of DG (Fig. 4c). Late senile group (IV) showed positive immune reaction for Ki67 in few nuclei of DG (Fig. 4d). Morphometric results: mean number of immune positive nuclei for Ki67 was 57.5100 ± 8.14101 in the new born group; 69.5455 ± 10.87533 in the adult group; 74.0909 ± 6.84769 in the early senile group and 18.1818 ± 3.60051 in the late senile group. Statistical analysis of these results revealed that there was statistically significant decrease in the mean of the number of immune positive nuclei of Ki67 as a proportion of the total number of cells in the late senile group (IV) compared to the new born group (I) as P value <0.001. Post-hoc test comparisons using LSD test indicated that the mean of the number of immune positive nuclei of Ki67 as a proportion of the total number of cells in the new born and adult group was statistically significant different from older age groups (early and late senile). While the mean number of immuno-positive nuclei count of Ki67 was statistically significant different from all other age groups.
Immuno-expression of Ki67 (arrow heads) is positive immune reaction in numerous nuclei of DG in (a) new born group and (b) adult group, is positive immune reaction in few nuclei of DG in (c) Early senile group and (d) Late senile group. [Immuno peroxidase technique for Ki67, Scale Bar = 50 μm] and mean number of immuno-positive nuclei for Ki67 count in the different studied groups
Mean number of dentate granule cells, immature cells and astrocytes (Fig. 5 & Table 1)
There was statistically significant decrease in the mean number of mature granule cells from the new born group (I) towards the adult and late senile groups (II&IV) as P value <0.001. There was statistically significant decrease in the mean number of immature granule cells from the new born group (I) towards the late senile group (IV) as (P value <0.001). Also, there was statistically significant increase between the adult group (II) and early senile group (III) (P value <0.001). Statistically significant increase in the mean number of astrocytes cell count was reported the new born group (I) towards the adult, early senile and late senile groups (II, III, IV) (P value <0.001).
Discussion
Neurogenesis in the postnatal human brain occurs in two neurogenic niches; the subventricular zone (SVZ) in the wall of the lateral ventricles and the subgranular zone (SGZ) of the hippocampus. The extent to which this physiological process continues into adulthood is an area of ongoing research (Dennis et al. 2016). Subgranular zone of the hippocampus contains the microenvironment that is permissive for neuronal development to occur. This microenvironment is called the neurogenic “niche.” The niche comprises the precursor cells themselves, their immediate progeny and immature neurons, other glial cells and endothelia, very likely immune cells, microglia, and macrophages, and an extracellular matrix (Mercier et al. 2002). This study was conducted to through more light on dentate gyrus neuro-cytomorphological and immunohistochemical alterations during postnatal development and to evaluate correlated interneuronal modulating functions.
The present work revealed that new born group (I) dentate gyrus H&E stained sections consisted of molecular layer, granule cell layer and the polymorphic layer forming upper blade and lower blade. It occupied the area between Cornu ammonis 3 (CA3) and the Para-hippocampal gyrus. It had a toothed shape and the hippocampal sulcus appeared as a deep groove in adult (II), early senile (III) and late senile (IV) groups. However, in late senile group (IV) the subgranular zone appeared bullous. These findings were in agreement with Amaral et al. (2007) & Witter (2007). Basically in agreement with Cummings et al. (2005) & Seri et al. (2001) the present results revealed that granule cell layer of DG contained compactly arranged mature granule cells with rounded pale vesicular nuclei. The present work noticed that SGZ contained oval shaped neurons with darkly stained nuclei and paucity of cytoplasm; Kempermann et al. (2004) described them as an early stage of immature neurons. Llorens-Martín et al. (2016) confirmed that these maturational stages are identified on the basis not only of the expression of specific molecular markers but also of their unique morphological features. Chohan et al. (2011) proved that immature granule cells are unique neuro-precursor cells that maintain mitotic activity in adulthood; they ultimately differentiate into mature granule neurons with rounded pale vesicular nuclei and pale acidophilic cytoplasm. Statistically significant decrease in GCL thickness was evident in late senile group (IV) compared to the new born group (I), this was in line with Wisse et al. (2014). Varela-Nallar et al. (2010) explained this with age dependent loss of afferents from the entorhinal cortex, reduced rates of precursor cells proliferation and reactive oxygen species (ROS) theory of aging. This research investigated differential cell count of GCL that confirmed this interpretation. Interestingly, the present observation showed that early senile group (III) revealed significant increase in the number of immature neurons in the SGZ. Subsequently, this explains the non-significant difference in GCL thickness between adult and early senile groups. Li et al. (2008) clarified that compensatory increase of immature cell proliferation rate accoumpany neurodegenerative disorders as Alzheimer’s disease. However, the newly generated neurons in dentate gyrus do not differentiate to mature neurons.
