Abstract
Calcium ion is essential for cellular functions including signal transduction. Uncontrolled calcium stress has been linked causally to a variety of neurodegenerative diseases. Thapsigargin, which inhibits Ca2+-ATPase in the endoplasmic reticulum (ER) and blocks the sequestration of calcium by the ER, induced apoptotic cell death (chromatin condensation and nuclear fragmentation) accompanied by GRP78 protein expression and caspase-3 activation in rat fetal cortical neurons (days in vitro 9–10). Blockade of N-methyl-d-aspartate (NMDA) receptors with NMDA antagonists induced apoptosis without GRP78 protein expression. Apoptosis accompanied both caspase-9 and caspase-3 activation. We then examined whether GSK-3 is involved in thapsigargin-induced cell death by using GSK-3 inhibitors. We assayed the effects of selective GSK-3 inhibitors, SB216763, alsterpaullone and 1-azakenpaullone, on thapsigargin-induced apoptosis. These inhibitors completely protected cells from thapsigargin-induced apoptosis. In addition, GSK-3 inhibitors inhibited caspase-9 and caspase-3 activation accompanied by thapsigargin-induced apoptosis. These results suggest that thapsigargin induces caspase-dependent apoptosis mediated through GSK-3β activation in rat cortical neurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous N-methyl-d-aspartate (NMDA) receptor activity supports the survival of developing neurons [1–3]. The NMDA receptor, a subtype of the glutamate receptor, acts via the receptor-gated cation channel, which is permeable to Ca2+. Blockade of NMDA receptors with NMDA antagonists induces apoptosis in cultured cortical neurons through the Bax-cytochrome C-caspase pathway [4]. Loss of activity-dependent survival signals is recognized as a stimulus for neuronal apoptosis and may play a significant role in neurodegeneration [5].
Calcium ion is essential for cellular functions including signal transduction [6]; however, uncontrolled calcium stress has been linked causally to a variety of neurological diseases, including ischemia, excitotoxicity and Alzheimer’s disease [7, 8].
Impaired function of the endoplasmic reticulum (ER), commonly referred to as ER stress, is an important factor in the neuropathology of a wide variety of neurological disorders [9, 10]. Several agents, such as thapsigargin, can be used to induce ER stress experimentally. Thapsigargin inhibits Ca2+-ATPase in the ER [11, 12], which blocks the sequestration of calcium by the ER, causing increases in the intracellular concentration of calcium, accumulation of unfolded or misfolded proteins and activation of caspase-3-mediated apoptosis. The cellular signals involved in ER stress-mediated cell death are not fully understood.
Recent reports suggest that glycogen synthase kinase-3 (GSK-3) affects many fundamental cellular functions, including the cell cycle, gene transcription, cytoskeletal integrity and apoptosis [13–16]. The phosphatidylinositol-3 kinase/Akt signaling pathway is a signaling system implicated in the survival of neurons that leads to the inhibition of GSK-3 by increasing Ser9 phosphorylation [13, 17, 18]. We reported recently that thapsigargin-induced apoptosis is prevented by GSK-3 inhibitors in PC12 cells [19].
As described above, recent data have implicated ER stress as an important factor in some pathological conditions such as ischemia; however, whether shared or unique apoptotic cascades are activated by blocking NMDA receptor vs. ER stress in cortical neurons has not previously been investigated. We therefore examined whether GSK-3 is involved in thapsigargin-induced cell death using GSK-3 inhibitors in cultured rat cortical cells.
