Abstract
The brain undergoes many structural and functional changes during aging. Some of these changes are regulated by estrogens which act mainly through their intracellular receptors, estrogen receptor ERα and ERβ. The expression of these receptors is regulated by several factors including their own ligand estrogen, and others such as growth hormone and thyroid hormone. The levels of these factors decrease during aging which in turn influence estrogen signaling leading to alterations in brain functions. In the present paper, we review the effects of aging on brain structure and function, and estrogen action and signaling during brain aging. The findings suggest key role of estrogen in the maintenance of brain functions during aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The brain undergoes many biochemical and structural changes involving both functional reorganization and compensation during aging. Majority of these changes are regulated by estrogen which is derived from either circulation or steroidogenesis in the brain [1–4]. Estrogen acts through nongenomic as well as genomic pathways. Whereas the nongenomic pathway is not well understood, the genomic pathway is mediated by intracellular receptors, estrogen receptor ERα and ERβ. The level of ERα and ERβ is influenced by a number of factors including age, cell density, growth hormone, thyroid hormone and gonadal steroids [5]. Consequently, the estrogen-mediated functions change in the brain leading to different diseases. Experimental studies using animal models and cell culture suggest that these diseases can be delayed or prevented if estrogen action is maintained. This article focuses on recent research findings in four areas—aging of brain, diverse actions of estrogen in the brain, effect of aging on estrogen signaling in brain and effect of estrogen during aging of brain.
Brain aging
Structural changes
The most striking feature of aging brain is its shrinkage, weight loss and expansion of the ventricular volume. However, the age-related shrinkage of brain shows regional specificity [6]. The major factor responsible for age-dependent brain shrinkage is loss of white matter, which occurs due to damage of myelinated fibers and is closely correlated with the age-associated cognitive decline [7]. Other age-associated changes in the brain include increase in the number of microglia and astrocytes [8], reduction in dendritic arbors and dendritic spines of cortical pyramidal neurons [9–11]. Changes in dendrites include both shortening and fewer dendritic branches. Hippocampal circuits are also vulnerable to degeneration during normal aging and Alzheimer’s disease (AD), though such effects show species specificity. Analysis of synapses in old rats shows age-dependent reduction, while similar analyses in humans and monkeys indicate no loss of synapses in the hippocampus [12, 13].
Another inevitable consequence of brain aging includes neuronal death in neocortex and hippocampus, though the extent of neuronal loss during aging is much debated. The idea of significant neuronal loss during normal aging of human cortex evolved after examining the cortices of subjects between 18 and 95 years of age [14]. Data obtained from this and other similar type of studies suggested that most of the neocortical areas and hippocampal subfields lose 25–50% of their resident neurons in old age. However, this view has been modified after the development of relatively more accurate procedures for counting neurons [15, 16]. Careful applications of stereological techniques to several species including humans have led to the conclusion that the old brain shows no evidence of neuronal loss in the major areas of entorhinal cortex and CA1 region of hippocampus, which are involved in memory function. However, some age-related neuronal loss occurs in the hilus of dentate gyrus and subiculum [15].
Other structural changes occurring in the old brain include intracellular deposition of lipofuscin pigment (made up of peroxidized proteins and lipids), formation of neurofibrillary tangles, senile plaques, neuropil threads, granulovacuolar degeneration, hirano bodies and infarcts. However, except the lipofuscin pigment, all other deposits are considered as the hallmark of AD, though they also exist at lower density in the normal old brain [17].
Neurochemical changes
There are several evidences suggesting that neurotransmitter systems are affected differentially by aging. The most consistent age-related change in the neurochemical system is the loss of glutamate receptors. A significant decrease in the mRNA level of glutamate receptor is reported in the rat cerebral cortex [18]. Among different glutamate receptors, N-methyl-d-aspartate (NMDA) receptor levels change in the prefrontal cortex of aged macaque monkeys and rats [19, 20]. In addition, the expression levels of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid and NMDA receptor subunit proteins, GluR2 and NR1 decrease significantly in neurons, suggesting corticocortical links between temporal and frontal cortices in aged monkeys. In vitro experiments also demonstrate an age-dependent decrease of glutamate receptor-dependent synaptic activation in prefrontal cortex layer 2/3 of pyramidal cells of the aged monkey [21]. However, so far there is no conclusive evidence for the age-dependent alteration in kainate (a glutamate receptor) and γ-amino butyric acid (GABA) receptors [6].
Besides different receptors, the levels of neurochemicals, their metabolites and presynaptic markers also show age-dependent changes. For example, the levels of metabolites of acetylcholine, dopamine (DA) and noradrenaline (NA) are reduced in the cerebral cortex of aged rats and monkeys [22]. The level of GABA also decreases in the old rat brain. In the rat hippocampus, the expression of presynaptic protein synaptophysin declines with age, and the degree of decline correlates with deficits in spatial memory [23]. The cholinergic and monoaminergic systems projecting from the basal forebrain and brainstem also show certain degree of functional impairment during aging [24]. Thus, age-related neurochemical alterations display region specificity that affects brain functions in old age.
