Abstract
Nosologically, Alzheimer disease (AD) is not a single disorder. A minority of around 400 families worldwide can be grouped as hereditary in origin, whereas the majority of all Alzheimer cases (approx. 25 million worldwide) are sporadic in origin. In the pathophysiology of the latter type, a number of susceptibility genes contribute to the disease among which are allelic abnormalities of the apolipoprotein E4 gene pointing to a link between disturbed cholesterol metabolism and sporadic AD. Cholesterol is a main component of membrane composition enriched in microdomains and is functionally linked to the proteolytic processing of amyloid precursor protein (APP). In sporadic AD, a marked diminution of both membrane phospholipids and cholesterol has been found. Evidence has been provided that high plasma cholesterol may protect from AD. In contrast to these well documented abnormalities observed in AD patients, it was assumed that an elevated cholesterol concentration might favour the generation of β-amyloid and, thus, AD. However, a series of in vitro-and in vivo-studies did not provide evidence for the assumption that an enhanced cholesterol concentration increased βA4-production. A harsh reduction of membrane cholesterol only caused a “beneficial” effect of APP metabolism. However, this experimentally induced condition may not be compatible to sporadic AD. The application of statins in sporadic AD did not yield results to assume that this therapeutic strategy may prevent or treat successfully sporadic AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer disease (AD) is the most prominent neurodegenerative disorder. With respect to its nosology, AD is not a single disorder although both its clinical phenotype and morphologic brain abnormalities are fairly uniform. Evidence has been provided that a very small proportion of 404 families worldwide (by August 2006) of all Alzheimer cases is caused by missense mutations in the presenilin gene 1 on chromosome 14 (315 families ∼78%), in the presenilin gene 2 on chromosome 1 (18 families ∼4%), and in the amyloid precursor protein (APP) gene on chromosome 21 (71 families ∼18%) (http://molgen-www.uia.ac.be//ADMutations//) leading to autosomal dominant familial AD with an early onset. These mutations explain well the genetically induced excess formation of the APP derivative βA4 which aggregates to form amyloid deposited as neuritic plaques [1]. In contrast, the great majority of patients suffering from AD (approx. 25 million patients worldwide) is sporadic in origin.
Beside age as the main risk factor, a number of susceptibility genes were found contributing to the generation of sporadic AD. Of greatest significance in this respect are a single nucleotide polymorphism in the gene coding for 11β-hydroxysteroid dehydrogenase I [2], and allelic abnormalities of the apolipoprotein E4 (APOE) gene on chromosome 19 (for review see 3) affecting cholesterol metabolism in that plasma total and low-density lipoprotein cholesterol were found to be elevated [4–6].
The association of APO E4 allele and an increased risk of AD may point on a link between elevated cholesterol and AD. However, several clinico-epidemiological studies did not yield uniform results. Increased cholesterol levels at ages 70 were not found to be associated with an elevated risk of dementia between ages 79 and 88 [7, 8]. In contrast, exposure to vascular risk factors inclusive enhanced cholesterol during midlife increased the risk of dementia in late life which was reduced by lipid-lowering agents [9, 10]. In a predictive study, most AD patients carrying the APOE 4 gene were found to have lower total cholesterol levels than controls in a case–control study [11]. Otherwise, APOE-4 allele carriers with high serum levels of total cholesterol and high systolic blood pressure at midlife were at an elevated risk to develop AD in older ages. This risk was highest in subjects carrying the APOE-4 allele and having high serum cholesterol and increased systolic blood pressure [12–14]. In all studies cited above, the diagnosis of AD was made on the basis of clinico-psychometric tests only demonstrating the existence of dementia symptoms without detailed differentiation of its origin. However, the ascertainment of the causatively different AD needs neuroimaging, EEG and cerebrospinal fluid (CSF) markers additionally.
Based on the above epidemiological studies, on the strong connection found between elevated plasma cholesterol and βA4 generation in transgenic mice [15], and on the association of both APP and βA4 with cholesterol-rich membrane domains [16, 17], it was assumed that both the generation and clearance of βA4 are regulated by cholesterol [18]. Finally, this concept that high concentration of brain cholesterol leads inevitably to abnormal βA4 accumulation as the main cause of AD [1] prompted investigatiors to use statins for prevention and treatment of AD in experimental and clinical studies. However, a number of issues do not support this concept. (1) It has not been proven that excess formation of βA4 is necessary for the generation and the development of sporadic AD [19]. (2) Transgenic mice models of AD may represent its hereditary type and have not been shown to be valid for sporadic AD. (3) Serum cholesterol has not been demonstrated to penetrate the blood–brain barrier, i.e. provided cholesterol were increased in the serum compartment in sporadic AD, it might have no impact on its concentration in the brain compartment ([20, 21]; see also below). (4) The state of the neuronal membranes of AD patients, in particular its composition of phospholipids and cholesterol, may not have been considered in the therapeutic strategy using statins.
It becomes, thus, obvious that the role of brain cholesterol is conflicting for the development of sporadic AD. Therefore, in this short review, its role is discussed in terms of the physiological significance and in terms of the pathophysiological impact to sporadic AD, also contributing to the question whether or not the use of statins to reduce brain cholesterol is of benefit for patients suffering from sporadic AD.