Materials and methods
Materials
SB216763 was purchased from Tocris Cookson (Bristol, UK). Alsterpaullone, roscovitine and mouse anti-β-actin monoclonal antibody were purchased from Sigma Chemical Co. (St Louis, USA). 1-Azakenpaullone was purchased from Calbiochem (La Jolla, USA). (+)-5-Methyl-10,11-dihydro-5H-dibenzo cyclohepten-5,10-imine (MK801) was generously supplied by Merck Sharp Dohme (Rahway, USA). Rabbit anti-GRP78 polyclonal antibody was purchased from Santa Cruz Biotechnology Inc. (California, USA). Hoechst 33258 dye (bis-benzimide) was purchased from Molecular Probes (Oregon, USA). Ac-Asp-Glu-Val-Asp-4-methyl-coumaryl-7-amide (Ac-DEVD-MCA), Ac-Leu-Glu-His-Asp-4-methyl-coumaryl-7-amide (Ac-LEHD-MCA) and 7-amino-4-methyl-coumarin (AMC) were acquired from the Peptide Institute (Osaka, Japan). Thapsigargin and other chemicals were purchased from Wako Pure Chemical Industries (Osaka, Japan).
Cell culture
Cerebral cortical cells were obtained and cultured essentially as described by Dichter [20] from fetal rats (Wistar) after 18–19 days of gestation. Whole cerebral neocortices were removed from fetal mice, taking care to discard the hippocampal formation, basal ganglia and most of the meninges. The tissue was then minced, incubated in 0.25% trypsin for 30 min at 37 °C, and then Dulbecco’s modified Eagle’s medium (DMEM) plus fetal calf serum was added. The tissue was triturated with Pasteur pipette. Clumps of cells were removed by filtering through a double layer of lens paper.
The dissociated cortical cells were cultured on poly-d-lysine-coated 35 mm dishes (Falcon 3001) (2 × 106 cells/dish) or cover glasses in DMEM containing 10% fetal calf serum. Ten micromolar of cytosine-β-d arabinofuranoside was added to the culture medium on day 3 after plating. The cells were cultured for 9–10 days and then used in the experiments. We examined the relative proportion of neurons to other cell types by staining the cells with anti-neuronal nuclei (NeuN) monoclonal antibody and anti-glial fibrillary protein (GFAP) visualized with an immunohistochemical staining kit, Vectastain ABC kit (Vector). The relative proportion of neurons (NeuN+) to glia cells (GFAP+) was about 95%.
Cell treatment and cell viability
The cells were washed twice with Tris-buffered salt solution containing (in mM): NaCl 120, KCl 5.4, CaCl2 1.8, MgCl2 0.8, Tris–HCl 25 and glucose 15, at pH 6.5 (washing buffer) and then replaced with 2 ml of serum (5%)-containing or serum-free DMEM. We also used washing buffer at pH 6.5 to avoid glutamate neurotoxicity which depends on extracellular pH. Cell treatment with various reagents was carried out for 48 h at 37 °C. Morphological cell changes were observed by phase-contrast microscopy during treatment. Cell viability was checked by Trypan blue (0.4%) exclusion.
Quantitation of apoptosis by nuclear morphological changes
Apoptotic cell death was determined by staining cells with Hoechst dye H33258. Cells were fixed with a 10% formalin neutral phosphate buffer solution (pH 7.4) for 5 min at room temperature. After washing the cells with distilled water, they were stained with 8 μg/ml of H33258 for 5 min. Nuclear morphology was observed under a fluorescent microscope (Olympus IX70 model). Apoptosis was quantitated by scoring the percentage of cells with apoptotic nuclear morphology at the single cell level. Condensed or fragmented nuclei were scored as apoptotic. A total of five to seven randomly selected fields were captured using Polaroid PDMC II software. At least 200 cells were counted per condition, and each experiment was repeated in at least three different cultures.
Western blotting
Primary cultured cells were scraped off the dish and collected by centrifugation (400 × g for 5 min), followed by homogenization in ice-cold buffer (50 mM Tris–HCl buffer containing 50 mM NaCl, 10 mM EGTA, 5 mM EDTA, 2 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM benzamide and 10% protease inhibitor cocktail, pH 7.4). The cell suspension was placed on ice for 30 min and centrifuged at 18,000 × g for 30 min. The supernatant was diluted with an equal volume of sample buffer containing 62.5 mM of Tris–HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS), 10% glycerol, 50 mM of dithiothreitol and 0.1% bromophenol blue. Protein concentration was determined by the bicinchoninic acid assay. Each sample (10 μg/lane) was loaded and separated using 7.5% SDS-polyacrylamide gel electrophoresis. Proteins were transferred to a nitrocellulose membrane and blocked with Tris-buffered saline (TBS-T; 20 mM Tris and 0.1% Tween) containing 5% skim milk for 1 h at room temperature, and then incubated with anti-GRP78 in TBS-T overnight at 4 °C. After washing for 5 min with three changes of TBS-T, the membrane was incubated with a phosphatase-conjugated goat anti-rabbit antibody for 1 h at room temperature in TBS-T. After washing for 5 min with three changes of TBS-T, immunoreactive bands were visualized with a Western blot detection kit BCIP/NBT system. Equal amounts of protein extracts were also analyzed by Western blot analysis with anti-actin antibody.