Functional changes
Several age-dependent studies show impairments in gait control, sleeping cycle, learning and memory. However, Burke and Mackay [25] described the memory impairment with advancing age as a selective deficit rather than a general decline in all cognitive functions. The memory capabilities that depend on the hippocampal function (spatial memory) are particularly vulnerable with increasing age. The application of functional magnetic resonance imaging technique in aged humans demonstrated a decrease in the cortical activity [26]. Studies on spontaneous activities of cortical neurons indicate a reduced firing rate in old age [6]. However, the lack of age-related changes in the spontaneous neuronal firing rate in some areas of the hippocampus suggests that the loss of spontaneous neuronal activities may be restricted to specific circuits [27]. Apart from the decrease in firing rate, modifications also occur in the neuronal firing pattern in the area governing the circadian rhythm (suprachiasmatic nuclei) [6]. In the CA1 region, the significant loss of synapses matches with a decrease in the evoked synaptic potential and a reduction in the evoked GABA-mediated inhibitory postsynaptic potentials. However, there also occur compensatory changes for maintaining the magnitude of synaptic potential [28]. Such changes include an increase in the excitatory postsynaptic potential of NMDA receptor signaling in CA1 area of the aged rat. These compensatory alterations in synaptic function may account for relatively minimal age-related functional changes in the brain [29].
Estrogen action in brain
Numerous studies support the beneficial effect of estrogens on the function and viability of neurons and learning and memory processes [1–3]. Estrogen action in various regions of the brain influences reproductive process, higher cognitive functions, pain mechanism, fine motor skills, mood, temperature regulation, sleep and susceptibility to neurodegenerative disorders [30].
Actions on brain micromorphology
Estrogen significantly affects the microstructure of different brain regions [31–33]. The increase in synaptic spine and dendritic density, which depends on circulating estrogen levels, is correlated with the superior performance in behavioral and memory tests [34, 35]. The depletion of estrogen in adult female rats by removal of ovaries results in loss of spines from certain hippocampal cells, whereas the ovariectomized rats receiving exogenous estrogen show normal number of hippocampal spines. These changes are mediated through the estrogen dependent increase in NMDA receptor and its phosphorylation in rat hippocampal neurons [36, 37].
Neurotrophic action
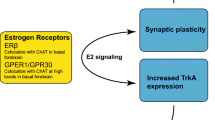
In addition to affecting the microstructure of brain, estrogen regulates the level of neurotrophins such as nerve growth factor (NGF), which is essential for early development, differentiation and growth of neurons. Receptors for both estrogen and neurotrophins are located on same neurons in the rodent basal forebrain, hippocampus and cerebral cortex [38]. The functional significance of this co-localization is supported by the observation that estrogen increases the expression of p75NGFR, a 75-kDa transmembrane protein which binds with low affinity to NGF and other neurotrophins. Estrogen also regulates the levels of brain-derived neurotrophic factor, insulin like growth factor-1 (IGF-1), transforming growth factor beta and related receptors TrkA and TrkB [39–41].
Neuroprotective action
Estrogen protects the neuronal damage through many ways. It reverses the effect of oxidative stress in neuronal cell culture by increasing the intracellular concentration of glutathione, a natural free radical scavenger. It modulates the activity of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase [42]. The antioxidant property of estrogen has been related primarily to its basic chemical property such as the presence of a hydroxyl group in steroid ring A of the estrogen molecule. Any modification or absence of this hydroxyl group leads to the loss of neuroprotective effect [43–45]. Estrogen replacement in young and middle aged rats after the removal of ovaries significantly decreases ischemic injury compared to vehicle-treated controls [46–48]. Such estrogen dependent protection against ischemia induced neuronal damage occurs by inhibiting the release of free calcium from intracellular stores and the influx of calcium from the extracellular space and thus preventing activation of apoptotic signaling [49]. Other mechanisms by which estrogen prevents the neuronal death involve inhibition of apoptosis by increasing the level of antiapoptotic proteins or repressing the level of pro-apoptotic proteins [50, 51]. Estrogen treatment also increases the clearance of amyloid β by microglia [52], protects against glucose deprivation [53], decreases the inflammatory reactions by blocking expression of pro-inflammatory factors [54], helps in laminin reorganization after injury and regulates the permeability of blood–brain barrier [55, 56].
Learning and memory
The variation in plasma concentration of estrogen during menstrual cycle is responsible for cyclic modulation of mood and cognitive activities. Systematic analysis of the impact of estrogen loss and replacement in human demonstrated that verbal memory declines with the loss of estrogen [57]. In Morris water maze test of rodents, retention/consolidation of spatial memory varies with alterations in estrous cycle and this hippocampus-dependent task is sexually dimorphic [58]. Although all studies do not show enhancement in estrogen dependent spatial memory tasks, results indicate that such enhancement may be limited to working versions of spatial memory [59–61]. The sexually dimorphic differences also exist in human cognitive functions, e.g., women excel in verbal memory, verbal fluency and fine motor skills, whereas men excel in visuospatial skills and gross motor coordination [62]. These cognitive changes may occur due to differences in the exposure of male and female brains to sex steroids during early development.
Modulation of neurotransmitter system
Serotonergic system
Estrogen regulates components of the serotonin system such as increase in the expression of tryptophan hydroxylase, serotonin transporter mRNA, 5-HT1A mRNA, 5-HT2A mRNA and 5-HT2A receptor binding [63–66] in dorsal and medial raphe of midbrain, amygdala, hypothalamus, hippocampus and many other brain areas of primates and rodents. Estrogen also causes a rapid decrease in the coupling of G proteins to 5-HT1A receptor system, resulting in the reduction of inhibitory effect of 5-HT1A agonists on lordosis behavior, hyperphagia, and oxytocin and corticotropin responses [67].
