Abstract
GABA (γ-aminobutyric acid) is the major inhibitory neurotransmitter in the central nervous system, and changes in GABAergic neurotransmission modulate the activity of neuronal networks. Gephyrin is a scaffold protein responsible for the traffic and synaptic anchoring of GABAA receptors (GABAAR); therefore, changes in gephyrin expression and oligomerization may affect the activity of GABAergic synapses. In this work, we investigated the changes in gephyrin protein levels during brain ischemia and in excitotoxic conditions, which may affect synaptic clustering of GABAAR. We found that gephyrin is cleaved by calpains following excitotoxic stimulation of hippocampal neurons with glutamate, as well as after intrahippocampal injection of kainate, giving rise to a stable cleavage product. Gephyrin cleavage was also observed in cultured hippocampal neurons subjected to transient oxygen-glucose deprivation (OGD), an in vitro model of brain ischemia, and after transient middle cerebral artery occlusion (MCAO) in mice, a model of focal brain ischemia. Furthermore, a truncated form of gephyrin decreased the synaptic clustering of the protein, reduced the synaptic pool of GABAAR containing γ2 subunits and upregulated OGD-induced cell death in hippocampal cultures. Our results show that excitotoxicity and brain ischemia downregulate full-length gephyrin with a concomitant generation of truncated products, which affect synaptic clustering of GABAAR and cell death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Excessive activation of glutamatergic synapses plays an important role in neuronal death in different disorders of the nervous system, including brain ischemia, epilepsy, and neurodegenerative disorders [1, 2]. Brain ischemia is also characterized by a downregulation of GABAergic synaptic transmission, both at the pre- and postsynaptic levels [3, 4], but the mechanisms involved are not fully understood. Recent studies showed a decrease in the interaction of GABAA receptors (GABAAR) with the scaffold protein gephyrin in cultured hippocampal neurons subjected to oxygen-glucose deprivation (OGD), an in vitro model of brain ischemia, by a mechanism dependent on protein phosphatase activity [5]. The reduced interaction of GABAAR with the submembrane gephyrin lattice after OGD [6–8], together with the dephosphorylation of the receptors, facilitates their internalization by a clathrin-dependent mechanism [5, 9].
The gephyrin molecule contains an N-terminal G-domain, a C-terminal E-domain, and a linker region (C-domain) that connects the G and E domains [10, 8]. Crystallographic studies using isolated G- and E-domains showed that they form trimeric and dimeric structures, respectively. This interaction is thought to account for the formation of a hexagonal gephyrin scaffold that anchors GABAAR at the synapse by reducing their lateral mobility on the membrane [11, 12]. The GABAAR subunits α1-α3, β2 and β3 were shown to interact directly with gephyrin [13–17], and morphological analysis showed that α1-α3 subunits, together with the γ2 subunit, are colocalized on the postsynaptic membrane together with gephyrin [18]. Gephyrin clustering was recently shown to be modulated by extracellular signal-regulated kinases 1 and 2 (ERK1/2) and by glucogen synthase kinase 3β (GSK3β)-dependent phosphorylation. Furthermore, neuronal activity under physiological conditions regulates gephyrin clustering through Ca2+-dependent proteolysis mediated by calpains [19, 20].
The excessive calpain activity due to [Ca2+]i overload contributes to neuronal death in brain ischemia and in several chronic neurodegenerative conditions [21, 22]. Calpains were shown to cleave the vesicular GABA transporters in a model of transient focal ischemia as well as in cultured hippocampal neurons subjected to excitotoxic conditions, and the truncated transporters are not targeted to the synapse [23]. The plasma membrane GABA transporter GAT1 is also cleaved by calpains in the N- and C-terminus, including a region involved in the interaction of the transporter with a high-density PDZ anchoring matrix [24]. Furthermore, calpain activity contributes to the downregulation of several GABAAR subunits in hippocampal neurons subjected to OGD, an in vitro model of brain ischemia [5]. In this work, we investigated the alterations in gephyrin protein levels in hippocampal neurons subjected to excitotoxic conditions and in brain ischemia, and the impact on neuronal survival. Our results show that gephyrin cleavage by calpains leads to the formation of stable cleavage products which disassemble the synaptic gephyrin lattice. The cleavage of gephyrin reduces the synaptic clustering of GABAAR, and the consequent downregulation of inhibitory synapses upregulates neuronal death in hippocampal neurons subjected to OGD.
Material and Methods
Hippocampal and Striatal Cultures
Cultures of rat hippocampal and striatal neurons were prepared from E18-E19 and E16 Wistar rat embryos, respectively, as previously described [25, 23, 5]. Briefly, the dissected tissue was treated with trypsin (0.06 %, for 15 min at 37 °C; GIBCO Invitrogen) in Ca2+- and Mg2+-free Hank’s balanced salt solution (HBSS; 5.36 mM KCl, 0.44 mM KH2PO4, 137 mM NaCl, 4.16 mM NaHCO3, 0.34 mM Na2HPO4·2H20, 5 mM glucose, 1 mM sodium pyruvate, 10 mM HEPES, and 0.001 % phenol red). The hippocampi/striata were washed with HBSS containing 10 % fetal bovine serum to stop trypsin activity and then washed once in HBSS to remove serum and avoid glial growth. Finally, the hippocampi/striata were transferred to Neurobasal medium (GIBCO Invitrogen) supplemented with B27 (1:50 dilution; GIBCO Invitrogen), glutamate (25 μM), glutamine (0.5 mM), and gentamicin (0.12 mg/mL), and the cells were dissociated mechanically before plating on poly-d-lysine-coated 6 microwell plates (MW6) at a density of 9 × 104 cells/cm2, or on poly-d-lysine-coated glass coverslips at a density of 80.0 × 103 cells/cm2. The cells were kept at 37 °C in a humidified incubator with 5 % CO2/95 % air, for 7 or 14 days in vitro (DIV). Low-density hippocampal cultures were prepared by plating the dissociated cells at a final density of 1–5 × 104 cells/dish on poly-d-lysine-coated glass coverslips in neuronal plating medium (MEM supplemented with 10 % horse serum, 0.6 % glucose and 1 mM pyruvic acid). After 2–4 h, coverslips were flipped over an astroglial feeder layer in Neurobasal medium (GIBCO-Life Technologies) supplemented with SM1 supplement (1:50 dilution, STEMCELL Technologies), 25 μM glutamate, 0.5 mM glutamine, and 0.12 mg/ml gentamycin (GIBCO-Life Technologies). The neurons grew face down over the feeder layer but were kept separate from the glia by wax dots on the neuronal side of the coverslips. To prevent overgrowth of glial cells, neuron cultures were treated with 5 μM cytosine arabinoside (Sigma-Aldrich) after 3 DIV. Cultures were maintained in a humidified incubator with 5 % CO2/95 % air, at 37 °C, for up to 2 weeks, feeding the cells once per week.
Excitotoxic Stimulation with Glutamate
Hippocampal neurons were exposed to 125 μM glutamate for 20 min in Neurobasal medium and further incubated in culture conditioned medium for the indicated periods of time. Pre-incubations of 2 h were used when cells were treated with the calpain inhibitors MDL2817 (Calbiochem) and ALLN (Calbiochem) (50 μM). Under control conditions, hippocampal neurons were not exposed to glutamate.
Preparation of Hippocampal Culture Extracts
Hippocampal cultures were washed twice with ice-cold PBS, and once more with PBS buffer, supplemented with 1 mM dithiothreitol (DTT) and a cocktail of protease inhibitors (0.1 mM phenylmethylsulfonyl fluoride (PMSF) and CLAP [1 μg/ml chymostatin, 1 μg/ml leupeptin, 1 μg/ml antipain, and 1 μg/ml pepstatin; SIGMA]). The cells were then lysed with RIPA (150 mM NaCl, 50 mM Tris–HCl, pH 7.4, 5 mM EGTA, 1 % Triton, 0.5 % DOC, and 0.1 % SDS, pH 7.5) supplemented with 50 mM sodium fluoride (NaF), 1.5 mM sodium orthovanadate (Na3VO4), and the cocktail of protease inhibitors. After centrifugation at 16,100×g for 10 min at 4 °C, protein in the supernatants was quantified using the Bicinchoninic acid (BCA) assay kit (Pierce), and the samples were denatured with 2× concentrated denaturating buffer (125 mM Tris, pH 6.8, 100 mM glycine, 4 % SDS, 200 mM DTT, 40 % glycerol, 3 mM Na3VO4, and 0.01 % bromophenol blue).