Caspase activity
Caspase activity was measured as described previously [2]. Briefly, cells were washed with phosphate-buffered saline and suspended in 50 mM of Tris–HCl buffer (pH 7.4) containing 1 mM of EDTA and 10 mM of EGTA. They were then treated with 10 μM of digitonin for 10 min. Lysates were obtained by centrifugation at 10,000 × g for 5 min, and cleared lysates containing 50–100 μg protein were incubated with 50 μM of enzyme substrate Ac-DEVD-MCA (caspase-3) or Ac-LEHD-MCA (caspase-9) for 1 h at 37 °C. The reaction was terminated by the addition of monoiodoacetic acid (5 mM). AMC levels were measured using a spectrofluorometer (Hitachi F4500, Japan) with excitation at 380 nm and emission at 460 nm, and the activity was expressed as pmol of AMC released/min/mg protein.
Statistical analysis
Statistical significance was assessed by one-way ANOVA and by post-hoc Scheffe’s comparisons.
Results
Thapsigargin- and NMDA antagonist-induced apoptosis
We used thapsigargin to induce ER stress experimentally. Thapsigargin inhibits Ca2+-ATPase in the ER, which blocks the sequestration of calcium by the ER. In addition, we used MK801, an NMDA receptor antagonist, to induce apoptosis by blocking Ca2+-dependent survival [2]. After treating cortical cells with thapsigargin (0.1 μM) for 48 h, cells showed apoptotic morphology, including shrunken cell bodies by phase-contrast observation, chromatin condensation and nuclear fragmentation detected by staining with H33258 (Fig. 1). Cell treatment with MK801 (10 μM) in the absence of serum induced apoptosis showing apoptotic morphology, including shrunken cell bodies, chromatin condensation and nuclear fragmentation. The loss of cell viability was also confirmed by trypan blue exclusion (data not shown).
Thapsigargin- and MK801-induced apoptosis. Phase-contrast (a–d) and H33258 fluorescence (e–h) microscopy of rat cortical cells. Cells were incubated with 0.1 μM thapsigargin (b, f) or 10 μM MK801 (d, h) in the absence (c, d, g, h) or presence (a, b, e, f) of 5% dialyzed bovine fetal serum for 48 h at 37 °C. Arrowheads indicate healthy neurons. Arrows indicate neurons with apoptotic morphology. Scale bars = 10 μm
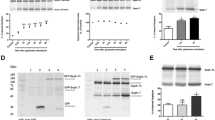
Up to 40–50% of cells showed apoptotic death by treatment with either thapsigargin (0.1 μM) or MK801 (10 μM) (Fig. 2a). Up to 20% neurons showed apoptosis depending on experimental conditions by serum withdrawal.
a Thapsigargin- and MK801-induced apoptosis. Cells were incubated with 0.1 μM thapsigargin in the presence of 5% dialyzed bovine fetal serum, or 10 μM MK801 in the absence of serum for 48 h at 37 °C. Each value represents the mean ± S.E.M. Asterisk represents P < 0.05 (vs. untreated cells). Hash represents P < 0.05 [vs. untreated cells (serum-free)]. b Effects of thapsigargin and MK801 on GRP78 protein expression. Cells were incubated with (lane 2) or without (lane 1) 0.1 μM thapsigargin in the presence of 5% dialyzed bovine fetal serum, and incubated with (lane 4) or without (lane 3) 10 μM MK801 in the absence of serum for 24 h at 37 °C. Cells were lysed and the lysate was immunoblotted with anti-GRP78 or anti-β-actin antibody
Cell treatment with thapsigargin (0.1 μM) for 24 h induced the expression of GRP78 protein, indicating ER stress response (Fig. 2b). On the other hand, cell treatment with MK801 (10 μM) did not induce the expression of GRP78 protein (Fig. 2b).