Formation and function of cholesterol in the healthy adult brain
In the mammalian central nervous system (CNS), cholesterol is synthesized exclusively by de novo synthesis reaction from acetyl-CoA in the 3-hydroxy-3-methylglutaryl-CA (HMG-CoA) reductase reaction by oligodendrocytes, astrocytes and neurons [22]. In vitro studies showed a cholesterol shuttle from astrocytes to neurons that is mediated by apoE [23]. Plasma lipoproteins are unable to cross the blood–brain barrier [20, 21]. Serum cholesterol levels have been demonstrated to have no effect on HMG-CoA reductase and its activity in the brain [24], and on total brain cholesterol levels [25].
Cholesterol synthesis via the HMG-CoA reductase reaction needs additional proteins such as seladin-1 which is highly expressed in almost all neurons, and energy [20, 26, 27]. The formation/availability of both acetyl-CoA and energy as ATP in the brain has been demonstrated to be controlled by the neuronal insulin/insulin receptor signal transduction cascade [28]. Cholesterol is removed from the brain by the neural rate-limiting enzyme cholesterol 24-hydroxylase which mediates the hydroxylation of cholesterol to 24-hydroxycholesterol the concentration of which was found in the brain compared to other organs and that can pass the blood–brain barrier. Cholesterol 24-hydroxylase is encoded by the CYP46 gene [29–31].
Cholesterol serves as the basic compound from which neurosteroids derive [32]. Glia-derived cholesterol has been demonstrated to promote synaptogenesis in nervous tissue [23]. Cholesterol has been found to stimulate neurite outgrowth in rabbit dorsal root ganglion neurons what was accentuated by apoE [23a]. Synaptic plasticity depends on a well-balanced cholesterol homeostasis mediated by apoE [33]. The latter also has been shown to play an important role in the translocation of cholesterol from astrocytes to neurons in mouse brain under long-term (24 weeks) environmental stimulation [23b]. Its greatest significance, however, has cholesterol as an important component of plasma membranes of every brain cell. Its concentration in the CNS has been found to be higher than in any other tissue and accounts for 23% of the sterol of the whole body pool [22]. In cell membranes, cholesterol is asymmetrically distributed in the cytofacial and exofacial layer with higher concentration in the former ensuring fluidity and function [34].
Concentration and distribution of membrane cholesterol are tightly regulated by the cell [35] whereby around 75% of free cholesterol resides in the cytofacial layer and around 25% in the exofacial layer. Cholesterol plays an essential role in the regulation of synaptic function and plasticity [36]. In the exofacial leaflet of the lipid bilayer, microdomains (lipid rafts) are located that are enriched in cholesterol, glycosphingolipids and acylated proteins. Beside its essential role in signal transduction, they are assumed to be involved in processing of the APP [37–39].
The asymmetric distribution of cholesterol in the exofacial and cytofacial layers along with predominance of fatty acids in the exofacial layer maintains the distance between the two layers and, thus, the biophysical properties of membranes, also supported by the asymmetric distribution of the phospholipids, ethanolamine phosphoglyceride (PE) mainly facing the inside, and choline phoshoglyceride the outside of the membrane [40]. Intercalated in this fluid structure are proteins such as ion channels, receptors, membrane-bound enzymes etc [41]. Both structure and function of membranes have been found to be also ensured by a normal cellular energy state [42].
Cerebral membranes, cholesterol and aging
There is ample evidence to show that aging is the main risk factor for neurodegenerative processes such as sporadic AD. With aging, a multitude of inherent variations in fundamental metabolic processes mainly in glucose/energy metabolism, and in related metabolism, and its control are set into motion at the cellular, molecular and genetic levels which frequently result in functional imbalancies of regulative systems [43]. Age-related changes in membrane composition inclusive cholesterol will have to be discussed in more detail. Two different aspects may be of significance for membrane function: Loss of lipids, and displacement of constituents within the bilayer. In human brain, the concentrations of the major membrane lipids decreased by 18% and 21% (phospholipids), by 18% and 19% (cholesterol), and by 11% and 18% (gangliosides) in frontal and temporal cortices between 20 and 100 years of age [44, 45]. In another study, cholesterol started to fall beyond the age of 69 years in cerebral gray and white matter, and in very old age in the hippocampus, too [46]. In contrast, the cholesterol concentration did not change in cerebral cortex and hippocampus of 24-month-old (aged) rats [47]. With respect to the relation of unsaturated and saturated fatty acids in membranes, a shift was found in favour of the latter [48]. The decreased insertion of arachidonic acid in membrane lipids associated with an increase in arachidonoyl-CoA [49], and the increase of cholesterol in the exofacial layer associated with a reduction in the cytofacial layer altering cholesterol asymmetry [34] were found to be age-related changes. All together, these changes are in agreement to that normal aging changes both structure and function in brain membranes leading to varied function of e.g. ion channels, membrane fluidity, receptors, etc. The latter are markedly modified in structure [49a] and number comprising the insulinergic [43], acetylcholinergic [49b] glutamatergic [49c] and dopaminergic transduction systems [49d]. These changes may have marked impacts in the development of multifold disturbances accompanying neurodegenerative diseases in general and sporadic AD in particular.