Astrocytes are critical for neuronal survival in central nervous system; as they regulate energy metabolism and maintain blood-brain barrier (Dringen et al. 2000; Zonta et al. 2003). Examination of H&E stained sections of early and late senile groups revealed observable increase of astrocytes, the present statistical data confirmed these findings. Astrocytes appeared with small rounded nuclei surrounded by lightly stained cytoplasm in agreement with Vinters and Kleinschmidt-DeMasters (2018). Borlongan et al. (2000) described this result as reactive gliosis or astrogliosis which is characterized by compensatory accelerated synthesis of GFAP. Rodríguez-Arellano et al. (2016) added that reactive astrogliosis is associated with dystrophic neurite plaques. Glial fibrillary acidic protein (GFAP) is the principal intermediate filament in mature astrocytes. It is thought to be important in modulating astrocyte motility and shape by providing structural stability to astrocytic processes (von Bohlen Und Halbach 2007). Immunohistochemical stained sections revealed increase of GFAP optical density in astrocytes in late senile group (IV) compared to new born group (I). In addition, increase in number and size of astrocytes in their GFAP-immunoreactivity with astrocytic foot processes edema around the blood vessels with dilated perivascular spaces were observed in late senile group (IV). These findings were in agreement with Larsson et al. (2004) and Xiaoli et al. (2006).
Using Ki67 as a marker for proliferating cells proved significant reliability as it is expressed during mitosis and has a short half-life. Kim et al. (2009) stated that Ki67 is a good marker for detecting proliferating cells during neurogenesis of the adult brain. In the current study, examination of immunohistochemical stained sections for Ki67 revealed that positive nuclei were abundant in the new born group, decreased in the adult one, reached low values in the early senile group then almost disappeared in late senile group. The present study confirmed this finding as statistically significant decrease in number of immune positive nuclei of Ki67 in the late senile group was reported compared to the new born group. This work observed that Ki67 immuno-reactive cells were dispersed in almost all of the layers of dentate gyrus with more concentration in the SGZ. These observations were in agreement with Ambrogini et al. (2004); Liu et al. (2000) who proved that granular cells neurogenesis is pronounced within the first three postnatal weeks and continues into adulthood at a reduced rate. They added that, after proliferation of neural precursor cells within the SGZ, newborn cells migrate toward the GCL. The recent work of Boldrini et al. (2018) proved that ongoing hippocampal neurogenesis is mandatory to sustain higher memory and cognitive functions. Ki67 positive nuclei observed in this study were similar to the nuclei of granule cells in size and shape. This suggests that these nuclei may be destined to become granule cells. Skilling et al. (2017) stated that neurogenesis related circuits modifications could steer granule cells integration into dentate gyrus-neocortex networks.