We then examined whether ER stress-induced apoptosis was accompanied by the activation of caspase-9 and caspase-3. Cell treatment with thapsigargin (10 and 100 nM) for 24 h activated caspase-9 (Fig. 3a) and caspase-3 (Fig. 3b) activity in a dose-dependent manner.
Thapsigargin-induced caspase-9 and caspase-3 activation. Cells were incubated with thapsigargin (10–100 nM) for 24 h at 37 °C. Caspase-9 (a) and caspase-3 (b) activity was measured as described in Sect. ”Materials and methods.” Data are shown as the mean ± S.E.M. Asterisk represents P < 0.05 (vs. untreated cells)
Protective effect of GSK-3 inhibitors on thapsigargin-induced apoptosis
Glycogen synthase kinase-3 is the principal physiological substrate of Akt (also known as protein kinase B) and the activity of GSK-3 is inhibited by Akt-mediated phosphorylation in response to trophic stimulation. To investigate directly the role of endogenous GSK-3 activity in cell death in response to thapsigargin treatment, we assayed the effects of selective GSK-3 inhibitors, SB216763, alsterpaullone and 1-azakenpaullone, on thapsigargin-induced apoptosis. Incubation with alsterpaullone, SB216763 and 1-azakenpaullone inhibited thapsigargin-induced apoptosis (Fig. 4). SB216763 has been reported to inhibit GSK-3 activity with no significant activity toward other protein kinases, including cyclin-dependent protein kinases [21, 22]. On the other hand, alsterpaullone is also known to inhibit cyclin-dependent protein kinase 5 [23, 24]; however, a cyclin-dependent protein kinase 5 selective inhibitor, roscovitine, did not prevent thapsigargin-induced apoptosis (data not shown), suggesting that alsterpaullone prevented apoptosis by inhibiting GSK-3 activity.
Protective effect of GSK-3 inhibitors on thapsigargin-induced cell injury. Cells were incubated with 0.1 μM of thapsigargin in the presence or absence of alsterpaullone (Als, 1 μM), SB216763 (SB, 5 μM) or 1-azakenpaullone (Aza, 2 μM) for 48 h at 37 °C. Each value represents the mean ± S.E.M. Asterisk represents P < 0.05 (vs. thapsigargin-only-treated cells)
Effect of GSK-3 inhibitors on thapsigargin-induced caspase activation
As shown in Fig. 3, thapsigargin activated caspase-3 and caspase-9 activity; therefore, we assayed the effect of alsterpaullone on thapsigargin-induced caspase activation. Cell treatment with thapsigargin (0.1 μM) for 24 h activated caspase-9 (Fig. 5a) and caspase-3 (Fig. 5b), and alsterpaullone inhibited the activation of both caspases.