Oxygen-Glucose Deprivation
Hippocampal neurons (14–15 DIV) were incubated in a glucose-free saline buffer (116 mM NaCl, 25 mM sucrose, 10 mM HEPES, 5.4 mM KCl, 0.8 mM MgSO4, 1 mM NaH2PO4, 1.8 mM CaCl2, and 25 mM NaHCO3), under an anaerobic atmosphere (10 % H2, 85 % N2, and 5 % CO2) (Forma Anaerobic System, Thermo Scientific) at 37 °C, for the indicated time periods. After the OGD challenge, cultures were incubated in culture-conditioned medium and returned to the humidified 95 % air/5 % CO2 incubator for 8 h. Control neurons were washed and incubated in the saline buffer described above, supplemented with 25 mM glucose, and kept in the humidified 95 % air/5 % CO2 incubator at 37 °C for the indicated time periods. When appropriate, the calpain inhibitor MDL28170 (50 μM; Merck-Millipore) was added 2 h before and during OGD, and was also present during the period after the ischemic injury.
Western Blot
Protein samples were separated by SDS-PAGE, in 12 % polyacrylamide gels, transferred to polyvinylidene fluoride (PVDF) membranes (Milipore, MA), and immunoblotted, as previously described [5]. Blots were incubated with primary antibodies overnight at 4 °C, washed, and exposed to alkaline phosphatase-conjugated secondary antibodies (1:20,000 dilution for anti-rabbit IgG and 1:10,000 dilution for anti-mouse IgG) for 1 h at room temperature. Alkaline phosphatase activity was visualized by enhanced chemifluorescence (ECF) on the Storm 860 gel and blot imaging system, and quantified using the Image Quant software (GE Healthcare). The following primary antibodies were used: anti-gephyrin (1:1000, Synaptic Systems) and anti-spectrin (1:1000, Merck-Millipore). The anti-synaptophysin (1:10000, Synaptic Systems) antibody was used as loading control.
Activation of Synaptic and Extrasynaptic NMDA Receptors
Activation of synaptic versus extrasynaptic NMDAR was performed as previously described [26, 27], in a basal saline buffer containing 132 mM NaCl, 4 mM KCl, 1.4 mM MgCl2, 2.5 mM CaCl2, 6 mM glucose, and 10 mM HEPES. To stimulate synaptic glutamate release, cultured hippocampal neurons (14 DIV) were treated with 50 μM bicuculline (Tocris), 2.5 mM 4-aminopyridine (4-AP; Tocris) and 10 μM glycine for 20 min, and were then allowed to recover for 4 h in the original culture medium. To activate the extrasynaptic pool of NMDA receptors, cultured hippocampal neurons (14 DIV) were treated with 50 μM bicuculline, 2.5 mM 4-AP, 10 μM MK 801 and 10 μM glycine for 5 min, and were then washed with a similar solution but in the absence of MK 801. Thereafter, neurons were incubated with 100 μM NMDA for 20 min and were further incubated in the original culture medium for 4 h. Cell extracts were prepared and described above for Western blot experiments.
Intra-Hippocampal Injection of Kainate
Intrahippocampal injection of kainate was performed as previously described [23]. Briefly, wild type adult male mice (C56BL6) were deeply anesthetized with avertin (2,2,2 Tribromoethanol, 2-methyl-2-butanol), placed in a stereotaxic apparatus, and given a unilateral injection of 1 nmol of kainate (in 0.3 μl of PBS) into the hippocampal CA1 region using a 10 μl motorized syringe (Hamilton), after drilling a small hole with a surgical drill. The coordinates of the injection were: anterior-posterior -2.3 mm, medial lateral -1.5 mm, and dorsal-ventral -1.7 mm from the bregma. Two minutes after the needle insertion, kainate was injected at a constant flow rate of 0.05 μl/min. The needle remained in place for an additional 2 min to prevent reflux of fluid. The body temperature of mice was monitored and maintained at 37 °C during surgery and up to 30 min after the injection, using a homeothermic heating blanket (Harvard Apparatus) with feedback regulation. The death rate in this experiment was less than 5 %. Mice were sacrificed 4 or 8 h after injection, and a 2 mm section was taken around the hippocampus with the help of a 1 mm coronal mouse matrice. The slices were immediately frozen with dry ice, and the contralateral and the damaged ipsilateral areas of the hippocampal slices were taken using a Harris Unicore 2 mm tip (Pelco International). Samples were then homogenized in RIPA buffer supplemented with 50 mM NaF, 1.5 mM Na3VO4 and the cocktail of protease inhibitors (PMSF, DTT, and CLAP), and processed for western blot.
Middle Cerebral Artery Occlusion (MCAO)
Experiments were conducted in accordance with protocols approved by the Malmö/Lund Ethical Committee for Animal Research (M332-09, M243-07). Nine to 11 weeks old C57BL/6 J male mice (weight 21.5 to 27.9 g; Taconic, Denmark) were housed under diurnal conditions with free access to water and food, before and after surgery.
Focal cerebral ischemia was induced by the transient occlusion of the right middle cerebral artery (MCA), using the intraluminal filament placement technique as described previously [28]. Briefly, adult male mice were anesthetized by inhalation of 2.5 % isoflurane (IsobaVet, Schering-Plough Animal Health, England) in O2:N2O (30:70). Anesthesia was subsequently reduced to 1.5–1.8 % isoflurane and sustained throughout the occlusion period. Body temperature was kept at approximately 37 °C throughout the surgery period. In order to monitor regional cerebral blood flow (rCBF), an optical fiber probe (Probe 318-I, Perimed, Sweden) was fixed to the skull at 2 mm posterior and 4 mm lateral to the bregma and connected to a laser Doppler flow meter (Periflux System 5000, Perimed, Sweden). A filament composed of 6–0 polydioxanone suture (PSDII, Ethicon) with a silicone tip (diameter of 225 to 275 μm) was inserted into the external carotid artery and advanced into the common carotid artery. The filament was retracted, moved into the internal carotid artery and advanced until the origin of the MCA, given by the sudden drop in rCBF (approximately 70 % of baseline). After 45 min, the filament was withdrawn and reperfusion observed. The animals were placed in a heating box at 37 °C for the first 2 h post-surgery and thereafter transferred into a heating box at 35 °C, to avoid post-surgical hypothermia. Thirty minutes after the onset of reperfusion, 0.5 ml of 5 % glucose were administered subcutaneously. Temperature and sensorimotor deficits were assessed at 1 and 2 h and in the morning after the surgery. Forty-eight hours after MCAO, mice were anesthesized, perfused with saline, and sacrificed. Brains were removed and frozen in isopentane (−60 °C), and the regions of interests (infarct core, peri-infarct area, and contralateral cortex) were dissected at −60 °C. Samples were then homogenized and processed for Western blot.
Neuron Transfection with Calcium Phosphate
Transfection of cultured hippocampal neurons (DIV12) with EGFP, EGFP-tagged full-length gephyrin (EGFP-geph.FL), or with a truncated form of gephyrin (EGFP-geph.T) [29] was performed by the calcium phosphate coprecipitation method. Briefly, 2 μg of plasmid DNA were diluted in Tris-EDTA (TE) pH 7.3 and mixed with 2.5 M CaCl2. This DNA/TE/calcium mix was added to 10 mM HEPES-buffered saline solution (270 mM NaCl, 10 mM KCl, 1.4 mM Na2 HPO4, 11 mM dextrose, 42 mM HEPES, and pH 7.2). The precipitates were allowed to form for 30 min at room temperature, protected from light, with vortex mixing every 5 min, to ensure that the precipitates had similar small sizes. Meanwhile, cultured hippocampal neurons were incubated with cultured-conditioned medium with 2 mM kynurenic acid (Sigma). The precipitates were added drop-wise to each well and incubated for 2 h at 37 °C, in an incubator with 95 % air/5 % CO2. The cells were then washed with acidic 10 % CO2 equilibrated culture medium containing 2 mM kynurenic acid and returned to the 95 % air/5 % CO2 incubator for 20 min at 37 °C. Finally, the medium was replaced with the initial culture-conditioned medium, and the cells were further incubated in a 95 % air/5 % CO2 incubator for 48 h at 37 °C to allow protein expression. Where indicated the cells were then subjected to OGD for 90 min, and 8 h after the insult they were fixed to proceed with the cell death assay.