Cholesterol metabolism in sporadic AD brain
One major metabolic abnormality in sporadic AD is perturbed cerebral glucose metabolism [50–52]. At the cellular level, the diminished cerebral glucose utilization may be mediated by reduced capacities of key enzymes working in glycolytic glucose breakdown leading to both reduced formation and oxidation of acetyl-CoA [53–55]. The reduced availability of acetyl-CoA has been found to reduce both the synthesis of acetylcholine in the presynaptic neuron [56] and the formation of ATP [57, 58]. In context with this article, both less acetyl-CoA and the depletion of ATP may have marked effects on the activity of HMG-CoA reductase which is inactivated by the ATP-dependent activation of the AMP-mediated kinase [59] although mRNA HMG-CoA reductase was not found to be modified [60]. This concerted action may cause a reduced level of cholesterol in brain tissue what is mirrored in the cerebral spinal fluid [61]. Since seladin-1 which participates in the formation of cholesterol has been demonstrated to be downregulated in AD brain [26], an increased synthesis of cholesterol in sporadic AD brain may not be assumed to occur. Otherwise, the cholesterol-catabolising enzyme CYP 46 has been found to be abnormaly induced in glial cells [62] the gene of which shows polymorphism associated with AD [63]. As a result of the increased cholesterol catabolism, its metabolite 24S-hydroxycholesterol was found to be enhanced in both plasma [64, 65] and CSF [66].
The depletion of ATP, as was found in SAD brain [57, 58], may have marked impacts on the maintenance of the non-equilibrium distribution of Na+, K+ and Ca2+ in that the intracellular concentrations of both Na+ and Ca2+ may rise whereas K+ is released from the cell. The increase of intracellular cytosolic Ca2+ concentration causes the activation of phospholipases which degrade phospholipids ([67], for review [68, 69]). Energy depletion has been assumed to enhance also the catabolism of membrane cholesterol [42].
Membrane composition in sporadic AD brain
It has been well documented in several studies that membranes undergo marked changes in sporadic SAD. First data were reported on the catabolic metabolites glycerophosphocholine and glycerophosphoethanolamine which both were found to be enhanced [70]. The degradation of structural membrane compounds such as phosphatidylcholine and phosphatidylethanolamine associated with an increase in both glycerophosphocholine and glycerophosphoethanolamine in a nearly stoichiometric relationship pointed to an increased activity of phospholipases [71]. Regional differences became obvious in the phospholipid contents as a whole in that decreases were found in frontal white matter and the caudate nucleus whereas the Alzheimer-specific areas frontal and temporal cortex and hippocampus showed insignificant decreases only [72]. Interestingly, the same authors reported on an Alzheimer-specific change of the fatty acid composition involving an increase of saturated and a decrease of unsaturated fatty acids [48]. Gangliosides were found to be reduced in temporal cortex, hippocampus and frontal white matter, but phospholipids did not show significant changes. Likewise, cholesterol decreased insignificantly by around 10% in temporal lobe and caudate nucleus [73]. A stronger reduction of brain membrane cholesterol by 30% [39] was found to be associated with an 50% increase in BACE1 in the soluble fractions [74]. Reduced cholesterol has been demonstrated to accentuate the membrane disturbing effects of βA4 on individual hippocampal membranes from AD patients [75, 76]. In AD brains exhibiting morphological changes of an early disease stage, GM1 ganglioside-bound βA4 was found [77] which, in in vitro-studies, showed a confirmation different from that of soluble βA4 and which accelerated the rate of amyloid fibril formation of soluble βA4 [78–80]. The increase in membrane-bound βA4 concentration triggered its conformational transition from helix-rich to β-sheet-rich structures [81]. Marked structural membrane changes in temporal gyrus membranes of AD patients became obvious by lipid and protein analyses showing an unchanged phospholipids: protein mass ratio but a decrease by 30% of the unesterified cholesterol: phospholipids ratio [82].
More detailed studies on cholesterol metabolism/concentration in sporadic AD brain revealed no uniform data. No differences were found in the 3-hydroxy-3-methylglutaryl-CoA reductase mRNA in Alzheimer and control brain pointing to a robust capacity to synthisize cholesterol in AD brain [60]. However, the activity of HMG-CoA reductase is reduced due to an energy-deficit [59]. The undisturbed or even elevated synthesis of cholesterol associated with its increased catabolism (see above) might explain the higher concentration/turnover of free cholesterol in (damaged) tangle-bearing neurons compared to adjacent tangle-free neurons [83]. These findings may indicate a dysregulation of cholesterol homeostasis what may include cholesterol metabolism in the membrane. The cholesterol-binding protein caveolin involved in cellular cholesterol transport has been found to be increased in both mRNA and protein [84]. As a result of this dysregulated metabolism, the depletion of cholesterol in the—normally cholesterol-enriched-lipid rafts—may be assumed [85, 86]. Both, dysregulation of metabolism and disorganization of membrane structure may have considerable impact on the function of proteins, and the formation of its metabolites such as βA 1–40 and βA 1–42 [87]. Otherwise, both, ceramides and cholesterol increased in association with disease severity in membranes of brain areas affected by AD (middle frontal gyrus) but not in a non-vulnerable brain region (cerebellum) [88]. Ceramides derive from sphingolipids which are enriched in membrane microdomains as cholesterol is (see above).