Effects of a GSK-3 inhibitor on thapsigargin-induced caspase-9 and caspase-3 activation. Cells were incubated with 0.1 μM of thapsigargin in the presence of alsterpaullone (1 μM) for 24 h at 37 °C. Caspase-9 (a) and caspase-3 (b) activity were measured as described in Sect. ”Materials and methods.” Data are shown as the mean ± S.E.M. Asterisk represents P < 0.05 (vs. thapsigargin-only-treated cells)
Discussion
We showed in this report that GSK-3 inhibitors protected cortical cells from thapsigargin-induced apoptosis. GSK-3 inhibitors inhibited thapsigargin-induced caspase-9 and caspase-3 activation, suggesting that GSK-3 is involved in the caspase pathway. Glycogen synthase kinase-3 activity is known to be suppressed when it becomes phosphorylated on serine 9 by the activation of Akt. Insulin growth factor-I, which is known to activate the phosphatidylinositol-3 kinase/Akt signaling pathway, protected cortical neurons from thapsigargin-induced apoptosis (data not shown). The mechanism by which ER stress activates GSK-3β activity is not clear. Song et al. [25] reported that ER stress signaling induces the protein phosphatase 2A-dependent dephosphorylation of GSK-3β, and the subsequent GSK-3β-dependent induction of apoptosis in human SH-SY5Y neuroblastoma cells. Pro-apoptotic substrates for GSK-3β have not been well-characterized, although Linseman et al. [26] reported that GSK-3 phosphorylates Bax, a pro-apoptotic Bcl-2 family member that stimulates the intrinsic (mitochondrial) death pathway by eliciting cytochrome c release from mitochondria, and promotes its mitochondrial localization in human embryonic kidney 293 cells. We reported previously that GSK-3 inhibitors completely protected cortical neurons from the NMDA antagonist-induced apoptosis induced possibly by blocking the trophic effect of NMDA receptor and decreasing the intracellular calcium ion levels of the cells [16, 27]. As shown in Fig. 2b, the blockade of NMDA receptor induced apoptosis without expressing GRP78. NMDA receptor antagonist-induced apoptosis involves apoptotic events that are induced by changes in outer mitochondrial membrane permeability in rat cortical neurons [4]. Glutamate plays an important role in neuronal survival in the developing brain largely through facilitating the entry of Ca2+ [28, 29]. On the other hand, impaired function of the ER is an important factor in the neuropathology of a wide variety of neurological disorders [9, 10].
Feng et al. [30] reported recently that cleavage and activation of caspase-3, -9, releasing of cytochrome C from mitochondria, upregulation of GRP78 expression were induced in K562 cells after exposure of thapsigargin, suggesting that mitochondria participates in ER stress-induced apoptosis in K562 cells. Turner et al. [31] reported that A1 adenosine receptor activation and NMDA receptor blockade lead to early postnatal cell injury by mechanisms that involve inhibition of intracellular Ca2+ signaling, and that thapsigargin enhanced the cell injury induced by A1 adenosine receptor activation and NMDA receptor blockade.
ER stress may also activate GSK-3 and induce apoptosis via a mitochondrial apoptotic pathway accompanied by caspase-9 and caspase-3 activation [32]. GSK-3 may serve as a common site of apoptotic signaling downstream of both the inhibition of the trophic effect of NMDA receptor possibly by reducing the intracellular calcium level and the induction of ER stress by disturbing Ca2+ homeostasis in ER.
We showed in this report that GSK-3 inhibitors protected cortical neurons from thapsigargin-induced apoptosis. GSK-3 inhibitors may deserve to be evaluated as therapeutic agents in ER stress-induced neurological disorders such as ischemia
References
Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW (1999) Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283:70–74
Takadera T, Matsuda I, Ohyashiki T (1999) Apoptotic cell death and caspase-3 activation induced by N-methyl-d-aspartate receptor antagonists and their prevention by insulin-like growth factor I. J Neurochem 73:548–556
Hwang JY, Kim YH, Ahn YH, Wie MB, Koh JY (1999). N-Methyl-d-aspartate receptor blockade induces neuronal apoptosis in cortical culture. Exp Neurol 159:124–130
Yoon WJ, Won SJ, Ryu BR, Gwag BJ (2003) Blockade of ionotropic glutamate receptors produces neuronal apoptosis through the Bax-cytochrome C-caspase pathway: the causative role of Ca2+ deficiency. J Neurochem 85:525–533