Production of Adeno-Associated Viral Vectors Serotype 1 (AAV1) and Transduction of Hippocampal Neurons
AAV1 vectors were produced by polyethylenimine (PEI)-based triple transfection of HEK293 cell cultures using a protocol adapted from Konermann et al. [30]. Briefly, HEK293 cells were grown in complete medium containing Dulbecco’s Modified Eagle Medium (DMEM)/Nutrient Mixture F-12 medium with GlutaMax (Life technologies), 10 % heat-inactivated HyClone FBS (Thermo Scientific), and 1 % 1 M HEPES (Life Technologies) at 37 °C in a humidified incubator with 5 % CO2/95 % air. Twenty-four hours before transfection, 10 × 106 HEK293 cells were plated onto a 15-cm dish. The cells were transfected with pDF6 helper plasmid, pAAV1 serotype packaging vector, and pAAV vector carrying the gene of interest, in a ratio of 2:1.6:1, mixed with 434 μl of serum-free DMEM and 130 μl of PEI “Max” (MW 40,000, Polysciences Europe) (1 mg/ml). The DNA/DMEM/PEI mix was vortexed for 10 s, incubated at room temperature for 15 min, added to 22 ml of complete medium, and used to replace the medium on the 15-cm dish of HEK293 cells. At 48 h posttransfection, the viral supernatant was collected and filtered through a 0.45-μm PVDF filter (Fisherbrand) and stored at −80 °C. Two recombinant AAV1 vectors were generated, one encoding for eGFP the other encoding for Calpastatin.
AAV1 vectors (500 μl/well) were added directly to primary cultures of hippocampal neurons, maintained in 6-well multiwell plates, on DIV 9. The cells were further incubated in culture medium lacking virus for 5 days before preparation of the extracts.
Immunocytochemistry
Cells were fixed in 4 % sucrose/paraformaldehyde and permeabilized with 0.3 % Triton X-100 in PBS. The neurons were then incubated with PBS supplemented with 10 % BSA for 1 h at room temperature, to block nonspecific staining, and incubated with primary antibodies diluted in PBS supplemented with 3 % BSA, overnight at 4 °C. The cells were then washed six times with PBS and incubated with the appropriate secondary antibodies, for 1 h at 37 °C. The coverslips were mounted in a fluorescent mounting medium (DAKO, Denmark), and imaging was performed on a Zeiss LSM510 confocal microscope, using a 63× oil objective. For each set of experiments, the images were acquired using identical exposure settings. The regions of interest used in the quantification were blindly chosen using the MAP2 channel. The quantification was performed using the ImageJ software (NIH). After determination of the threshold and subtraction of the background, the results were normalized for the length of the region of interest. At least 12 cells per condition were analyzed for each preparation.
The primary antibodies used were anti-VGAT (1:500, Ab: 75-87aa; Synaptic Systems, Germany), anti-MAP2 (1:10,000, Abcam), anti-gephyrin (1:500, Synaptic Systems), and anti-GFP (1:500, MBL). The secondary antibodies used were anti-Chicken IgG conjugated with AMCA Fluor 350 (1:200, Invitrogen), anti-mouse IgG conjugated with Alexa Flour 568 (1:500, Invitrogen), anti-rabbit IgG conjugated with Alexa Flour 568 (1:500, Invitrogen), and anti-guinea pig IgG conjugated with Alexa 647 (Invitrogen).
For surface labeling of GABAAR γ2 subunits, before fixation, cells were incubated with primary antibodies (anti-GABAAR γ2, 1:500, Merck-Millipore), diluted in conditioned Neurobasal medium, washed twice and then fixed.
Cell Death Assay
Hippocampal neurons were cultured for 14 days on poly-d-lysine-coated glass coverslips as previously described [5]. After the experiments cells were fixed in 4 % sucrose/4 % paraformaldehyde (in PBS), washed twice with PBS and were then incubated with Hoechst 33342 (0.5 μg/ml) to stain nuclei. Analysis of the nuclear morphology was performed on a Zeiss Axiovert 200 florescence microscope, under a 40× objective.
Statistical Analysis
The immunoreactivity obtained in each experimental condition was calculated as a percentage of the control. Data are presented as mean ± SEM of at least three different experiments, performed in independent preparations. Statistical analysis of the results was performed using one-way ANOVA analysis followed by the Dunnet or Bonferroni multiple comparison test, as indicated in the figure caption.
Results
Gephyrin is Cleaved After Excitotoxic Stimulation with Glutamate
To investigate the effect of excitotoxic stimulation on gephyrin protein levels, cultured hippocampal and striatal neurons (7 DIV) were stimulated with 125 μM glutamate for 20 min and were further incubated in culture conditioned medium for different periods of time. Stimulation of cultured hippocampal neurons under these conditions causes 40–50 % of cell death (Almeida et al., 2005), and Western blot experiments, using an antibody against the C-terminal region of gephyrin (G-domain), showed a time-dependent cleavage of the protein, with a t1/2 of 6 h, giving rise to a product of about 47 kDa (Geph.T) (Fig. 1a). The time-dependent downregulation of the full-length gephyrin (by 30 % at 1 h) was associated with an upregulation of the 47 kDa immunoreactive band, suggesting that this is a cleavage product of gephyrin. Accordingly, the total amount of gephyrin (full length + truncated gephyrin) did not change at the different time points analyzed after the excitotoxic insult (Fig. 1b).
Excitotoxic injury leads to the cleavage of gephyrin in cultured hippocampal and striatal neurons. Hippocampal (a, b) (7 DIV) or striatal (c) (7 DIV) neurons were subjected to excitotoxic stimulation with glutamate (125 μM for 20 min), and the extracts were prepared after incubation in culture conditioned medium for the indicated periods of time. In the experiments shown in d hippocampal neurons (14 DIV) were transfected or not with EGFP-tagged full length gephyrin (GFP-gephyrin FL) and were stimulated with 50 μM glutamate for 20 min. In this case, extracts were prepared after incubation of the cells for 1 h in culture-conditioned medium. Samples were analysed by western blot with an antibody against the G-domain of gephyrin (or with an anti-GFP antibody as indicated in panel (d)), and the results are expressed as a percentage of total gephyrin (FL and truncated gephyrin) (a, c) or as total gephyrin protein levels (b; full length + truncated gephyrin). e Cultured hippocampal neurons (14 DIV) were incubated under conditions that allow the specific activation of synaptic or extrasynaptic NMDA receptors, as described in material and methods. Gephyrin protein levels were determined by western blot, and the results are expressed as a percentage of the total amount of gephyrin. The results are the average ± SEM of five to seven independent experiments performed in distinct preparations. The images shown in panel (d) are representative of two independent experiments. Statistical analysis was performed using one-way ANOVA, followed by the Dunnet’s multiple comparison test. N.S. non significant;*p < 0.05,**p < 0.01, ***p < 0.001 when compared with the control
Since the striatum is enriched in GABAergic neurons vulnerable to excitotoxic injury, we tested the effect of glutamate stimulation on gephyrin protein levels in cultured rat striatal neurons. The cells were stimulated with 125 μM glutamate for 20 min and further incubated during 1.5 or 8 h in culture conditioned medium. The results also showed an increase in the percentage of cleaved gephyrin by about 20 % when determined 8 h after injury (Fig. 1c).