Dopaminergic system
Like serotonin system, estrogen influences the dopaminergic system involved in motor function, motivation, reward, cognition and hypothalamic–pituitary axis control [68, 69]. The level and turnover of DA fluctuate during the estrous cycle [70]. However, administration of estrogen following ovariectomy increases the release of DA [71, 72], and concentrations of D1 and D2 receptor in the striatum [73]. Estrogen inhibits the release of DA from the median eminence [74], but induces the release and turnover of striatal DA. Re-uptake of DA increases in the rat preoptic-septal tissue, but decreases in the hypothalamus [75].
Cholinergic system
Administration of estrogen to ovariectomized rats increases the activity of choline acetyl transferase (ChAT) in the basal forebrain, and two of its projection areas, CA1 region of hippocampus and frontal cortex. ChAT is involved in the synthesis of acetylcholine [76]. In the noradrenergic system, both α- and β-adrenergic receptors are upregulated by 17β-estradiol in ovariectomized female rats [75, 77]. However, β-adrenergic receptors are eventually downregulated due to a hormone dependent increase in noradrenergic activity. The synaptic uptake of NA decreases when estrogen is administered alone [78], but increases when estrogen is followed by progesterone in rats [77].
Effect of aging on estrogen signaling in brain
Estrogen mediates numerous responses via three distinct types of signaling, namely genomic, nongenomic and ligand-independent pathways.
Genomic pathway
The classical genomic pathway involves signaling through intracellular receptors, ERα (NR3A1) and ERβ (NR3A2). Both ERα and ERβ are ligand-activated transcription factors belonging to the nuclear receptor superfamily of steroid receptors [79]. In the absence of ligand (estrogens), ERs are sequestered in a multiprotein inhibitory complex within the nuclei. A recent study shows the localization of ERβ exclusively in the mitochondria of target cells [80]. The binding of ligand induces conformational changes in ERs such as homo- or hetero-dimerization of receptors and high affinity binding to specific estrogen responsive elements (EREs) located as cis-acting enhancers within the regulatory regions of target genes. The DNA-bound receptors contact general transcription apparatus either directly or indirectly via coregulators, cointegrators and other proteins having histone modification activities. It is generally accepted that the ER–coactivator interaction stabilizes the formation of transcription pre-initiation complex and facilitates the remodeling of chromatin at ERE. Depending upon the cell and promoter context, the DNA-bound receptor exerts either positive or negative effects on the expression of downstream target genes [1, 79, 81].
Estrogen receptorα and ERβ can also modulate the expression of target genes that do not have ERE in their promoter regions. ERE independent pathway implies the interaction of liganded ERs with other transcription factors such as Fos and Jun proteins at AP1 binding sites and Sp1 transcription factor in GC-rich promoter sequences [82, 83]. These actions of ERs depend on ligand, cell and receptor subtype [84]. Repression of interleukin-6 (IL-6) gene by estrogen involves the interaction of ERs with two transcription factors, nuclear factor κB (NF-κB) and CCAAT/enhancer binding protein β. The interaction of ERα with c-rel subunit of NF-κB prevents the binding of NF-κB to IL-6 promoter resulting in the repression of IL-6 expression [84].
Nongenomic pathway
Nongenomic actions of estrogens are mediated by the binding of hormone to either a subpopulation of classical ERs, which are located at the plasma membrane [85, 86] and exist as functional dimers when activated by estrogens [87] or novel membrane ERs such as ER-X [88]. Nongenomic pathway involves the activation of various protein kinase cascades such as src, ras, MEK and MAPK [89, 90].
Ligand-independent pathway
In addition to ligand mediated activation, ER functions can be modulated by ligand-independent pathway through extracellular signals. Signaling through peptide growth factors such as epidermal growth factor, IGF-1 and cAMP activates ER and target gene transcription. ERα and ERβ also act as the target of growth factor dependent phosphorylation which occurs through MAPK signaling pathway [3], after phosphorylation both ERα and ERβ interact with different coactivators for activation of target genes [91].
Signal transduction pathways also connect the nongenomic actions of estrogens to genomic responses [89]. The nongenomic pathway stimulates a second messenger system, which phosphorylates various cellular substrates including transcriptional regulators like cAMP response element (CRE) binding protein (CREB) by protein kinase A or serum response factor (SRF)–Elk-1 complex by MAPK/ERK [89]. These transcription regulatory proteins CREB and SRF–Elk-1 bind to DNA regulatory regions namely CRE and serum response element, respectively. The resulting cascades are capable of regulating non-ERE-containing genes. Thus, stimulation of second messenger system can regulate both ER dependent and ligand-independent genomic actions, independent to each other.
Brain aging and ERs
The level of ERα and ERβ is determined by a balance between its synthesis and degradation during aging of the brain. The binding of ER to its cognate ligand varies in specific regions of the brain of young and old rats [92]. Aged female rats show decreased binding of 17β-estradiol in preoptic area, but no difference is found in amygdala, medial basal hypothalamus and pituitary [93]. In contrast, other groups have reported decreased binding in hypothalamus, preoptic area and pituitary of old female rats [94, 95]. Such discrepancy in results needs to be resolved by additional approaches. Further complexity in the interpretation of these results was added after the discovery of ERβ which has almost similar affinity of binding with 17β-estradiol as ERα.