As a result in between, it may be deduced that both phospholipids and cholesterol in membranes diminish in sporadic AD brain. However, whereas the biochemical processes in the breakdown of membrane constituents by phospholipases due to acute pathological conditions such as ischemia and hypoglycaemia have been clearly demonstrated (for review [89]), there is no direct evidence of the maintenance of an elevated activity of phospholipases in chronic diseases such as sporadic AD, and in its post mortem state. The activity of the major catabolic enzyme phospholipase A2 was found to be decreased and the extend of the reduction correlated with the disease process [90]. Otherwise, the activities of lysophospholipid acyltransferase which recycles lysophospholipids into phospholipids, and glycerophosphocholine phosphodiesterase contributing to phospholipid resynthesis were found to be enhanced [90a] suggesting compensatory mechanism to reduce the primarily occurring phospholipid loss in the incipient disease state.
Plasma cholesterol and AD
Although the cholesterol metabolism in the brain has been found to be regulated independently from non-nervous tissues (see above), some findings point to an interrelationship between these two compartments. In a late-life/post mortem autopsy study, a strong linear association was found between increasing late-life HDL-cholesterol and increasing number of neuritic plaques in neocortex and hippocampus and neurofibrillary tangles in neocortex [91]. In contrast, AD patients revealed significantly higher LDL cholesterol and significantly lower HDL cholesterol related to the amount of βA 1–42, but not βA 1–40 in AD brains [92]. The latter findings may be in agreement with data showing that high cholesterol in late life was associated with a decreased dementia risk [8]. In a subject sample representing a cognitive continuum from normal cognitive function to mild dementia, low HDL-cholesterol was found to be associated with a higher risk of dementia whereas high HDL-cholesterol was associated with a larger hippocampal volume (less hippocampal atrophy as an index of AD pathology) and was assumed to be protective against dementia/AD [93].
The use of statins—a rational therapy for Alzheimer disease?
Pharmacology
Statins are inhibitors of the 3-hydroxy-3-methylglutaryl-CoA reductase, representing the rate-limiting enzyme in cholesterol biosynthesis. Statins exist in two different forms: (1) the lactone form is lipophilic comprising e.g. lovastatin, simvastatin and cerivastatin which are able to pass the blood–brain barrier; (2) the acid from is hydrophilic comprising e.g. atorvastatin, fluvastatin and pravastatin which do not pass the blood–brain barrier to any significant extent [94, 95]. Both lovastatin and simvastatin were found to reduce the cholesterol content in the cytofacial membrane leaflet, and lovastatin in the exofacial membrane leaflet, too. Interestingly, pravastatin—although not passing the blood–brain barrier—reduced cholesterol in the exofacial membrane leaflet, and was also shown to affect gene regulation in the brain [96, 97].
Effects on membranes, tau-protein and APP/β-amyloid in experimental in vitro- and in vivo-studies
Lovastatin treatment (100 mg/kg/day) over a period of 23 days caused a marked reduction in brain cholesterol content associated with decreased pyrene-excimer fluorescence indicating altered membrane function in young and middle-aged mice [98]. This cholesterol-induced reduced membrane lipid fluidity was assumed to dysregulate membrane-bound allosteric enzymes, membrane permeability and to modulate the phospholipid–protein interaction [99, 100]. Treatment of hippocampal neurons with lovastatin (≥10 μM) induced cell death within 72 h [101]. The latter is a generally observed phenomenon after reduction of cholesterol [20]. Before complete destroyment of the neuritic network a decrease in dendritic outgrowth, attenuated axonal branching and depolymerization of microtubules associated with hyperphosphorylation of tau protein were observed [101–103].
In numerous studies, a combination of statins and methyl-β-cyclodextrin of both different concentrations and duration was used to markedly reduce the cholesterol level in membranes and to induce effects on APP/β-amyloid metabolism. Methyl-β-cyclodextrins have been demonstrated to selectively extract cholesterol from plasma membranes [104, 105], i.e. the application of both compounds may cause a harsh membrane damage. In vivo- and in vitro-studies showed that a reduction of membrane cholesterol content deteriorated membrane properties such as fluidity, and that the cholesterol content of membranes was negatively correlated with the membrane perturbing effect of β-amyloid [76]. Synaptic plasticity was impaired in that the formation of neurodegenerative fragmentation and teardrop varicose widening of neurites were found to be associated with a damage of long-term potentation [33]. However, most studies focus on the relationship between membrane cholesterol content and β-amyloid formation to demonstrate the beneficial effect of statins as a therapeutic strategy in the treatment of AD. In different cell lines (HEK cells, neuroblastoma SH-SY5Y cells, human astroglioma cells, primary neurons), around 60% of cholesterol was removed from the cell by methyl-β-cyclodextrin or was reduced by around 50% by lovastatin. This harsh membrane damage resulted in a drastic increase of secreted-α-secretase cleaved soluble APP, in a decreased secretion of βA4-peptides by around 20% and in increase membrane fluidity. It is deduced from the data that cholesterol reduction promotes the non-amyloidogenic α-secretase pathway and that this strategy may be useful for the prevention of or therapy for AD [106]. In other studies, α-secretase cleaved APP (secreted APP) was found to be unchanged or reduced [107, 108] and βA4-formation was reduced between 50% and 70% when cellular cholesterol was diminished by 50–70% by lovastatin, or methyl-β-cyclodextrin, or by both. It was concluded that cholesterol is required for the formation of βA4 and that this process may be inhibited by the depletion of cholesterol [108, 109]. However, in studies more relevant to the reduction of cholesterol in sporadic AD brain membranes (see above), the latter was diminished by 30% only what was accompanied by an increase in β-secretase, a higher β-secretase/APP colocalization and an increased Aβ-production. In contrast, a harsh reduction of cholesterol by more than 35% of control induced less Aβ-production most likely due to an overall marked disruption of membrane integrity [74, 109a], not described in postmortem AD brain (see above).