To further characterize the effect of excitotoxic stimulation on the cleavage of gephyrin, cultured hippocampal neurons were transfected with GFP-gephyrin, and the cleavage of the protein was analysed by western blot with an antibody against GFP. Stimulation with glutamate increased the protein levels of a gephyrin cleavage product containing the N-terminal region of the protein fused with GFP (Fig. 1d, left panel, lane 4). Under the same conditions, there was also an upregulation of the cleavage product containing the C-terminal region of the protein, detected by the anti-gephyrin antibody (Fig. 1d, right panel, lane 4).
It has been hypothesized that the extrasynaptic pool of NMDA receptors is preferentially coupled to the activation of the excitotoxic machinery [27, 31]. To better understand the role NMDA receptors (NMDAR) in glutamate-evoked cleavage of gephyrin, we stimulated cultured hippocampal neurons under conditions that allow activating synaptic or extrasynaptic NMDAR [27, 26]. Gephyrin protein levels were analyzed by Western blot at 8 h after stimulation of NMDA receptors. The results show that gephyrin cleavage was specifically induced by stimulation of the extrasynaptic pool of NMDAR (Fig. 1e).
To determine whether gephyrin cleavage also occurs under excitotoxic conditions in vivo, adult mice were subjected to middle cerebral artery occlusion (MCAO), a model of transient focal ischemia [28]. Adult mice were subjected to a 45 min occlusion of the left middle cerebral artery, and extracts were prepared from the ipsilateral and contralateral brain hemispheres (cerebral cortex, hippocampus, and striatum) 48 h after injury. Gephyrin cleavage was observed in the striatum (peri-infarct area) and cerebral cortex (infarct core), which are the brain areas affected in this model, as assessed by Western blot using the antibody against gephyrin C-terminal region (Fig. 2).
Cleavage of gephyrin in the core and penumbra regions after transient focal ischemia. Adult C56BL/6 mice were subjected to transient 45 min MCAO/sham and gephyrin, and synaptophysin protein levels were determined in the infarct core, penumbra, and contralateral cortex 48 h after the lesion, by Western blot. The ratio between gephyrin protein levels and the loading control (synaptophysin) was calculated, and the results obtained in the contralateral hemisphere of sham operated mice were set to 100 %. The results are the average ± SEM of 3–4 independent experiments performed in different animals. Statistical analysis was performed using one-way ANOVA followed by Bonferroni’s multiple comparison test; ***p < 0.001, when compared for the indicated comparisons
Calpain Cleaves Gephyrin Under In Vitro and In Vivo Excitotoxic Conditions
Calpains play a key role in neuronal death following excitotoxic injury and in brain ischemia [21], and the gephyrin molecule contains several putative calpain cleavage sites as predicted by the GPS-CCD program (calpain cleavage detector based on the algorithm for group-based prediction system, available at http://ccd.biocuckoo.org/) [32]. Accordingly, in vitro experiments showed that gephyrin is a substrate of calpains in the brain [33, 19]. Therefore, we investigated whether these proteases are involved in gephyrin cleavage following an excitotoxic insult with glutamate. Incubation of cultured hippocampal neurons (7 DIV) with the chemical calpain inhibitors ALLN or MDL28170 prevented glutamate-evoked gephyrin cleavage, when analyzed 8 h after the insult (Fig. 3a).
Calpains mediate the cleavage of gephyrin in hippocampal neurons subjected to excitotoxic conditions and after OGD. a Cultured hippocampal neurons were preincubated (2 h) with the calpain inhibitors MDL 28170 (100 μM) or ALLN (50 μM), before and during glutamate stimulation (Glu; 125 μM, 20 min). The cells were further incubated in culture-conditioned medium for 1.5 h with calpain inhibitors and extracts were analyzed by western blot with an anti-gephyrin antibody. b Cultured hippocampal neurons were subjected to OGD (90 min) in the presence or in the absence of the calpain inhibitors MDL 28170 (100 μM) or ALLN (50 μM). c Cultured hippocampal neurons infected or not with AVV type 1 virus expressing calpastatin or GFP were subjected to OGD (90 min) and western blot analysis was performed 8 h after the insult. The cleavage of gephyrin was quantified, and neuronal infection was confirmed using specific antibodies against GFP and calpastatin. The results are expressed as a percentage of total gephyrin protein levels (FL and truncated gephyrin) and are the average ± SEM of 4 to 7 independent experiments performed in different preparations. Statistical analysis was performed using one-way ANOVA followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01,***p < 0.001
To determine whether calpains also play a role in gephyrin cleavage in an in vitro model of brain ischemia, we tested the effect of MDL28170 in hippocampal neurons subjected to a transient incubation in the absence of oxygen and glucose (OGD) (Hertz, 2008). Hippocampal neurons were subjected to OGD for 90 min, which induces the death of about 40 % of the neurons when determined 7–12 h after the insult [5]. Incubation of hippocampal neurons with MDL28170 prevented gephyrin cleavage induced by OGD (Fig. 3b). Furthermore, the effects of OGD on the cleavage of gephyrin were abrogated in hippocampal neurons transduced with calpastatin, the endogenous calpain inhibitor, while no effect was observed in cells transduced with GFP (Fig. 3c). Taken together, the results indicate that gephyrin is cleaved by calpains after excitotoxic injury and following an in vitro ischemic insult.
In additional experiments, we investigated the role of calpains in gephyrin cleavage under excitotoxic conditions in vivo, by injecting kainate in the hippocampus of wild type mice and in mice overexpressing calpastatin [23, 34, 35]. Gephyrin protein levels were determined at 4 and 8 h after kainate injection in the CA1 region of the hippocampus. In agreement with the results obtained in cultured hippocampal neurons subjected to excitotoxic conditions, kainate injection induced gephyrin cleavage in the ipsilateral hemisphere (as compared to the contralateral hemisphere) at 4 and 8 h after the insult in the wild type mice. The effect of kainate was significantly decreased in transgenic mice overexpressing calpastatin, by about 71 or 50 % when determined 4 and 8 h after the excitotoxic lesion, respectively (Fig. 4a, b). Under the latter conditions, there was also a significant reduction in cell death induced by kainate injection in the hippocampus [23].
Calpains mediate the cleavage of gephyrin in the hippocampus under excitotoxic conditions in vivo. Adult wild type mice (Wt) (C56BL6) or calpastatin-transgenic mice (hCast) were injected with kainate (1 nmol in 0.3 μl phosphate buffer) in the right hippocampus and 4 h (a) or 8 h (b) later the ipsilateral, and the contralateral hippocampi were collected from the coronal sliced brain. Hippocampal extracts were analysed by western blot using an antibody against gephyrin, and gephyrin cleavage was expressed as a percentage of total gephyrin (FL and truncated). The results are the average ± SEM of 3–5 independent experiments performed in different preparations. Statistical analysis was performed using one-way ANOVA followed by Bonferroni’s multiple comparison test.*p < 0.05,**p < 0.01, ***p < 0.001
Gephyrin Cleavage Decreases GABAA Receptor Clustering at the Synapse
Cleavage of gephyrin under excitotoxic conditions gives rise to a cleavage product with an apparent molecular weight of 47 kDa which interacts with an antibody raised against the gephyrin N-terminus (Figs. 1 and 2). The complementary cleavage product was also detected following excitotoxic stimulation of cultured hippocampal neurons transfected with GFP-gephyrin (Fig. 1d). This indicates that the calpain cleavage site is located at the end of the gephyrin linker region or at N-terminus of the E-domain [29]. Accordingly, this region of the gephyrin molecule contains several putative calpain cleavage sites as predicted by the GPS-CCD program (e.g., residues 401 and 412). To investigate the impact of gephyrin cleavage on GABAergic synapses, we analyzed the clustering of the endogenous protein along dendrites in hippocampal neurons transfected with an EGFP-tagged truncated form of the protein, containing the G-domain and the linker region (gephyrin-GC). Under these conditions, the alteration in the distribution of the endogenous full-length protein can be specifically analyzed with an antibody against the gephyrin E-domain. In control experiments, hippocampal neurons were transfected with GFP. The results of Fig. 5a–c show that transfection of hippocampal neurons with truncated gephyrin significantly reduced the area and intensity of the clusters formed by the endogenous protein by 53.4 and 60.0 %, respectively, when compared with the control. These results indicate that the cleavage of gephyrin decreases the clustering of the protein along dendrites. In contrast, transfection with full-length gephyrin increased the area and intensity of the puncta containing endogenous and EGFP-tagged protein (Fig. 5a–c). Previous studies showed that synaptic targeting and clustering of recombinant gephyrin are not affected by the presence of EGFP [29, 20].