In situ hybridization studies using ERα specific probes demonstrate little or no change in ERα mRNA expression in the preoptic area and hypothalamus of old rats [96–99]. Further, immunohistochemical studies show that the number of cells expressing ERα protein does not change in the median preoptic nucleus (MPN) of adult and old female rats [100]. However, the number of cells expressing ERα protein increases in anteroventral periventricular nucleus (AVPV) but decreases in ventromedial nucleus of old rats [101]. A recent report from our laboratory indicates that ERα protein level does not vary with age, but shows sex dependent differences in the cerebral cortex of AKR mice [102].
The expression of ERβ mRNA shows no effect of age in MPN, paraventricular or periventricular preoptic nuclei [99], but decreases significantly in supraoptic nucleus. The immunoreactivity of ERβ also shows no change in principal nucleus of the bed nucleus of the stria terminalis, but increases in AVPV of old rats [103]. We have recently reported an age-dependent decrease in the level of ERβ protein in the cerebral cortex of AKR mice [102].
Estrogen effects in aging brain
In addition to age-related changes in ER expression, the response to estrogen varies with age. As described earlier, experiments using laboratory animals and cell culture suggest beneficial effect of estrogen treatment on the brain; however, almost all of these studies involve young or middle aged animals. Studies using senescent laboratory animals suggest that estrogen treatment may or may not have the same effect in old brain as in adult [104–107]. Estrogen treatment in the gonadectomized aged rats has been shown to be responsible for the reversal of hippocampus related memory impairment, blocking of long-term depression, decreased cytosolic calcineurin activity [58, 108], increased level of growth associated protein-43 and choline acetyltransferase [109].
Estrogen is also involved in the modulation of expression of amyloid precursor protein (APP) associated with AD in old brain. Of the various APP isoforms (APP770, APP751 and APP695), the APP695 is predominantly found in the brain and its level remains high under non-pathological conditions. Experimental evidences suggest that the level of APP695 is upregulated by estrogen treatment in old female mouse cerebral cortex [110–112], suggesting that estrogen treatment in old age may shift the APP load in non-pathological condition.
In contrast to these beneficial effects, estrogen treatment in aged rats fails to induce an increase in spine number but has an impact on the molecular nature of CA1 axospinous synapses through enhancement of synaptic NR1 and NR2B expression, suggesting that estrogen can restore a partial youthful NMDA receptor profile in aged rats [113]. Similarly, Jezierski and Sohrabji [114] reported that aged forebrain is unresponsive to estrogen dependent neurotrophin expression. Estrogen treatment reduces the permeability of blood–brain barrier in the olfactory bulb of young but not old rats, and increases the permeability in the hippocampus of old females compared with age-matched untreated animals, suggesting that the hormonal decline leads to increased permeability of the blood–brain barrier, which is further exacerbated by estrogen treatment in specific regions [56].
The comparison of estrogen replacement effects between young adult and reproductively senescent animals suggests that estrogen replacement is beneficial when given to “surgically menopausal” (ovariectomized) animals. However, estrogen replacement appears to be deleterious for the acyclic reproductively senescent animals, where target organs such as the brain have been in a prolonged estrogen-deficient state [115].
Recent studies in non-human primates suggest that aged female monkeys (equivalent to perimenopausal women) are just as responsive as young monkeys with respect to estrogen-induced increase in CA1 spine number [116]. A behavioral analysis demonstrated significant estrogen-induced enhancement of cognitive function in aged ovariectomized rhesus monkeys [117]. The estrogen-treated group showed enhanced performance in hippocampus-dependent task (delayed non-matching to sample, DNMS) as well as the delayed response task (a prefrontal task that is sensitive to aging). Although the effects of estrogen on DNMS were moderate, estrogen treatment dramatically affected the delayed response performance, restoring it to that of young monkeys [117]. This indicates that in primates the prefrontal cortex might be at least as responsive to estrogen as the hippocampus, implicating a much broader array of cognitive functions than suggested by the rat hippocampal data.
Like the aged laboratory animals, results from clinical studies characterizing the cognition-enhancing and neuroprotective efficacy of estrogen in old age have revealed conflicting results. Earlier clinical investigations indicate that as compared to young women, postmenopausal women are more vulnerable to neurodegenerative diseases such as AD, Parkinson’s disease, stroke and memory dysfunction and estrogen replacement therapy (ERT) not only reduces the risk to AD but increases the verbal memory [104]. Using positron emission tomography, Rasgon et al. [118] reported that estrogen replacement may preserve regional cerebral metabolism and protect against metabolic decline in postmenopausal women, especially in the posterior cingulate cortex, and thus estrogen enhances the chance of neuronal survival. Another study showed differences between estrogen users and non-users in the cerebral blood flow in the hippocampus, parahippocampal gyrus and temporal lobe. These studies suggest that at least some areas of the brain involved in memory circuits and sensitive to AD are responsive to ERT in old females [119]. ERT in postmenopausal women also increases the choline containing compounds in parietal and hippocampus regions, indicating increased neuronal/glial membrane turnover and suggesting that neuroprotective effects of estrogen may involve modulation of cell integrity [120].