In most of the in vivo-studies investigating the cholesterol-lowering effects of statins transgenic animals were used. The results are incomplete (e.g. no determination of cholesterol content) and with respect to βA4-formation not uniform: reduction of the latter up to 60% were found as well as no changes, and increases up to 50% (for review [110]). In one study only, wild-type adult male guinea pigs were used which received simvastatin over three weeks in a 227–407 times higher dose than applied in human beings [111]. Whereas the plasma cholesterol level was found to be markedly reduced (16% of control), no significant change in total brain cholesterol level was found. However, the cholesterol precursor lathosterol was diminished indicating a reduction of de novo brain cholesterol synthesis. Simvastatin treatment reduced βA4 concentration in both CSF and brain tissue between around 50–60%.
Taken together, the results from the in vitro- and in vivo-studies discussed above do not provide evidence for the assumption that an enhanced cholesterol content in membranes increased βA4-production. All experiments started in normal conditions i.e. normal cholesterol concentration. The application of statins in combination with, or without methyl-β-cyclodextrin, caused “beneficial” effects of APP metabolism associated with reduced βA4-production first when the cholesterol content of membranes was reduced by 50–60% of normal. Applied to AD brain, the abnormal but moderate decrease of cholesterol (around −15% to −30%) might have to be diminished about 2-to 3-fold stronger to achieve a “beneficial” effect on APP/βA4-metabolism. It is not clear as yet whether or not a neuron would survive such a harsh procedure.
Another aspect might also be taken in account. APP (and presenilin) mutations are found to be rare in AD brain, and the hereditary form of the disease represents a very small proportion of AD patients only (see Introduction). Mutated APP used in cell culture studies or in transgenic animals is produced in excess causing an increased amount of cleaved APP, and, thus, an increased formation of Aβ. This process may be diminished by the harsh membrane damage due to cholesterol reduction by more than 35% of normal. This experimental condition may not be assumed to mirror the pathology of sporadic AD brain membranes.
Effects of statins in the treatment of dementia in human beings
Sporadic AD is the most frequent form of all dementias in the elderly, but other causations than that for sporadic AD (the causation(s) of which is (are) as yet unknown) will have to be considered for treatment strategies. To give one example only: vascular dementia is different from sporadic AD in origin, and, thus, different therapeutic strategies may have to be applied.
Studies on unclassified dementias
The most frequently cited articles in this context are from Wolozin et al. [112] and Jick et al. [113]. The latter retrospective study included 284 subjects who had a first-time diagnosis, 84% of whom were classified as having possible or probable AD. Different statins were applied over a period of more than four years. This treatment was associated with a substantial lowered risk of developing dementia whereby it was emphasized that no distinction between AD and other forms of dementia was possible. Wolozin et al. [112] evaluated the records of nearly 57,000 patients in a cross-sectional analysis retrospectively. The diagnoses were made by documentation of cognitive impairment, neuropsychological examination, computed tomography and magnetic resonance imaging of the brain. The diagnosis of AD referred to probable AD and did not exclude confounding vascular disease factors, i.e. a population of mixed causes of dementias was included in the evaluation comprising 753 patients both taking and not taking medications. As a result, a lower prevalence (60–73%) of dementia of the cohort taking statins during the study interval (October 1996 through August 1998) was found. In a subsequent case–control and retrospective cohort study on patients diagnosed as suffering from hypercholesterolemia or dementia (AD, vascular dementia, mixed-type dementia, Lewy body dementia), patients on statins showed an improvement on their MMSE score by 0.7 as compared to a decline by 0.5 in controls. The data also suggested that the use of statins was associated with a lower prevalence of vascular dementia and AD [114].
None of the above studies gave information on drugs used additionally to treat the different types of dementias.
Studies on classified dementias
In smaller samples of well diagnosed AD patients (n = 44), CSF markers such as βA40, βA42, tau-protein, lathosterol and cholesterol were included in the studies. A 26-week treatment with 80 mg/day simvastatin reduced serum cholesterol by around 50%, CFS lathosterol by around 10% and CSF 24S-hydroxycholesterol by around 10%. The patients were allowed to take acetylcholinesterase blockers during the 26-week study period. In a subgroup of mildly diseased AD patients (MMSE21-26: n = 8), CSF βA40 fell significantly accompanied by no changes in CSF βA42. Simvastatin treatment maintained the MMSE score at baseline level [115]. Nineteen AD-patients received simvastatin (20 mg/day) for 12 weeks in an open trial, leading to a slight increase of the ADAS-cog score, a decrease in serum total cholesterol and LDL cholesterol, and a reduction of both the α-secretase-cleaved and the β-secretase-cleaved APP, but without any variations in CSF βA42, tau-protein, and phospho-tau-protein [116], largely confirmed in a small, 1 year open-label study [117]. Sixty seven patients with mild to moderate AD were included in a 1-year pilot proof-of-concept, double blind, placebo-controlled, randomized study and treated with atorvastatin known not to pass the blood–brain barrier. The drug treatment was 80 mg/day atorvastatin while continuing to take cholinesterase inhibitors. Atorvastatin caused decreases in serum total cholesterol, LDL-cholesterol and VLDL-cholesterol. Improvement on the ADAS-cog score were observed after a 6-months treatment first what was maintained until 12 months. No significant changes were found during the period of 1-year treatment in several other psychometric test procedure applied [118].