Calretinin is a marker for specific non-pyramidal γ-aminobutyric acid (GABA)-ergic interneurons within hippocampus (Brandt et al. 2003). We observed that mean number of calretinin immuno-reactive cells revealed significant decrease in adult, early senile and late senile groups (II, III, and IV) compared to the new born group (I). These findings were reported by Andrews-Zwilling et al. (2010) who also stated that the population of CR-expressing cells within the granule cell layer of DG have not been characterized in suffecient detail. Takahashi et al. (2010) clarified that calretinin is a marker of various cell lines in the dentate gyrus: hilar mossy cells and interneurons of SGZ. However, calretinin-positive interneurons are expressed in all layers of hippocampal zones, including areas Cornu ammonis 1- Cornuammonis3 (CA1-CA3) and the DG (Brandt et al. 2003). Somogyi and Klausberger (2005) reported that axons of interneurons in the Cornu ammonis 1 (CA1) region of the hippocampus traverse the hippocampal sulcus and also innervate the dentate gyrus. de Guevara-Miranda et al. (2017) suggested that hippocampal interneurons are directly engaged in regulation of adult hippocampal neurogenesis based on their proximity to adult neural stem cells in the SGZ. Most hippocampal interneurons share a common characteristic they are GABAergic, producing and releasing this largely inhibitory neurotransmitter (Maccaferri and Lacaille 2003). During early postnatal development, newly generated neurons follow a sequence of events through which they form their synaptic connections (Ben-Ari 2002). Initially the neurons are “silent”, meaning that they have no spontaneous postsynaptic currents to the commonly applied agents e.g. GABA. Zhao et al. (2006) reported that upon the formation of GABAergic synapses they become sensitive to depolarization by GABA, followed by the development of glutamatergic inputs, and lastly a switch to hyperpolarization by GABA, a sign of neuronal maturity. Adult-generated neurons in SGZ pass a similar progression steps to initiate synaptic connectivity with hippocampal neuronal circuit (Espósito et al. 2005). Ables et al. (2010) added that GABAergic interneurons regulate the slow proliferation of putative neural stem cells which are the source cells of adult neurogenesis. Furthermore, Deisseroth et al. (2004) highlighted that as adult-generated cells differentiate into mature dentate gyrus granule cells; they not only respond to GABA but also receive GABAergic inputs. This input is important as it depolarizes the maturing cells and elevates their intracellular Ca2+ levels via activation of voltage-gated calcium channels. GABAergic interneurons have the power to regulate the differentiation of adult-generated DG cells. However, with this complex integration of interneurons into the hippocampal circuitry and proposed interneuron regulation of synaptic activity in the hippocampus, interneurons have received little attention in regard to how they affect the neurogenic niche and the generation of new neurons in the adult brain (Houser 2007). The biological mechanisms responsible for brain neuronal aging are not yet fully investigated; Ca2+ is widely believed to have a role in the process. Schwaller (2014) stated that Ca2+ homeostasis control appears to be a fundamental property of healthy neurons, so the Ca2+ binding proteins such as calretinin are of considerable importance. Although calretinin emerges as a multi-functional protein it is also associated with development i.e., cell proliferation and differentiation. Masiulis et al. (2011) suggested that various signaling factors regulate interneuron GABAergic activity and thus adult-neurogenesis in an activity-dependent manner. Herrup (2010) confirmed the interplay between interneurons and hippocampal neurogenesis. This research can suggest that there is a bidirectional crosstalk between the age-induced decline in interneurons and the decline in adult neurogenesis. The present study concludes that interneurons play a substantial role in modulating dentate gyrus neurogenesis which occurs throughout life and steadily decreases during aging. It is recommended to focus on the different stimuli and factors that potentiate neurogenesis to prevent or treat cognitive deficiencies associated with aging.