However, recent intervention trial (Women’s Health Initiative) concluded that the replacement of estrogen and other hormones prescribed to postmenopausal women does not improve global cognitive impairment and dementia [121]. The intervention trial also found that 65–79 years old women with 10–20 years postmenopausal state are less responsive to estrogen replacement than perimenopausal women in their early 1950s [122]. Postmenopausal women of 65 years and above, and free of probable dementia and treated with estrogen and progesterone had a negative impact on verbal memory and a trend to a positive impact on figural memory over time compared with placebo, but other cognitive domains were not affected. Both effects on memory were evident only after long-term therapy [122]. In another double-blind experiment, hysterectomized women (age 58–75 years) receiving placebo, estradiol or estradiol/progesterone treatment failed to show any beneficial effects in any of the cognitive tests (out of nine parameters) [123]. Therefore, this study does not support the notion that treatment with sex hormones has beneficial effects on cognition in old women. Taken together, these reports suggest a “short period of opportunity” as a function of age and duration of estrogen depletion, after which replacement is less effective. In fact, such age-associated alterations in response to estrogen might be a crucial factor for the failure of estrogen replacement to protect against cognitive impairment.
Conclusions
Aging is associated with alterations in brain structure and function. Estrogen action in brain influences many anatomical and neurochemical processes that go beyond their traditional role. So far the information about age-dependent changes in the membrane ER(s) is lacking, while very little is known about the changes occurring in nuclear ERs. Changes in ERs depend upon receptor subtypes and brain regions with the likely net outcome of a differential response to estrogen in the aging brain. Experimental evidences obtained from laboratory animals suggest that these effects may be of particular importance in the context of aging when circulating estrogen level decreases. However, the effect of estrogen supplementation in old females is not as beneficial as in adults, at least in the case of cognitive impairment, indicating the importance of detailed knowledge about age-dependent changes in estrogen signaling pathway and fidelity of other downstream interacting molecules.
References
McEwen BS, Alves SE (1999) Estrogen actions in the central nervous system. Endocr Rev 20:279–307
Thakur MK (1999) Estrogen and brain aging. J Anti-Aging Med 2:127–132
Lee SJ, McEwen BS (2001) Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu Rev Pharmacol Toxicol 41:569–591
Zwain IH, Yen SSC (1999) Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology 140:3843–3852
Reid G, Denger S, Kos M, Gannon F (2002) Human estrogen receptor-α: regulation by synthesis, modification and degradation. Cell Mol Life Sci 59:821–831
Wong TK (2002) Aging of cerebral cortex. McGill J Med 6:104–113
Gunning-Dixon FM, Raz N (2000) The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology 14:224–232
Mouton PR, Long JM, Lei D-L, Howard V, Jucker M, Calhoun ME, Ingram DK (2002) Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res 956:30–35
Jacobs B, Scheibel AB (1993) A quantitative dendritic dendritic analysis of Wernicke’s area in humans. Life span changes. J Comp Neurol 327:83–96
Coleman PD, Flood DG (1991) Net dendritic stability of layer II pyramidal neurons in F344 rat entorhinal cortex from 12 to 37 months. Neurobiol Aging 12:535–541
Anderson B, Rutledge V (1996) Age and hemisphere effects on dendritic structure. Brain 119:1913–1990
Adams I, Jones DG (1982) Quantitative ultrastructural changes in rat cortical synapses during early-, mid- and late-adulthood. Brain Res 239:349–363
Adams I (1987) Comparison of synaptic changes in the precentral and postcentral cerebral cortex of aging humans: a quantitative ultrastructural study. Neurobiol Aging 8:203–212
Brody H (1955) Organization of the cerebral cortex, III. A study of aging in the human cerebral cortex. J Comp Neurol 102:511–556
Morrison JH, Hof PR (1997) Life and death of neurons in aging brain. Science 278:412–419
Peters A, Morrison JH, Rosene DL, Hyman BT (1998) Are neurons lost from primate cerebral cortex during normal aging? Cereb Cortex 8:295–300
Anderton BH (1997) Changes in the ageing brain in health and disease. Phil Trans R Soc B 352:1781–1792
Carpenter MK, Parker I, Miledi R (1992) Messenger RNAs coding for receptors and channels in the cerebral cortex of adult and aged rats. Brain Res Mol Brain Res 13:1–5
Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC (1989) Age related changes in multiple neurotransmitter systems in monkey brain. Neurobiol Aging 10:11–19
Wenk GL, Walker LC, Price DL, Cork LC (1991) Loss of NMDA, but not GABA, binding in the brains of aged rats and monkeys. Neurobiol Aging 12:93–98
Luebke JL (2004) Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in monkey prefrontal cortex. Neuroscience 125:277–288
Hof PR, Morrison JH (2004) The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci 27:607–613
Woolley CS (1998) Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav 34:140–148
Arnsten AFT (1999) Age-related cognitive deficits and neurotransmitters—the role of catecholamine mechanisms in prefrontal cortical cognitive decline. In: Peters A, Morrison JH (eds) Cerebral cortex, vol 14. Neurodegenerative and age-related changes in structure and functions of cerebral cortex. Plenum Publishing Co, New York, pp 89–110