Expression of a truncated form of gephyrin downregulates gephyrin clustering. Cultured hippocampal neurons (low density) were transfected with GFP, EGFP-tagged full length gephyrin (EGFP-geph.FL) or with a truncated form of gephrin containing the G-domain and the linker region (EGFP-geph.T). Gephyrin expression was evaluated by immunocytochemistry with an antibody against the C-terminus of the protein. The area (a) and intensity (b) of gephyrin puncta were analysed in the dendritic compartment and normalized for its length. The number (d) and area (e) of gephyrin puncta colocalizing with VGAT, as well as the % of colocalization (f) were calculated. Results are means ± SEM of at least three independent experiments (30 cells), performed in different preparations. Representative images are shown in panel (c). Statistical analysis was performed by one-way ANOVA, followed by Dunnett’s test. *p < 0.05; **p < 0.01; ***p < 0.001—significantly different when compared to control conditions
To further investigate the effect of gephyrin cleavage on GABAergic synapses, we compared the colocalization of the endogenous protein with the vesicular GABA transporter (VGAT), a presynaptic marker, in hippocampal neurons transfected with EGFP-tagged gephyrin-GC or with GFP (control). Expression of truncated gephyrin decreased the number of puncta containing endogenous gephyrin and VGAT to 51.8 % of the control (Fig. 5d). Furthermore, under the same conditions, there was a reduction in the area of the puncta and the percentage of dendritic gephyrin colocalizing with VGAT to 47.5 and 56.0 %, respectively (Fig. 5e, f). Taken together, these results indicate that gephyrin cleavage contributes to a disassembly of the GABAergic synapses. In contrast, transfection of hippocampal neurons with EGFP-tagged gephyrin increased significantly the number of gephyrin puncta that colocalized with VGAT (by 84.6 %), the area of the puncta containing the pre- and postsynaptic GABAergic markers (by 97.8 %), and the percentage of gephyrin that colocalized with VGAT along dendrites (by 104.3 %) (Fig. 5d–f).
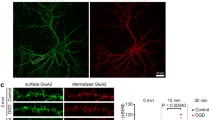
Given the role of gephyrin in anchoring GABAAR at the synapse, the cleavage of the protein under excitotoxic conditions is likely to affect the surface expression of the receptors. To address this question, we compared the surface distribution of GABAAR γ2 subunits in hippocampal neurons transfected with EGFP tagged gephyrin-GC or with GFP. GABAAR γ2 subunits are expressed in a large percentage of synaptic GABAAR [36]. In agreement with the effect on gephyrin clustering, transfection of gephyrin-GC decreased the number of GABAAR γ2 puncta, as well as the puncta area and intensity, to 60.6, 55.5, and 46.7 %, respectively (Fig. 6a–d). In contrast, transfection of hippocampal neurons with EGFP-tagged full-length gephyrin upregulated the total number of puncta containing the GABAAR γ2 subunit (by 47.4 %) and the intensity of the puncta (by 31.6 %), but no effect was observed in the puncta area (Fig. 6a–c and g). Together, these results show that dissociation of the gephyrin clusters induced by the truncated protein is correlated with a disassembly of surface GABAAR clusters. Colocalization experiments, characterizing the distribution of surface GABAAR γ2 subunits and the presynaptic marker VGAT, were performed to further evaluate the effect of gephyrin-GC on the surface distribution of plasma membrane-associated GABAAR. Transfection of hippocampal neurons with truncated gephyrin decreased the number and area of GABAAR γ2 puncta that colocalized with VGAT to 61.1 and 67.0 %, respectively (Fig. 6e, g). A significant reduction in the percentage of total GABAAR subunits that colocalized with the presynaptic marker was also observed (to 53.0 %) (Fig. 6g). In contrast, transfection with the EGFP-tagged full-length gephyrin increased the number GABAAR γ2 puncta that colocalized with VGAT (by 31.9 %) (Fig. 6e), while no effect was observed in the puncta area and on the percentage of GABAAR γ2 subunit colocalization with VGAT (Fig. 6f–g). These results show an effect of cleaved gephyrin on the surface expression of GABAAR which is likely to affect GABAergic synaptic transmission.
Expression of a truncated form of gephyrin downregulates the surface expression of GABAAR. a–g Cultured hippocampal neurons (low density) were transfected with GFP, EGFP-tagged full-length gephyrin (EGFP-geph.FL) or with a truncated form of gephyrin containing the G-domain and the linker region (EGFP-geph.T). Surface expression of the GABAAR γ2 subunits was evaluated by immunocytochemistry with an antibody against the GABAAR γ2 subunits (N-terminus) under non-permeabilizing conditions. The number of GABAAR γ2 subunit particles (a), puncta area (b), and intensity (c) were analysed in the dendritic compartment and normalized for its length. The number (e) and area of GABAAR γ2 puncta (f) colocalizing with VGAT, as well as the % of colocalization (g), were calculated. Results are means ± SEM of at least three independent experiments (30 cells), performed in different preparations. Representative images are shown in panel (d). Statistical analysis was performed by one-way ANOVA, followed by Dunnett’s test. **p < 0.01; ***p < 0.001—significantly different when compared to control conditions
Gephyrin Cleavage Increases OGD-Induced Neuronal Death
The activity of GABAergic synapses is an important modulator of neuronal demise in brain ischemia [5]. In particular, the GABAAR γ2 is present in the majority of postsynaptic GABAARs which mediate phasic inhibition [36]. Therefore, we hypothesized that the cleavage of gephyrin in brain ischemia (Figs. 2 and 3b and c) could contribute to neuronal death by downregulating the inhibitory synaptic transmission. To address this question, we determined the impact of transfection with EGFP-geph-GC on the survival of hippocampal neurons exposed to OGD. Under control conditions, hippocampal neurons were transfected with GFP or with the full-length form of gephyrin (EGFP-geph). Hippocampal neurons transfected with GFP showed an increase in cell death to 51.5 % after 90 min of OGD, when assessed 8 h after the insult. When similar experiments were performed in hippocampal neurons transfected with EGFP-geph-GC, the same insult induced a more significant increase in cell death to 82.5 % (Fig. 7a, b). As expected, trasfection with full length gephyrin (EGFP-gephyrin) slightly reduced neuronal injury induced by OGD although the effect was not statistically significant. As expected, non-transfected cells showed similar rates of cell death, showing the specificity of the effects resulting from the transfection with the full-length and truncated forms of gephyrin (Fig. 7a, c).
Expression of a truncated form of gephyrin increases OGD-induced neuronal death. a–b Cultured hippocampal neurons were transfected with GFP, EGFP-tagged full length gephyrin (EGFP-geph.FL) or with a truncated form of gephyrin containing the G-domain and the linker region (EGFP-geph.T), and subjected to OGD for 90 min before incubation in culture conditioned medium for 12 h. Where indicated (sham), the cells were treated under control conditions. The transfected cells were identified by immunocytochemistry with an anti-GFP antibody, and the viability of transfected (b) and non-transfected (c) cells was evaluated by fluorescence microscopy with Hoechst 33342. Representative images are shown in panel (a). The arrows point to the nuclei of hippocampal neurons transfected with GFP, the EGFP-tagged full length gephyrin (EGFP-geph.FL) or with the truncated form of gephyrin (EGFP-geph.T). For each experimental condition, two coverslips were analyzed and at least 40 cells were counted per coverslip. Results are means ± SEM of at least three independent experiments, performed in different preparations. Statistical analysis was performed by one-way ANOVA, followed by Bonferroni test. *p < 0.05, **p < 0.01, ***p < 0.001—significantly different when compared to Sham condition or for the indicated comparison. N.S. not significant
Taken together, the results indicate that the dominant negative effect of truncated gephyrin destabilizes GABAergic synapses and the resulting impairment of the inhibitory signaling renders the cells more vulnerable to the toxic injury resulting from OGD.