Abbreviations
- Ki67:
-
Kiel 67
- DG:
-
dentate gyrus
- GFAP:
-
glial fibrillary acidic protein
- PBS:
-
phosphate buffered saline
- LSD:
-
least significant difference
- GCL:
-
granule cell layer
- SGZ:
-
subgranular zone
- GABA:
-
gamma aminobutyric acid
- H&E:
-
hematoxylin and eosin
References
Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, Radtke F, Hsieh J, Eisch AJ (2010) Notch 1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci 30:10484–10492. https://doi.org/10.1523/JNEUROSCI.4721-09.2010
Amaral DG, Scharfman HE, Lavenex P (2007) The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies). Prog Brain Res 163:3–22. https://doi.org/10.1016/S0079-6123(07)63001-5
Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R (2004) Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res 1017:21–31. https://doi.org/10.1016/j.brainres.2004.05.039
Andersen P, Soleng AF, Raastad M (2000) The hippocampal lamella hypothesis revisited1. Brain Res 886:165–171. https://doi.org/10.1016/s0006-8993(00)02991-7
Andersen P., Morris R., Amaral D., Bliss T. & O’Keefe J. 2007. The hippocampus book. Oxford University Press, New York & Oxford. ISBN: 9780195100273
Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y (2010) Apolipoprotein E4 causes age- and tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci 30:13707–13717. https://doi.org/10.1523/JNEUROSCI.4040-10.2010
Bancroft J.D. & Gamble M. 2008. Theory and practice of histological techniques. Churchill Livingstone, London. ISBN 9780443102790
Ben-Ari Y (2002) Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci 3:728–739. https://doi.org/10.1038/nrn920
Bertoldi K, Cechinel LR, Schallenberger B, Meireles L, Basso C, Lovatel GA, Bernardi L, Lamers ML, Siqueira IR (2017) Aging process alters hippocampal and cortical secretase activities of Wistar rats. Behav Brain Res 317:374–381. https://doi.org/10.1038/nrn920
Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Hen R (2018) Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22(4):589–599. https://doi.org/10.1016/j.stem.2018.03.015
Borlongan CV, Yamamoto M, Takei N, Kumazaki M, Ungsuparkorn C, Hida H, Sanberg PR, Nishino H (2000) Glial cell survival is enhanced during melatonin-induced neuroprotection against cerebral ischemia. FASEB J 14(10):1307–1317. https://doi.org/10.1096/fasebj.14.10.1307
Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick-Sander A, Von Der Behrens W, Kempermann G (2003) Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci 24:603–613. https://doi.org/10.1016/s1044-7431(03)00207-0
Buckland M.D., Hall L., Mowlem A. & Whatley B.F. 2013. A guide to laboratory animal technology, William Heinemann Medical Books LTD, London, kindle ed. ISBN 0433045906
Capilla-Gonzalez V, Herranz-Pérez V, García-Verdugo JM (2015) The aged brain: genesis and fate of residual progenitor cells in the subventricular zone. Front Cell Neurosci 9(365):1–11. https://doi.org/10.3389/fncel.2015.00365
Chohan MO, Li B, Blanchard J, Tung YC, Heaney AT, Rabe A, Iqbal K, Grundke-Iqbal I (2011) Enhancement of dentate gyrus neurogenesis, dendritic and synaptic plasticity and memory by a neurotrophic peptide. Neurobiol Aging 32:1420–1434. https://doi.org/10.1016/j.neurobiolaging.2009.08.008
Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ (2005) Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci 102:14069–14074. https://doi.org/10.1073/pnas.0507063102
de Guevara-Miranda DL, Millón C, Rosell-Valle C, Pérez-Fernández M, Missiroli M, Serrano A, Pavón F, Rodríguez de Fonseca F, Martínez-Losa M, Álvarez-Dolado M, Santín L, Castilla-Ortega E (2017) Long-lasting memory deficits in mice withdrawn from cocaine are concomitant to neuroadaptations in hippocampal basal activity, GABAergic interneurons and adult neurogenesis. Dis Models Mech 10(3):323–336. https://doi.org/10.1242/dmm.026682
Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC (2004) Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42:535–552. https://doi.org/10.1016/s0896-6273(04)00266-1
Dennis CV, Suh LS, Rodriguez ML, Kril JJ, Sutherland GT (2016) Human adult neurogenesis across the ages: an immunohistochemical study. Neuropathol Appl Neurobiol 42:621–638. https://doi.org/10.1111/nan.12337