Olney JW, Wozniak DF, Jevtovic-Todorovic V, Farber NB, Bittigau P, Ikonomidou C (2002) Drug-induced apoptotic neurodegeneration in the developing brain. Brain Pathol 12:488–498
Tsien RW, Tsien RY (1990) Calcium channels, stores, and oscillations. Annu Rev Cell Biol 6:715–760
Paschen W, Frandsen A (2001) Endoplasmic reticulum dysfunction—a common denominator for cell injury in acute and degenerative diseases of the brain? J Neurochem 79:719–725
Choi DW (1988) Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci 11:465–469
Lindholm D, Wootz H, Korhonen L (2006) ER stress and neurodegenerative diseases. Cell Death Differ 13:385–392
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103
Lytton J, Westlin M, Hanley MR (1991) Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem 266:17067–17071
Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP (1990) Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci USA 87:2466–2470
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785–789
Hetman M, Cavanaugh JE, Kimelman D, Xia Z (2000) Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci 20:2567–2574
Grimes CA, Jope RS (2001) The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol 65:391–426
Takadera T, Sakamoto Y, Ohyashiki T (2004) NMDA receptor 2B-selective antagonist ifenprodil-induced apoptosis was prevented by glycogen synthase kinase-3 inhibitors in cultured rat cortical neurons. Brain Res 1020:196–203
Yao R, Cooper GM (1995) Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 267:2003–2006
Pap M, Cooper GM (1998) Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem 273:19929–19932
Takadera T, Yoshikawa R, Ohyashiki T (2006) Thapsigargin-induced apoptosis was prevented by glycogen synthase kinase-3 inhibitors in PC12 cells. Neurosci Lett 408:124–128
Dichter MA (1978) Rat cortical neurons in cell culture: culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res 149:279–293
Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC (2000) Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol 7:793–803
Cross DA, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD (2001) Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem 77:94–102
Sandal T, Stapnes C, Kleivdal H, Hedin L, Doskeland SO (2002) A novel, extraneuronal role for cyclin-dependent protein kinase 5 (CDK5): modulation of cAMP-induced apoptosis in rat leukemia cells. J Biol Chem 277:20783–20793
Leost M, Schultz C, Link A, Wu YZ, Biernat J, Mandelkow EM, Bibb JA, Snyder GL, Greengard P, Zaharevitz DW, Gussio R, Senderowicz AM, Sausville EA, Kunick C, Meijer L (2000) Paullones are potent inhibitors of glycogen synthase kinase-3beta and cyclin-dependent kinase 5/p25. Eur J Biochem 267:5983–5994
Song L, De Sarno P, Jope RS (2002) Central role of glycogen synthase kinase-3beta in endoplasmic reticulum stress-induced caspase-3 activation. J Biol Chem 277:44701–44708
Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML, Heidenreich KA (2004) Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci 24:9993–10002
Takadera T, Ishida A, Ohyashiki T (2006) Ketamine-induced apoptosis in cultured rat cortical neurons. Toxicol Appl Pharmacol 210:100–107
Balazs R, Jorgensen OS, Hack N (1988) N-methyl-d-aspartate promotes the survival of cerebellar granule cells in culture. Neuroscience 27:437–451
Yan GM, Ni B, Weller M, Wood KA, Paul SM (1994) Depolarization or glutamate receptor activation blocks apoptotic cell death of cultured cerebellar granule neurons. Brain Res 656:43–51
Feng XQ, You Y, Xiao J, Zou P (2006) Thapsigargin-induced apoptosis of K562 cells and its mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi 14:25–30
Turner CP, Pulciani D, Rivkees SA (2002) Reduction in intracellular calcium levels induces injury in developing neurons. Exp Neurol 178:21–32
Brewster JL, Linseman DA, Bouchard RJ, Loucks FA, Precht TA, Esch EA, Heidenreich KA (2006) Endoplasmic reticulum stress and trophic factor withdrawal activate distinct signaling cascades that induce glycogen synthase kinase-3beta and a caspase-9-dependent apoptosis in cerebellar granule neurons. Mol Cell Neurosci 32:242–253
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takadera, T., Fujibayashi, M., Kaniyu, H. et al. Caspase-dependent Apoptosis Induced by Thapsigargin was Prevented by Glycogen Synthase Kinase-3 Inhibitors in Cultured Rat Cortical Neurons. Neurochem Res 32, 1336–1342 (2007). https://doi.org/10.1007/s11064-007-9310-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-007-9310-4