Discussion
In this work, we show a role for calpains in the cleavage of gephyrin under excitotoxic conditions, both in cultured hippocampal neurons and in vivo, giving rise to a stable cleavage product. The results obtained in hippocampal neurons transfected with a peptide corresponding to the gephyrin G-domain and linker region suggest that the gephyrin cleavage products act as dominant negatives to disassemble the gephyrin lattice responsible for anchoring GABAAR at the synapse. Furthermore, the resulting downregulation of GABAergic synapses was found to increase neuronal death in hippocampal neurons subjected to OGD. In a previous study, we showed that OGD decreases the interaction of the surface GABAAR α1 subunit with gephyrin by a calcineurin-dependent mechanism [5]. Under the same conditions, the PP1/PP2A-mediated dephosphorylation of GABAAR β3 subunits was reported to favor the internalization of the receptors [5]. Together, the effects of calpain and calcineurin decrease the anchoring of GABAAR at the synapse, and the increased receptor mobility may allow them to reach the sites where they are internalized, thereby decreasing GABAAR surface expression.
A role for calpains in the cleavage of gephyrin under excitotoxic conditions in vivo and in vitro, as well as in in vitro brain ischemia, is suggested by the following pharmacological and non-pharmacological evidence: (i) the glutamate-evoked cleavage of gephyrin was blocked by the calpain inhibitors ALLN and MDL28170; (ii) the cleavage of gephyrin in hippocampal neurons subjected to OGD was inhibited by MDL28170 as well as in the presence of increased protein levels of calpastatin, an endogenous calpain inhibitor; (iii) the cleavage of gephyrin induced by kainate injection in the hippocampal CA1 region was inhibited in transgenic mice overexpressing calpastatin; (iv) the gephyrin molecule contains several putative calpain cleavage sites as predicted by the GPS-CCD program. In agreement with this hypothesis, incubation of hippocampal extracts with calpain-1 was also shown to induce gephyrin cleavage, giving rise to a stable cleavage product of about 50 kDa [19, 33]. Similarly, gephyrin cleavage under excitotoxic conditions and in brain ischemia gives rise to a stable (>8 h) cleavage product with ~47 kDa.
The postsynaptic multimeric, hexagonal gephyrin aggregates are thought to arise from the dimerization of gephyrin E-domain and trimerization of the G-domain [11, 37–39]. The results of the Western blot experiments suggest that gephyrin cleavage under excitotoxic and ischemic conditions occurs near the C-terminus of the linker region or in the first amino acids of the gephyrin E-domain. To investigate the impact of gephyrin cleavage on GABAergic synapses, we transfected differentiated hippocampal neurons with a truncated form of gephyrin containing the G-domain and the linker region, and analyzed the changes in GABAergic synapses. The results showed a decrease in the area and intensity of the puncta formed by the endogenous protein. A decrease in the number of synaptic puncta and their area was also observed, showing a dominant-negative type of effect by the truncated proteins to disassemble the postsynaptic clusters of endogenous gephyrin. The same effect was previously reported in developing hippocampal neurons [29] and suggests a constant turnover of gephyrin in postsynaptic clusters present in mature GABAergic synapses. A dynamic behavior of the postsynaptic gephyrin scaffold was previously suggested using different experimental approaches [40–42].
The GABAAR γ2 subunit plays a key role in synaptic clustering of the receptors [43], being expressed in a large fraction of postsynaptic GABAARs that mediate phasic inhibition [36]. Although gephyrin does not interact directly with GABAAR γ2 subunits, it is thought that the scaffold protein might stabilize the γ2-containing receptor clusters at the synapse [12]. In agreement with this model, we observed a decrease in synaptic clustering of GABAAR γ2 subunits in hippocampal neurons transfected with the truncated form of gephyrin containing the G-domain and the linker region, which also decreased the clustering of the endogenous gephyrin at the synapse. Interestingly, expression of the gephyrin E-domain was also shown to decrease the clustering of the scaffold protein along dendrites [29]. Therefore, under excitotoxic condition and in brain ischemia, the gephyrin cleavage products containing N-terminus (G-domain and linker region) and the C-terminus (E-domain) are likely to act together as dominant negatives in the inhibition of gephyrin clustering. This effect, together with the decrease in total full length gephyrin protein levels, is expected to contribute to the loss of synaptic GABAAR containing γ2 subunits, with consequent downregulation of phasic inhibition. The modulation of the surface clustering of GABAAR by the truncated forms of gephyrin may depend on the subunit composition of the receptors since expression of truncated forms of gephyrin did not affect the clustering of receptors containing α2 subunits [29]. However, the latter studies were performed in hippocampal neurons cultured for 6 or 10 days before transfection, and this may suggest that early in development the clustering of GABAAR is not critically regulated by gephyrin.
S-nitrosylation of gephyrin under excitotoxic conditions and in brain ischemia may also regulate the clustering of the protein at GABAergic synapses [44]. Accordingly, upregulation of the neuronal isoform of nitric oxide synthase (nNOS) was found to decrease the size of postsynaptic gephyrin clusters. Therefore, the NMDA receptor mediated [Ca2+]i overload with a consequent activation of nNOS [45] may contribute to the disassembly of the synaptic gephyrin clusters through S-nitrosylation of the protein, with a consequent loss of synaptic clustering of GABAAR. A distinct mechanism that may also contribute to downregulate the synaptic clustering of GABAAR in OGD is suggested by the observed decrease in the interaction of gephyrin with surface GABAAR containing α1 subunits mediated by calcineurin [5]. Although the calcineurin target(s) was not investigated in detail, the effects were attributed to the dephosphorylation of GABAAR. These effects of calcineurin may be downstream of the activation of NMDA receptors since the influx of Ca2+ through these receptors was shown to increase the mobility and the rapid dispersal of GABAAR [46]. Alternatively, OGD may affect the activity of the protein kinase(s) involved in the phosphorylation of the amino acid residue targeted by calcineurin.
The diffusion of GABAAR away from the synapse following excitotoxic injury is not necessarily associated with an upregulation of tonic inhibition by extrasynaptic receptors. First, the synaptic population of GABAAR is characterized by a lower affinity for the neurotransmitter GABA when compared with the extrasynaptic receptors, due to their distinct molecular composition [36, 47] and, therefore, the neurotransmitter concentration in the extrasynaptic regions may not be high enough to allow the activation of synaptic receptors displaced to extrasynaptic sites. Furthermore, the latter population of receptors may be internalized, by a mechanism dependent on the dephosphorylation of β3 subunits, as shown for synaptic GABAAR in hippocampal neurons subjected to OGD [5, 9]. In contrast with the OGD-induced loss of synaptic clustering of GABAAR, which is accompanied by receptor internalization, the decrease in synaptic clustering of GABAAR following sustained network activity is not accompanied by an upregulation in receptor internalization [48]. Differences in the phosphorylation of GABAAR β3 subunits may account for the distinct fate of the receptors after leaving the synapse.
Transfection of hippocampal neurons with a truncated form of gephyrin, which disassembles the clusters of gephyrin and downregulates the expression of GABAAR at the synapse, significantly increased neuronal death induced by OGD. These results point to a key protective role of GABAergic activity under ischemic conditions, which depends on the stability of gephyrin clustering. The disassembly of the gephyrin lattice may allow the lateral diffusion of the receptors on the membrane, followed by their internalization. Expression of a phosphomimetic mutant of GABAAR β3 subunits, which is not internalized upon OGD, also reduced cell death in this in vitro model of brain ischemia [5]. Together, our results showing neuroprotective effects of GABAergic neurotransmission in OGD strongly suggest that strategies for stabilizing the gephyrin clusters at the synapse may constitute a good target for ischemia therapy. However, an effective strategy should also prevent the calpain-mediated cleavage of the vesicular GABA transporters, which affects their delivery to the synapse by removing a targeting sequence [23, 49]. Cleavage of glutamic acid decarboxylase, the enzyme that mediates the rate limiting step in the synthesis of GABA from glutamate, also decreases the synthesis of the neurotransmitter [50, 51].