Dokter M, von Bohlen und Halbach O (2012) Neurogenesis within the adult hippocampus under physiological conditions and in depression. Neural Regen Res 7:552–559. https://doi.org/10.3969/j.issn.1673-5374.2012.07.013
Dringen R, Gutterer JM, Hirrlinger J (2000) Glutathione metabolism in brain. Eur J Biochem 267:4912–4916. https://doi.org/10.1046/j.1432-1327.2000.01597.x
Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF (2005) Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci 25:10074–10086. https://doi.org/10.1523/JNEUROSCI.3114-05.2005
Gulyás AI, Hájos N, Freund TF (1996) Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci 16(10):3397–3411. https://doi.org/10.1523/JNEUROSCI.16-10-03397.1996
Herrup K (2010) Reimagining Alzheimer’s disease—an age-based hypothesis. J Neurosci 30:16755–16762. https://doi.org/10.1523/JNEUROSCI.4521-10.2010
Houser CR (2007) Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog Brain Res 163:217–811. https://doi.org/10.1016/S0079-6123(07)63013-1
Huusko N, Römer C, Ndode-Ekane XE, Lukasiuk K, Pitkänen A (2015) Loss of hippocampal interneurons and epileptogenesis: a comparison of two animal models of acquired epilepsy. Brain Struct Funct 220(1):153–191. https://doi.org/10.1007/s00429-013-0644-1
Kempermann G, Jessberger S, Steiner B, Kronenberg G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27(8):447–452. https://doi.org/10.1016/j.tins.2004.05.013
Kiernan JA (2015) Histological and histochemical methods: theory and practice, 5th edn. Scion Publishing Ltd, Banbury, pp 103–118
Kim JS, Jung J, Lee HJ, Kim JC, Wang H, Kim SH, Shin T, Moon C (2009) Differences in immunoreactivities of Ki-67 and doublecortin in the adult hippocampus in three strains of mice. Acta Histochem 111:150–156. https://doi.org/10.1016/j.acthis.2008.05.002
Larsson A, Wilhelmsson U, Pekna M, Pekny M (2004) Increased cell proliferation and neurogenesis in the hippocampal dentate gyrus of old GFAP-/-vim-/- mice. Neurochem Res 29(11):2069–2073. https://doi.org/10.1007/s11064-004-6880-2
Li B, Yamamori H, Tatebayashi Y, Shafit-Zagardo B, Tanimukai H, Chen S, Iqbal K, Grundke-Iqbal I (2008) Failure of neuronal maturation in Alzheimer disease dentate gyrus. J Neuropathol Exp Neurol 67:78–84. https://doi.org/10.1097/nen.0b013e318160c5db
Liu XS, Tilwalli S, Ye G, Lio PA, Pasternak JF, Trommer BL (2000) Morphologic and electrophysiologic maturation in developing dentate gyrus granule cells. Brain Res 856:202–212. https://doi.org/10.1016/s0006-8993(99)02421-X
Llorens-Martín M, Rábano A, Ávila J (2016) The ever-changing morphology of hippocampal granule neurons in physiology and pathology. Front Neurosci 9(526):1–20. https://doi.org/10.3389/fnins.2015.00526
Maccaferri G, Lacaille JC (2003) Interneuron diversity series: hippocampal interneuron classifications – making things as simple as possible, not simpler. Trends Neurosci 26:564–571. https://doi.org/10.1016/j.tins.2003.08.002
Masiulis I, Yun S, Eisch AJ (2011) The interesting interplay between interneurons and adult hippocampal neurogenesis. Mol Neurobiol 44:287–302. https://doi.org/10.1007/s12035-011-8207-z
Mercier F, Kitasako JT, Hatton GI (2002) Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol 451:170–188. https://doi.org/10.1002/cne.10342
Paxinos G., Watson C., Petrides M., Rosa M. &Tokuno H. 2012. The marmoset brain in stereotaxic coordinates. Elsevier Academic Press. San Diego. ISBN 978-0124158184
Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, McBain CJ (2017) Hippocampal GABAergic inhibitory interneurons. Physiol Rev 97(4):1619–1747. https://doi.org/10.1152/physrev.00007.2017
Polak J.M.,Van Noorden S. 2003. Introduction to immunocytochemistry. 3rd edition, BIOS Scientific Publishers, Oxford, pp 141. ISBN 1859962084
Rodríguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A (2016) Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience 323:170–182. https://doi.org/10.1016/j.neuroscience.2015.01.007
Schwaller B (2014) Calretinin: from a “simple” Ca2+ buffer to a multifunctional protein implicated in many biological processes. Front Neuroanat 8:1–7. https://doi.org/10.3389/fnana.2014.00003