Burke DM, Mackay DG (1997) Memory, language and ageing. Phil Trans R Soc B 352:1845–1856
Reuter-Lorenz PA, Lustig C (2005) Brain aging. Reorganizing discoveries about aging mind. Curr Opin Neurobiol 15:245–251
Palombi PS, Caspary DM (1996) Physiology of aged Fischer 344 rat inferior colliculus: responses to contralateral monaural stimuli. J Neurophysiol 76:3114–3125
Jouvenceau A, Dutar P, Billard JM (1998) Alteration of NMDA receptor mediated synaptic responses in CA1 area of the aged rat hippocampus: contribution of GABAergic and cholinergic deficits. Hippocampus 8:627–637
Johnson SC, Saykin AJ, Baxter LC (2000) The relationship between fMRI activation and cerebral atrophy: comparison of normal aging and Alzheimer’s diseases. Neuroimage 11:179–187
McEwen B (2002) Estrogen actions throughout the brain. Recent Prog Horm Res 57:357–384
Toran-Allerand CD (1984) On the genesis of sexual differentiation of the general nervous system: morphogenetic consequences of steroidal exposure and possible role of alpha-fetoprotein. Prog Brain Res 61:63–98
Murphy DD, Segal M (1996) Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci 16:4059–4068
Wang L, Andersson S, Warner M, Gustafsson J-A (2002) Estrogen receptor (ER)β knockout mice reveal a role for ERβ in migration of cortical neurons in the developing brain. Proc Natl Acad Sci USA 100:703–708
Gould E, Woolley CS, Frankfurt M, McEwen BS (1990) Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci 10:1286–1291
Simpkins JW, Singh M, Bishop J (1994) The potential role for estrogen replacement therapy in the treatment of the cognitive decline and neurodegeneration associated with Alzheimer’s disease. Neurobiol Aging 15(Suppl. 2):S195–S197
Gazzaley AH, Weiland NG, McEwen BS, Morrison JH (1996) Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci 16:6830–6838
Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA (1997) Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci 17:1848–1859
Toran-Allerand CD (1996) The estrogen/neurotrophin connection during neural development: is colocalization of estrogen receptor with neurotrophins and their receptor biologically relevant? Dev Neurosci 18:36–42
Sohrabji F, Miranda RC, Toran-Allerand CD (1994) Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci 14:459–471
Agrati P, Ma ZQ, Patrone C, Picotti GB, Pellicciari C, Bondiolotti G, Bottone MG, Maggi A (1997) Dopaminergic phenotype induced by oestrogens in a human neuroblastoma cell line. Eur J Neurosci 9:1008–1016
Carrer HF, Cambiasso MJ (2002) Sexual differentiation of the brain: genes, estrogen and neurotrophic factors. Cell Mol Neurobiol 22:479–496
Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S (2005) Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the women’s health initiative. Endocr Rev 26:308–312
Behl C, Lezoualc’h F (1998) Estrogens with an intact phenolic group prevent death of neuronal cells following glutathione depletion. Restor Neurol Neurosci 12:127–134
Behl C, Widmann M, Trapp T, Holsboer F (1995) 17-Beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun 216:473–482
Patrone C, Andersson S, Korhonen L, Lindholm D (1999) Estrogen receptor-dependent regulation of sensory neuron survival in developing dorsal root ganglion. Proc Natl Acad Sci USA 96:10905–10910
Wise PM, Dubal DB (2000) Estradiol protects against ischemic brain injury in middle-aged rats. Biol Reprod 63:982–985
Wise PM, Dubal DB, Wilson ME, Rau SW, Bottner M, Rosewell KL (2001) Estradiol is a protective factor in the adult and aging brain: understanding of mechanisms derived from in vivo and in vitro studies. Brain Res Rev 37:313–319
Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM (2006) Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERα in estradiol mediated protection against delayed cell death. Endocrinology 147:3076–3084
McCullough LD, Hurn PD (2003) Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab 14:228–235
Pike CJ (1999) Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer’s disease. J Neurochem 72:1552–1563
Meda C, Vegeto E, Pollio G, Ciana P, Patrone C, Pellicciari C, Maggi A (2000) Oestrogen prevention of neural cell death correlates with decreased expression of mRNA for the pro-apoptotic protein nip-2. J Neuroendocrinol 12:1051–1059
Li R, Shen Y, Yang LB, Lue LF, Finch C, Rogers J (2000) Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex. J Neurochem 75:1447–1454
Zhang Y, Howard BV, Cowan LD, Yeh J, Schaefer CF, Wild RA, Wang W, Lee ET (2002) The effect of estrogen use on levels of glucose and insulin and the risk of type 2 diabetes in American Indian postmenopausal women: the strong heart study. Diabetes Care 25:500–504
Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP (2000) Antiinflammatory effects of estrogen on microglial activation. Endocrinology 141:3646–3656
Rozovsky I, Wei M, Stone DJ, Zanjani H, Anderson CP, Morgan TE, Finch CE (2002) Estradiol (E2) enhances neurite outgrowth by repressing glial fibrillary acidic protein expression and reorganizing laminin. Endocrinology 143:636–646
Bake S, Sohrabji F (2004) 17β-Estradiol differentially regulates blood brain barrier permeability in young and aging female rats. Endocrinolgy 145:5471–5475
Sherwin BB (2003) Estrogen and cognitive functioning in women. Endocr Rev 24:133–151
Foster TC, Sharrow KM, Kumar A, Masse J (2003) Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging 24:839–852