In addition to the role played in the cleavage of gephyrin and in the disassembly of the protein clusters under ischemic conditions, calpains were also shown to regulate the gephyrin postsynaptic cluster density in cultured hippocampal neurons under resting conditions [19]. Transfection of hippocampal neurons with calpastatin, the endogenous calpain inhibitor, increased the density but not the size of gephyrin clusters, indicating that Ca2+-dependent proteolysis of gephyrin reduces its availability for postsynaptic clustering.
In conclusion, in this work, we showed a key role for calpains in the cleavage of gephyrin under excitotoxic conditions and in an in vitro model of brain ischemia, giving rise to a stable cleavage product. These truncated forms of gephyrin contribute to the disassembly of the postsynaptic gephyrin clusters, with a consequent loss of the neuroprotective activity mediated by synaptic GABAAR. Therefore, inhibition of gephyrin cleavage might be a therapeutic target to preserve synaptic inhibition in brain ischemia. Given the recent evidence showing a downregulation of gephyrin during epileptogenesis in the CA1 region of the hippocampus [52], which is associated with the accumulation of gephyrin protein fragments [53, 54], strategies aiming at preventing calpain-mediated gephyrin cleavage may also be effective in epilepsy.
References
Szydlowska K, Tymianski M (2010) Calcium, ischemia and excitotoxicity. Cell Calcium 47(2):122–129. doi:10.1016/j.ceca.2010.01.003
Lai TW, Zhang S, Wang YT (2014) Excitotoxicity, and stroke: identifying novel targets for neuroprotection. Prog Neurobiol 115:157–188. doi:10.1016/j.pneurobio.2013.11.006
Schwartz-Bloom RD, Sah R (2001) gamma-Aminobutyric acidA neurotransmission and cerebral ischemia. J Neurochem 77(2):353–371
Wu C, Sun D (2014) GABA receptors in brain development, function, and injury. Metab Brain Dis 30(2):367–379. doi:10.1007/s11011-014-9560-1
Mele M, Ribeiro L, Inacio AR, Wieloch T, Duarte CB (2014) GABA(A) receptor dephosphorylation followed by internalization is coupled to neuronal death in in vitro ischemia. Neurobiol Dis 65:220–232. doi:10.1016/j.nbd.2014.01.019
Bedet C, Bruusgaard JC, Vergo S, Groth-Pedersen L, Eimer S, Triller A, Vannier C (2006) Regulation of gephyrin assembly and glycine receptor synaptic stability. J Biol Chem 281(40):30046–30056. doi:10.1074/jbc.M602155200
Yu W, Jiang M, Miralles CP, Li RW, Chen G, de Blas AL (2007) Gephyrin clustering is required for the stability of GABAergic synapses. Mol Cell Neurosci 36(4):484–500. doi:10.1016/j.mcn.2007.08.008
Tyagarajan SK, Fritschy JM (2014) Gephyrin: a master regulator of neuronal function? Nat Rev Neurosci 15(3):141–156. doi:10.1038/nrn3670
Smith KR, Muir J, Rao Y, Browarski M, Gruenig MC, Sheehan DF, Haucke V, Kittler JT (2012) Stabilization of GABAA receptors at endocytic zones is mediated by an AP2 binding motif within the GABAA receptor beta3 subunit. J Neurosci 32(7):2485–2498. doi:10.1523/JNEUROSCI.1622-11.2011
Prior P, Schmitt B, Grenningloh G, Pribilla I, Multhaup G, Beyreuther K, Maulet Y, Werner P et al (1992) Primary structure and alternative splice variants of gephyrin, a putative glycine receptor-tubulin linker protein. Neuron 8(6):1161–1170
Saiyed T, Paarmann I, Schmitt B, Haeger S, Sola M, Schmalzing G, Weissenhorn W, Betz H (2007) Molecular basis of gephyrin clustering at inhibitory synapses: role of G- and E-domain interactions. J Biol Chem 282(8):5625–5632. doi:10.1074/jbc.M610290200
Jacob TC, Moss SJ, Jurd R (2008) GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci 9(5):331–343. doi:10.1038/nrn2370
Tretter V, Jacob TC, Mukherjee J, Fritschy JM, Pangalos MN, Moss SJ (2008) The clustering of GABAA receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J Neurosci 28(6):1356–1365. doi:10.1523/JNEUROSCI.5050-07.2008
Tretter V, Kerschner B, Milenkovic I, Ramsden SL, Ramerstorfer J, Saiepour L, Maric HM, Moss SJ et al (2011) Molecular basis of the gamma-aminobutyric acid A receptor alpha3 subunit interaction with the clustering protein gephyrin. J Biol Chem 286(43):37702–37711. doi:10.1074/jbc.M111.291336
Mukherjee J, Kretschmannova K, Gouzer G, Maric HM, Ramsden S, Tretter V, Harvey K, Davies PA et al (2011) The residence time of GABAARs at inhibitory synapses is determined by direct binding of the receptor alpha1 subunit to gephyrin. J Neurosci 31(41):14677–14687. doi:10.1523/JNEUROSCI.2001-11.2011
Kowalczyk S, Winkelmann A, Smolinsky B, Forstera B, Neundorf I, Schwarz G, Meier JC (2013) Direct binding of GABAA receptor beta2 and beta3 subunits to gephyrin. Eur J Neurosci 37(4):544–554. doi:10.1111/ejn.12078
Maric HM, Mukherjee J, Tretter V, Moss SJ, Schindelin H (2011) Gephyrin-mediated gamma-aminobutyric acid type A and glycine receptor clustering relies on a common binding site. J Biol Chem 286(49):42105–42114. doi:10.1074/jbc.M111.303412
Sassoe-Pognetto M, Panzanelli P, Sieghart W, Fritschy JM (2000) Colocalization of multiple GABAA receptor subtypes with gephyrin at postsynaptic sites. J Comp Neurol 420(4):481–498
Tyagarajan SK, Ghosh H, Yevenes GE, Nikonenko I, Ebeling C, Schwerdel C, Sidler C, Zeilhofer HU et al (2011) Regulation of GABAergic synapse formation and plasticity by GSK3beta-dependent phosphorylation of gephyrin. Proc Natl Acad Sci U S A 108(1):379–384. doi:10.1073/pnas.1011824108
Tyagarajan SK, Ghosh H, Yevenes GE, Imanishi SY, Zeilhofer HU, Gerrits B, Fritschy JM (2013) Extracellular signal-regulated kinase and glycogen synthase kinase 3beta regulate gephyrin postsynaptic aggregation and GABAergic synaptic function in a calpain-dependent mechanism. J Biol Chem 288(14):9634–9647. doi:10.1074/jbc.M112.442616
Bevers MB, Neumar RW (2008) Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab 28(4):655–673. doi:10.1038/sj.jcbfm.9600595
Vosler PS, Brennan CS, Chen J (2008) Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol 38(1):78–100. doi:10.1007/s12035-008-8036-x
Gomes JR, Lobo AC, Melo CV, Inacio AR, Takano J, Iwata N, Saido TC, de Almeida LP et al (2011) Cleavage of the vesicular GABA transporter under excitotoxic conditions is followed by accumulation of the truncated transporter in nonsynaptic sites. J Neurosci 31(12):4622–4635. doi:10.1523/JNEUROSCI.3541-10.2011
Baliova M, Knab A, Franekova V, Jursky F (2009) Modification of the cytosolic regions of GABA transporter GAT1 by calpain. Neurochem Int 55(5):288–294. doi:10.1016/j.neuint.2009.03.012
Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, Carvalho RF, Carvalho AP et al (2005) Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ 12(10):1329–1343. doi:10.1038/sj.cdd.4401662
Caldeira MV, Curcio M, Leal G, Salazar IL, Mele M, Santos AR, Melo CV, Pereira P et al (2013) Excitotoxic stimulation downregulates the ubiquitin-proteasome system through activation of NMDA receptors in cultured hippocampal neurons. Biochim Biophys Acta 1832(1):263–274. doi:10.1016/j.bbadis.2012.10.009
Hardingham GE, Fukunaga Y, Bading H (2002) Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5(5):405–414. doi:10.1038/nn835