The data of the above studies do not support the assumption that the application of statins may be of benefit for AD patients. Remarkably, the clinical outcome of AD patients after a long-term treatment [115, 118] was very limited in improvement (8 out of 40 patients in [115]) and significance at the level of a trend [118]. These results are somewhat surprising in so far that a stronger improvement of clinical symptoms has been found in many studies using acetylcholinesterase inhibitors only. Therefore, drug interactions may not be excluded causing a reduced efficacy of acetyl-cholinesterase inhibitors by statins.
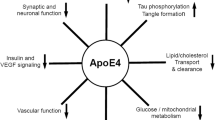
To summarize, the data discussed above do not support the assumption that the application of statins may prevent or treat AD and may inhibit β-amyloid production [119–122]. In contrast, statin treatment intensifies the disease-induced cholesterol deficits in membranes rendering the latter prone to collaps (Fig. 1). Otherwise, statins may be helpful in the treatment of vascular dementia and vascular-related cognitive impairment associated with cardiovascular disease and hypercholesterolemia, i.e. in secondary dementia [122].
References
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road of therapeutics. Science 297:353–356
de Quervain DJF, Poirier R, Wollmer MA, Grimaldi L M.E., Tsolaki M, Streffer JR, Hock C, Nitsch RM, Mohajeri MH, Pappassotiropoulos A (2004) Glucocorticoid-related genetic susceptibility for Alzheimer’s disease. Human Mol Genet 13:47–52
Tanzi RE, Bertram L (2001) New frontiers in Alzheimer’s disease genetics. Neuron 32:181–184
Davignon J, Gregg RE, Sing CF (1988) Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis 8:1–21
Mahley RW, Rall SC Jr (2000) Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genom Hum Genet 1:507–537
Hoshino T, Kamino K, Matsumoto M (2002) Gene dose effect of the APOE-epsilon 4 allele on plasma HDL cholesterol level in patients with Alzheimer’s disease. Neurobiol. Aging 23:41–45
Mainous AG III, Eschenbach SL, Well BJ, Everett CJ, Gill JM (2005) Fam Med 37:36–42
Mielke MM, Zandi PP, Sjogren M, Gustafson D, Ostling S, Steen B, Skoog I (2005) High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology 64:1689–1695
Dufouil C, Richard F, Fieret N, Dartigues JF, Ritchie K, Tzourio C, Amouyel P, Alferovitch A (2005) APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: the Three-City Study. Neurology 64:1531–1538
Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K (2005) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64:277–281
Jarvik GP, Wijsman EM, Kukull WA, Schellenberg GD, Yu C, Larson EB (1995) Interactions of apolipoprotein E genotype total cholesterol level, age, and sex in prediction of Alzheimer’s disease: A case–control study. Neurology 45:1092–1096
Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, Tuomilehto J, Nissinen A (1998) Serum total cholesterol, apolipoprotein E Σ4 allele, and Alzheimer’s disease. Neuroepidemiology 17:14–20
Kivipelto M, Helkala EL, Laakso MP, Hänninen T., Hallikainen M Alhainen K, Iievenen S, Mannermaa A, Tuomilhto J, Nissinen A, Soininen H (2002) Apolipoprotein E Σ4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med 137:149–155
Kivipelto M, Helkala EL, Laakso MP, Hänninen T., Hallikainen M, Alhainen K, Soininen H, Tuomiletho J, Nissien A (2001) Vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. Br Med J 322:1447–1451
Refolo LM, Pappolla MA, Malester B, La Francois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K (2000) Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis 7:321–331
Refolo LM, Wittenberg IS, Friedrich VL Jr, Robaki NK (1991) The Alzheimer amyloid precursor is associated with the detergent-insoluble cytoskeleton. J Neurosci 11:3888–3897
Lee SJ, Liyanage U, Bickel PE, Xia R, Landsbury PT Jr, Kosik KS (1998) A detergent-insoluble membrane compartment contains Aβ in vivo. Nat Med 4:730–734
Puglielli L, Tanzi RE, Kovacs DM (2003) Alzheimer’s disease: the cholesterol connection. Nat Neurosci 6:345–351
Joseph J, Shukitt-Hale B, Denisova NA, Martin A, Perry G, Smith MA (2001) Copernicus revisited: amyloid beta in Alzheimer’s disease. Neurobiol Aging 22:131–146
Michikawa M, Yanagisawa K (1999) Inhibition of cholesterol production but not of nonsterol isoprenoid products induces neuronal cell death. J Neurochem 72:2278–2285
Vance JE, Hayashi H, Karten B (2005) Cholesterol homeostasis in neurons and glial cells. Semin Cell Dev Biol 16:193–212
Dietschy JM, Turley SD (2004) Thematic review series: brain lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res 45:1375–1397