Sandstorm NJ, Williams CL (2001) Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci 115:384–393
Luine VN, Rodriguez M (1994) Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol 62:230–236
Li C, Brake W, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos A-M, Allen PB, Greengard P, Luine V, McEwen BS (2004) Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci USA 101:2185–2190
Hampson E (1990) Estrogen related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology 15:97–111
Gundlah C, Pecans-Thompson M, Schutzer WE, Bethea CL (1999) Ovarian steroid effects on serotonin 1A, 2A and 2C receptor mRNA in macaque hypothalamus. Brain Res Mol Brain Res 63:325–339
Sumner BE, Fink G (1998) Testosterone as well as estrogen increases serotonin2A receptor mRNA and binding site densities in the male rat brain. Brain Res Mol Brain Res 59:205–214
Rapp DK, DonCarlos L, Gracia F, Murna NA, Wolf WA, Battaglia G, Van de Kar LD (2000) Estrogen desensitizes 5-HT(1A) receptors and reduces levels of G(z), G(i1) and G(i3) proteins in the hypothalamus. Neuropharmacology 39:1823–1832
Osterlund MK, Halldin C, Hurd YL (2000) Effects of chronic 17β-estradiol treatment on the serotonin 5-HT1A receptor mRNA and binding levels in the rat brain. Synapse 35:39–44
Mize AL, Poisner AM, Alper RH (2001) Estrogens act in rat hippocampus and frontal cortex to produce rapid, receptor-mediated decreases in serotonin 5-HT(1A) receptor function. Neuroendocrinology 73:166–174
Di Paolo T (1994) Modulation of brain dopamine transmission by sex steroids. Rev Neurosci 5:27–41
Saunders-Pullman R, Gordon-Elliott J, Parides M, Fahn S, Saunders HR, Bessman S (1999) The effect of estrogen replacement on early Parkinson’s disease. Neurology 52:1417
Crowley WR, O’Donohue TL, George JM, Jacobowitz DM (1978) Changes in pituitary oxytocin and vasopressin during the estrous cycle and after ovarian hormones: evidence for mediation by norepinephrine. Life Sci 23:2579–2585
Di Paolo T, Daigle M, Barden N (1985) Decreased tuberoinfundibular and pituitary dopamine and dihydroxyphenylacetic acid concentrations in rats with estrogen-induced pituitary tumors. Neurosci Lett 54:251–256
Becker JB (1990) Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett 118:169–171
Tonnaer JA, Leinders T, Van Delft AM (1989) Ovariectomy and subchronic oestradiol-17 beta administration decreases dopamine D1 and D2 receptors in rat striatum. Psychoneuroendocrinology 14:469–476
Cramer OM, Parker CR Jr, Porter JC (1979) Estrogen inhibition of dopamine release into hypophysial portal blood. Endocrinology 104:419–422
Vacas MI, Cardineli DP (1980) Effect of estradiol on alpha- and beta-adrenoceptor density in medial basal hypothalamus, cerebral cortex and pineal gland of ovariectomized rats. Neurosci Lett 17:73–77
Luine VN (1985) Estradiol increases the choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol 89:484–490
Jonowsky DS, Davis JM (1970) Progesterone–estrogen effects on uptake and release of norepinephrine by synaptosomes. Life Sci 9:525–531
McEwen BS (1980) Gonadal steroids: humoral modulators of nerve-cell function. Psychoneuroendocrinology 16:151–164
Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson J-Å (2001) Mechanisms of estrogen action. Physiol Rev 81:1535–1565
Yang S-H, Liu R, Perez EJ, Wen Y, Stevens SM Jr, Valencia TM, Burn-Zinkernagel A-M, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW (2004) Mitochondrial localization of estrogen receptorβ. Proc Natl Acad Sci USA 101:4130–4135
McKenna NJ, O’Malley BW (2002) Nuclear receptor coactivators—an update. Endocrinology 143:2461–2465
Porter W, Saville B, Hoivik D, Safe S (1997) Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol Endocrinol 11:1569–1580
Safe S (2001) Transcriptional activation of genes by 17β-estradiol through estrogen receptor–Sp1 interactions. Vitam Horm 62:231–252
Ray A, Prefontaine KE, Ray P (1994) Down-modulation of interleukin-6 gene expression by 17β-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J Biol Chem 269:12940–12946
Lossel R, Wehling M (2003) Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol 4:46–56
Pappas TC, Gametchu B, Watson CS (1995) Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J 9:404–410
Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER (2004) Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol 18:2854–2865
Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES Jr, Nethrapalli IS, Tinnikov AA (2002) ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci 22:8391–8401
Bjornstom L, Sjoberg M (2005) Mechanisms of estrogen receptor signaling: convergence of genomic and non genomic actions on target genes. Mol Endocrinol 19:833–842
Duan R, Xie W, Burghardt RC, Safe S (2001) Estrogen receptor-mediated activation of the serum response element in MCF-7 cells through MAPK-dependent phosphorylation of Elk-1. J Biol Chem 276:11590–11598
Tremblay A, Tremblay GB, Labrie F, Giguere V (1999) Ligand-independent recruitment of SRC-1 to estrogen receptor β through phosphorylation of activation function AF-1. Mol Cell 3:513–519
Kanungo MS, Patnaik SK, Koul O (1975) Decrease in 17beta-oestradiol receptor in brain of aging rats. Nature 253:366–367
Wise PM, Camp P (1984) Changes in concentration of estradiol nuclear receptors in preoptic area, medial basal hypothalamus, amygdala and pituitary gland of middle-aged and old cycling rats. Endocrinology 114:92–98
Rubin BS, Fox TO, Bridges RS (1986) Estrogen binding in nuclear and cytosolic extracts from brain and pituitary of middle-aged female rats. Brain Res 383:60–67