Nygren J, Wieloch T (2005) Enriched environment enhances recovery of motor function after focal ischemia in mice, and downregulates the transcription factor NGFI-A. J Cereb Blood Flow Metab 25(12):1625–1633. doi:10.1038/sj.jcbfm.9600157
Lardi-Studler B, Smolinsky B, Petitjean CM, Koenig F, Sidler C, Meier JC, Fritschy JM, Schwarz G (2007) Vertebrate-specific sequences in the gephyrin E-domain regulate cytosolic aggregation and postsynaptic clustering. J Cell Sci 120(Pt 8):1371–1382. doi:10.1242/jcs.003905
Konermann S, Brigham MD, Trevino AE, Hsu PD, Heidenreich M, Cong L, Platt RJ, Scott DA et al (2013) Optical control of mammalian endogenous transcription and epigenetic states. Nature 500(7463):472–476. doi:10.1038/nature12466
Parsons MP, Raymond LA (2014) Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 82(2):279–293. doi:10.1016/j.neuron.2014.03.030
Liu Z, Cao J, Gao X, Ma Q, Ren J, Xue Y (2011) GPS-CCD: a novel computational program for the prediction of calpain cleavage sites. PLoS One 6(4), e19001. doi:10.1371/journal.pone.0019001
Kawasaki BT, Hoffman KB, Yamamoto RS, Bahr BA (1997) Variants of the receptor/channel clustering molecule gephyrin in brain: distinct distribution patterns, developmental profiles, and proteolytic cleavage by calpain. J Neurosci Res 49(3):381–388
Takano J, Tomioka M, Tsubuki S, Higuchi M, Iwata N, Itohara S, Maki M, Saido TC (2005) Calpain mediates excitotoxic DNA fragmentation via mitochondrial pathways in adult brains: evidence from calpastatin mutant mice. J Biol Chem 280(16):16175–16184. doi:10.1074/jbc.M414552200
Higuchi M, Tomioka M, Takano J, Shirotani K, Iwata N, Masumoto H, Maki M, Itohara S et al (2005) Distinct mechanistic roles of calpain and caspase activation in neurodegeneration as revealed in mice overexpressing their specific inhibitors. J Biol Chem 280(15):15229–15237. doi:10.1074/jbc.M500939200
Farrant M, Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci 6(3):215–229. doi:10.1038/nrn1625
Sola M, Kneussel M, Heck IS, Betz H, Weissenhorn W (2001) X-ray crystal structure of the trimeric N-terminal domain of gephyrin. J Biol Chem 276(27):25294–25301. doi:10.1074/jbc.M101923200
Sola M, Bavro VN, Timmins J, Franz T, Ricard-Blum S, Schoehn G, Ruigrok RW, Paarmann I et al (2004) Structural basis of dynamic glycine receptor clustering by gephyrin. EMBO J 23(13):2510–2519. doi:10.1038/sj.emboj.7600256
Kim EY, Schrader N, Smolinsky B, Bedet C, Vannier C, Schwarz G, Schindelin H (2006) Deciphering the structural framework of glycine receptor anchoring by gephyrin. EMBO J 25(6):1385–1395. doi:10.1038/sj.emboj.7601029
Hanus C, Ehrensperger MV, Triller A (2006) Activity-dependent movements of postsynaptic scaffolds at inhibitory synapses. J Neurosci 26(17):4586–4595. doi:10.1523/JNEUROSCI.5123-05.2006
Specht CG, Izeddin I, Rodriguez PC, El Beheiry M, Rostaing P, Darzacq X, Dahan M, Triller A (2013) Quantitative nanoscopy of inhibitory synapses: counting gephyrin molecules and receptor binding sites. Neuron 79(2):308–321. doi:10.1016/j.neuron.2013.05.013
Dobie FA, Craig AM (2011) Inhibitory synapse dynamics: coordinated presynaptic and postsynaptic mobility and the major contribution of recycled vesicles to new synapse formation. J Neurosci 31(29):10481–10493. doi:10.1523/JNEUROSCI.6023-10.2011
Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B (2005) Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci 25(3):594–603. doi:10.1523/JNEUROSCI.4011-04.2005
Dejanovic B, Schwarz G (2014) Neuronal nitric oxide synthase-dependent S-nitrosylation of gephyrin regulates gephyrin clustering at GABAergic synapses. J Neurosci 34(23):7763–7768. doi:10.1523/JNEUROSCI.0531-14.2014
Samdani AF, Dawson TM, Dawson VL (1997) Nitric oxide synthase in models of focal ischemia. Stroke 28(6):1283–1288
Muir J, Arancibia-Carcamo IL, MacAskill AF, Smith KR, Griffin LD, Kittler JT (2010) NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the gamma2 subunit. Proc Natl Acad Sci U S A 107(38):16679–16684. doi:10.1073/pnas.1000589107
Mtchedlishvili Z, Kapur J (2006) High-affinity, slowly desensitizing GABAA receptors mediate tonic inhibition in hippocampal dentate granule cells. Mol Pharmacol 69(2):564–575. doi:10.1124/mol.105.016683
Bannai H, Levi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, Mikoshiba K et al (2009) Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron 62(5):670–682. doi:10.1016/j.neuron.2009.04.023
Santos MS, Park CK, Foss SM, Li H, Voglmaier SM (2013) Sorting of the vesicular GABA transporter to functional vesicle pools by an atypical dileucine-like motif. J Neurosci 33(26):10634–10646. doi:10.1523/JNEUROSCI.0329-13.2013
Baptista MS, Melo CV, Armelao M, Herrmann D, Pimentel DO, Leal G, Caldeira MV, Bahr BA et al (2010) Role of the proteasome in excitotoxicity-induced cleavage of glutamic acid decarboxylase in cultured hippocampal neurons. PLoS One 5(4), e10139. doi:10.1371/journal.pone.0010139
Wei J, Wu JY (2008) Post-translational regulation of L-glutamic acid decarboxylase in the brain. Neurochem Res 33(8):1459–1465. doi:10.1007/s11064-008-9600-5
Gonzalez MI, Cruz Del Angel Y, Brooks-Kayal A (2013) Down-regulation of gephyrin and GABAA receptor subunits during epileptogenesis in the CA1 region of hippocampus. Epilepsia 54(4):616–624. doi:10.1111/epi.12063
Forstera B, Belaidi AA, Juttner R, Bernert C, Tsokos M, Lehmann TN, Horn P, Dehnicke C et al (2010) Irregular RNA splicing curtails postsynaptic gephyrin in the cornu ammonis of patients with epilepsy. Brain 133(Pt 12):3778–3794. doi:10.1093/brain/awq298
Fang M, Shen L, Yin H, Pan YM, Wang L, Chen D, Xi ZQ, Xiao Z et al (2011) Downregulation of gephyrin in temporal lobe epilepsy neurons in humans and a rat model. Synapse 65(10):1006–1014. doi:10.1002/syn.20928
Acknowledgments
This work was supported by FCT, FEDER, and COMPETE (PEst-C/SAU/LA0001/2013-2014; PTDC/NEU-NMC/0198/2012). We thank Professor Günter Schwarz (Institute of Biochemistry, University of Cologne, Germany) for the kind gift of the EGFP-geph.FL and EGFP-geph.T plasmids. We are also thankful to Elisabete Carvalho for the assistance in the preparation of cultured hippocampal neurons.
Compliance with Ethical Standards
ᅟ
Conflict of Interest
The authors declare that they have no conflict of interest.
Welfare of Animals
Experiments were performed according to the European Union Directive 86/609/EEC and the legislation Portaria n. 1005/92, issued by the Portuguese Government for the protection of animals used for experimental and other scientific purposes. The procedures were approved by the CNC-FMUC Ethical Committee for Animal Research (ORBEA 19–201325022013) and by DGAV (Reference: 421/2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
João T. Costa and Miranda Mele contributed equally to this work.
Rights and permissions
About this article
Cite this article
Costa, J.T., Mele, M., Baptista, M.S. et al. Gephyrin Cleavage in In Vitro Brain Ischemia Decreases GABAA Receptor Clustering and Contributes to Neuronal Death. Mol Neurobiol 53, 3513–3527 (2016). https://doi.org/10.1007/s12035-015-9283-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9283-2