Mauch DH, Nägler J, Schumacher S, Göritz EC, Otto A, Pfrieger FW (2001) CNS synaptogenesis promoted by glia-derived cholesterol. Science 294:1354–1357. (a) Handelmann GE, Boyles JK, Weisgraber KH, Mahley RH, Pitas RE (1992) Effects of apolipoprotein Eβ, very low-density lipoproteins, and cholesterol on the extension of neurites by rabbit dorsal root ganglion neurons in vitro. J Lipid Res 33:1677–1688. (b) Levi O, Lütjohann D, Devir A, von Bergmann K, Hartmann T, Michaelson DM (2005) Regulation of hippocampal cholesterol metabolism by apoE and environmental stimulation. J Neurochem. 95:987–997
Jurevics H, Hostettler J, Barrett C, Morell P, Toews AD (2000) Diurnal and dietary-induced changes in cholesterol synthesis correlate with levels of mRNA for HMG-CoA reductase. J Lipid Res 41:1048–1054
Kirsch C, Eckert GP, Koudinov AR, Müller W.E. (2003) Brain cholesterol, statins and Alzheimer’s disease. Pharmacopsychiatry 36(Suppl. 2): S113–S119
Greeve I, Hermans-Borgmeyer I, Brellinger C, Kasper D, Gomez-Isla T, Behl C, Levkau B, Nitsch RM (2000) The human DIMINUTO/DWARF 1 homolog seladin-1 confers resistance to Alzheimer’s disease-associated neurodegeneration and oxidative stress. J Neurosci 20:7345–7352
Pettegrew JW, Klunk WE, Panchalingam K, McClure RJ, Stanley JA (2000) Molecular insights into neurodevelopmental and neurodegenerative diseases. Brain Res Bull 53:455–469
Hoyer S (2004) Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol 490:115–125
Lütjohann D., Breuer O, Ahlborg G Nennesmo I, Siden A, Diczfalusy U, Björkhem I. (1996) Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc Natl Acad Sci USA 93:9799–9804
Björkhem I., Lütjohann D., Diczfalusy U, Stahle L, Ahlborg G, Wahren J (1998) Cholesterol homeostais in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J Lipid Res 39:1594–1600
Lund EG, Guileyardo JM, Russell DW (1999) cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc Natl Acad Sci USA 96:7238–7243
Rupprecht R, Holsboer F (1999) Neuroactive steroids: mechanism of action and neuropsychopharmacological perspectives. Trends Neurosci 22:410–416
Koudinov AR, Koudinova NV (2001) Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J 15:1858–1860
Igbavboa U, Avdulov NA, Schröder F., Wood WG (1996) Increasing age alters transbilayer fluidity and cholesterol asymmetry in synaptic plasma membranes of mice. J Neurochem 66:1717–1725
Liscum L, Munn NJ (1999) Intracellular cholesterol transport. Biochim Biophys Acta 1438:19–37
Schroeder F, Frolov AA, Murphey EJ, Atshaves BP, Jefferson JR, Pu LX, Wood WG, Foxworth WB, Kier AB (1996) Recent advances in membrane cholesterol domain dynamics and intracellular cholesterol trafficking. Proc Soc Exp Biol Med 213:150–177
Parkin ET, Turner AJ, Hooper NM (1999) Amyloid precursor protein, although partially detergent-insoluble in mouse cerebral cortex, behaves as an atypical lipid raft protein. Biochem J 344:23–30
Simons K, Toomre D, (2000) Lipid rafts and signal transduction. Nature Rev Mol Cell Biol 1:31–39
Ledesma MD, Da Silva JS, Schevchenko A, Wilm M, Doffi CG (2003) Proteomic characterisation of neuronal sphingolipid-cholesterol microdomains: role in plasminogen activation. Brain Res 987:107–116
Hirata F, Axelrod J (1980) Phospholipid methylation and biological signal transmission. Science 209:1082–1090
Spector AA, Yorek MA (1985) Membran lipid composition and cellular function. J Lipid Res 26:1015–1035
Wu Y, Sun FF, Tong DM (1996) Changes in membrane properties during energy depletion-induced cell injury studied with fluorescence microscopy. Biophys J 71:91–100
Hoyer S, Frölich L (2006) Brain insulin function and insulin signal transduction in sporadic Alzheimer’s disease. In Sun MK (ed) Research progress in Alzheimer’s disease and dementia. Nova Science, New York (in press)
Svennerholm L Boström K, Helander CG, Jungbjer B (1991) Membrane lipids in the aging human brain. J Neurochem 56:2051–2059
Svennerholm L, Boström K, Jungbjer B, Olsson L (1994) Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J Neurochem 63:1802–1811
Söderberg M., Edlund C, Kristensson K, Dallner G (1990) Lipid composition of different regions of the human brain during aging. J Neurochem 54:415–423
Zhang Y, Appelkvist EL, Kristensson K, Dallner G (1996) The lipid composition of different regions of rat brain during development and aging. Neurobiol Aging 17:869–875
Söderberg M, Edlung C, Kristensson K, Dallner G (1991) Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids 26:412–425