Seidler F, Roy TS, Slotkin TA (2002) Prenatal nicotine exposure evokes alterations of cell structure in hippocampus and somatosensory cortex. J Pharmacol Exp Ther 300:124–133. https://doi.org/10.1124/jpet.300.1.124
Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A (2001) Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21:7153–7160. https://doi.org/10.1523/JNEUROSCI.21-18-07153.2001
Skilling Q, Roach J, Althaus AL, Murphy GG, Sander L, Zochowski M (2017) Modifications in network structure and excitability may drive differential activity-dependent integration of granule cells into dentate gyrus circuits during Normal and pathological adult neurogenesis. In: The rewiring brain, pp 409–423. https://doi.org/10.1016/B978-0-12-803784-3.00019-6
Somogyi P, Klausberger T (2005) Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol 562:9–26. https://doi.org/10.1113/jphysiol.2004.078915
Suckow M.A., Weisbroth S.H. & Franklin C.L. 2006. In The Laboratory Rat, second. ed. Elsevier Academic Press, San Diego, CA. Chapter 4 – Morphophysiology, pp. 93–125. ISBN 9780120749034
Takahashi H, Brasnjevic I, Rutten BPF, Van Der Kolk N, Perl DP, Bouras C, Steinbusch HWM, Schmitz C, Hof PR, Dickstein DL (2010) Hippocampal interneuron loss in an APP/PS1 double mutant mouse and in Alzheimer’s disease. Brain Struct Funct 214:145–160. https://doi.org/10.1007/s00429-010-0242-4
Varela-Nallar L, Aranguiz FC, Abbott AC, Slater PG, Inestrosa NC (2010) Adult hippocampal neurogenesis in aging and Alzheimer’s disease. Birth Defects Res Part C Embryo Today Rev 90:284–296. https://doi.org/10.1002/bdrc.20193
Vinters H.V. & Kleinschmidt-DeMasters B.K. 2018. General pathology of the central nervous system. In Greenfield’s neuropathology, 9th edition. James Ironside, Arie Perry. CRC Press, Taylor& Francis group. Boca Raton.pp.25–82. ISBN9781498721288
von Bohlen Und Halbach O (2007) Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res 329:409–420. https://doi.org/10.1007/s00441-007-0432-4
Wisse LEM, Biessels GJ, Heringa SM, Kuijf HJ, Koek DL, Luijten PR, Geerlings MI (2014) Hippocampal subfield volumes at 7T in early Alzheimer’s disease and normal aging. Neurobiol Aging 35:2039–2045. https://doi.org/10.1016/j.neurobiolaging.2014.02.021
Witter MP (2007) The perforant path: projections from the entorhinal cortex to the dentate gyrus. Prog Brain Res 163:43–61. https://doi.org/10.1016/s0079-6123(07)63003-9
Wolf OT, Dyakin V, Vadasz C, De Leon MJ, McEwen BS, Bulloch K (2002) Volumetric measurement of the hippocampus, the anterior cingulate cortex, and the retrosplenial granular cortex of the rat using structural MRI. Brain Res Protocol 10(1):41–46. https://doi.org/10.1016/S1385-299X(02)00181-2
Xiaoli W, Yun X, Fang W, Lihua T, Zhilong L, Honglian L, Shenghong L (2006) Aging-related changes of microglia and astrocytes in hypothalamus after intraperitoneal injection of hypertonic saline in rats. J Huazhong Univ Sci Technol Medical Sci 26:231–234. https://doi.org/10.1007/BF02895824
Zhao C, Teng EM, Summers RG, Ming GL, Gage FH (2006) Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 26:3–11. https://doi.org/10.1523/JNEUROSCI.3648-05.2006
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132:645–660. https://doi.org/10.1016/j.cell.2008.01.033
Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G (2003) Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6:43–50. https://doi.org/10.1038/nn980
Acknowledgements
The authors would like to thank Dr. Wael Galal in Community Medicine Department, Zagazig University for this help in the statistical work and Dr. Hanaa S. Mousa in Histology Department, Zagazig University; for her help in the morphometric study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All procedures performed in the present study involving experimental animals were in accordance with the ethical standards of the Institutional Animal Care and Use Committee (IACUC), Faculty of Medicine, Zagazig University, Egypt.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghallab, A.M., Alazouny, Z.M., Samak, M.A. et al. Dentate gyrus neurogenesis across different ages in male rats: an immunohistochemical approach. Biologia 74, 905–914 (2019). https://doi.org/10.2478/s11756-019-00246-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-019-00246-7