Brown TJ, MacLusky NJ, Shanabrough M, Naftolin F (1990) Comparison of age and sex-related changes of in cell nuclear estrogen-binding capacity and progestin receptor induction in the rat brain. Endocrinology 126:2965–2972
Funabashi T, Kimura F (1994) Effects of estrogen and estrogen receptor messenger RNA levels in young and middle aged female rats: comparison of medial preoptic area and mediobasal hypothalamus. Acta Biol Hung 45:223–231
Miller MA, Kolb PE, Plans B, Raskind MA (1994) Estrogen receptor and neurotensin/neuromedin-N gene expression in the preoptic area are unaltered with age in Fischer 344 female rats. Endocrinology 135:1986–1995
Funabashi T, Kleopoulos SP, Brooks PJ, Kimura F, Pfaff DW, Shinohara K, Mobbs CV (2000) Changes in estrogenic regulation of estrogen receptor β mRNA and progesterone receptor mRNA in the female hypothalamus during aging: an in situ study. Neurosci Res 38:85–92
Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM (2002) Age differentially influences estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) gene expression in specific regions of the rat brain. Mech Ageing Dev 123:593–601
Maderia MD, Andrade JP, Paula-Barbosa MM (2000) Hypertrophy of the ageing rat medial preoptic nucleus. J Neurocytol 29:173–197
Chakraborty TR, Ng L, Gore AC (2003) Colocalization and hormone regulation of estrogen receptor α and NMDA receptor in the hypothalamus of female rats. Endocrinology 144:299–305
Sharma PK, Thakur MK (2006) Expression of estrogen receptor (ER) alpha and beta in mouse cerebral cortex: effect of age, sex and gonadal steroids. Neurobiol Aging 27:880–887
Chakraborty TR, Ng L, Gore AC (2003) Age related changes in estrogen receptor β in rat hypothalamus: a quantitative analysis. Endocrinology 144:4164–4171
Brinton RD (2001) Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer’s disease: recent insights and remaining challenges. Learn Mem 8:121–133
Gibbs RB, Gabor R (2003) Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res 74:637–643
Thakur MK, Sharma PK, Ghosh S, Mani ST (2004) Estrogen intervention in aging and longevity: problems and prospectives. Geriat Gerontol Int 4:S259–S261
Mani ST, Kumar RC, Thakur MK (2001) Age- and sex-related expression of norbin in the brain cortex of mice. Neurosci Lett 308:57–59
Bi R, Foy MR, Thompson RF, Baudry M (2003) Effects of estrogen, age, and calpain on MAP kinase and NMDA receptors in female rat brain. Neurobiol Aging 24:977–983
Ferrini M, Bisagno V, Piroli G, Deniselle MC, De Nicola AF (2002) Effects of estrogens on choline-acetyltransferase immunoreactivity and GAP-43 mRNA in the forebrain of young and aging male rats. Cell Mol Neurobiol 22:289–301
Chao HM, Spencer RL, Frankfurt M, McEwen BS (1994) The effects of aging and hormonal manipulation on amyloid precursor protein APP695 mRNA expression in the rat hippocampus. J Neuroendocrinol 6:517–521
Wei X, Zhang J (1999) Alzheimer’s disease related gene expression in the brain of senescence-accelerated mouse. Neurosci Lett 268:139–142
Thakur MK, Mani ST (2005) Estradiol regulates APP mRNA alternate splicing in the mice brain cortex. Neurosci Lett 381:154–157
Adams MM, Shah RA, Janssen WGM, Morrison JH (2001) Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci USA 98:8071–8076
Jezierski MK, Sohrabji F (2001) Neurotrophin expression in reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol Aging 22:304–319
Sohrabji F (2005) Estrogen: a neuroprotective or proinflammatory hormone? Emerging evidence from reproductive aging models. Ann NY Acad Sci 1052:75–90
Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH (2003) Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol 465:540–550
Rapp PR, Morrison JH, Roberts JA (2003) Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci 23:5708–5714
Rasgon NL, Silverman D, Siddharth P, Miller K, Ercoli LM, Elman S, Lavertsky H, Huang SC, Phelps ME, Small GW (2005) Estrogen use and brain metabolic change in postmenopausal women. Neurobiol Aging 26:229–235
Maki PM, Resnick SM (2000) Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging 21:373–383
Robertson DMW, Amelsvoort TV, Daly E, Simmons A, Whitehed M, Morris RG, Murphy KC, Murphy DGM (2001) Effects of estrogen replacement therapy on human brain aging: an in vivo 1H MRS study. Neurology 57:2114–2117
Gardiner SA, Morrison MF, Mozley PD, Mozley LH, Brensinger C, Bilker W, Newberg A, Battistini M (2004) Pilot study on the effect of estrogen replacement therapy on brain dopamine transporter availability in healthy, postmenopausal women. Am J Geriatr Psychiatry 12:621–630
Resnick SM, Maki PM, Rapp SR, Espeland MA, Burnner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA (2006) Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab 91:1802–1810
Wolf OT, Heinrich AB, Hanstein B, Kirschbaum C (2005) Estradiol or estradiol/progesterone treatment in older women: no strong effects on cognition. Neurobiol Aging 26:1029–1033
Vouimba RM, Fay MR, Fay JG, Thompson RF (2000) 17β estradiol supresses expression of long term depression in aged rats. Brain Res Bull 53:783–787
Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH (2004) Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: women’s health initiative memory study J Am Med Assoc 291:2947–2958
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thakur, M.K., Sharma, P.K. Aging of Brain: Role of Estrogen. Neurochem Res 31, 1389–1398 (2006). https://doi.org/10.1007/s11064-006-9191-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9191-y