Terracina L, Brunetti M, Avellini L, DeMedio GE, Trovarelli G, Gaiti A (1992) Arachidonic and palmitic acid utilization in aged rat brain areas. Mol Cell Biochem 115:35–42. (a) Changeux JP, Danchin A (1976) Selective stabilisation of developing synapses as a mechanism for the specification of neuronal network. Nature 264:705–712. (b) Lippa AS, Critchett DJ, Ehlert F, Yamamura HI, Enna SJ, Bartus RT (1981) Age-related alterations in neurotransmitter receptors: an electrophysiological and biochemical analysis. Neurobiol. Aging 2:3–8. (c) Baudry M, Arst DS, Lynch G (1981) Increased (3H) glutamate receptor binding in aged rats. Brain Res 223:195–198. (d) Seemann P, Bzowei NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, Bird ED, Riederer P, Jelliinger K, Watanabe W, Tourtellotte WW (1987) Human brain dopamine receptors in children and aging adults. Synapse 1:399–404
Hoyer S, Nitsch R, Oesterreich K (1991) Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer-type: a cross-sectional comparison against advanced late-onset dementia and incipient early-onset cases. J Neural Transm (PD-Sect) 3:1–14
Mielke R, Herholz K, Grond M, Heiss WD (1994) Clinical deterioration in probable Alzheimer’s disease correlates with progressive metabolic impairment of association areas. Dementia 5:36–41
Mielke R, Herholz K, Grond M, Kessler J, Heiss WD (1992) Differences of regional cerebral glucose metabolism between presenile and senile dementia of Alzheimer type. Neurobiol Aging 13:93–98
Perry EK, Perry RG, Tomlinson BE, Blessed G, Gibson PH, (1980) Coenzyme A-acetylating enzymes in Alzheimer’s disease: possible cholinergic “compartment” of pyruvate dehydrogenase. Neurosci Lett 18:105–110
Sorbi S, Bird ED, Blass JP (1983) Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol 13:72–78
Bigl M, Brückner M.K., Arendt T Bigl V, Eschrich K (1999) Activities of key glycolytic enzymes in the brains of patients with Alzheimer’s disease. J Neural Transm 106:499–511
Sims NR, Bowen DM, Allen SJ, Smith CCT, Neary D, Thomas DJ, Davison AN (1983) Presynaptic cholinergic dysfunction in patients with dementia. J Neurochem 40:503–509
Sims NR, Bowen DM, Neary D, Davison AN (1983) Metabolic processes in Alzheimer’s disease: adenine nucleotide content and production of 14C02 from (U14C) glucose in vivo in human neocortex. J Neurochem 41:1329–1335
Hoyer S (1992) Oxidative energy metabolism in Alzheimer brain. Studies in early-onset and late-onset cases. Mol Chem Neuropathol 16:207–224
Corton JM, Gillespie JG, Hardie DG (1994) Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol 4:315–324
Yasojima K, McGeer EG, McGeer PL (2001) 3-hydroxy-3-methylglutaryl-coenzyme A reductase mRNA in Alzheimer and control brain. NeuroReport 12:2935–2938
Mulder M, Ravid R, Swaab DF, deKloet ER, Haasdijk ED, Julk J, van der Boom J, Havekes LM (1998) Reduced levels of cholesterol, phospholipids, and fatty acids in cerebrospinal fluid of Alzheimer disease patients are not related to apolipoprotein E4. Alzheimer Dis Assoc Disord 12:198–203
Bogdanovic N, Bretillon L, Lund EG, Diczfalusy U, Lannfelt L, Winblad B, Russel DW, Björkhem I (2001) On the turnover of brain cholesterol in patients with Alzheimer’s disease. Hormonal induction of the cholesterol-catabolic enzyme CYP 46 in glial cells. Neurosci Lett 314:45–48
Kölsch H, Lütjohann D, Ludwig M, Schulte A, Ptok V, Jessen F, von Bergmann K, Rao ML, Maier W, Heun R (2002) Polymorphism in the cholesterol 24S-hydroxylase gene is associated with Alzheimer’s disease. Mol Psychiatr 7:899–902
Lütjohann D, Papassotiropoulos A, Björkhem I, Locatelli S, Bagli M, Oehring RD, Schlegel U, Jessen F, Rao ML, von Bergmann K, Heun R, (2000) Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J Lipid Res 41:195–198
Papassotiropoulos A, Lütjohann D., Bagli M, Locatelli S, Jessen F, Rao ML, Maier W, Björkhem I., von Bergmann K, Heun R (2000) Plasma 24S-hydroxycholesterol: a peripheral indicator of neuronal degeneration and potential state marker of Alzheimer’s disease. NeuroReport 11:1959–1962
Schönknecht P, Lütjohann D, Pantel J, Bardenheuer H, Hartmann T, von Bergmann K, Beyreuther K, Schröder J (2002) Cerebrospinal fluid 24S-hydroxycholesterol is increased in patients with Alzheimer’s disease compared to healthy controls. Neurosci Lett 324:83–85
Cooper MF, Webster GR (1970) The differentiation of phospholipase A1 and A2 in rat and human nervous tissues. J Neurochem 17:1543–1554
Siesjö B.K. (1981) Cell damage in the brain: A speculative synthesis. J Cereb Blood Flow Metab 1:155–185
Erecinska M, Silver IA (1989) ATP and brain function. J Cereb Blood Flow Metab 9:2–19
Pettegrew JW, Moossy J, Withers G, McKeag D, Panchalingam K (1988) 31P nuclear magnetic resonance study of the brain in Alzheimer’s disease. J Neuropathol Exp Neurol 47:235–248
Nitsch RM, Blusztajn JK, Pittas AG, Slack BE, Growdoin JH, Wurtman RJ (1992) Evidence for a membrane defect in Alzheimer disease brain. Proc Natl Acad Sci USA 89:1671–1675
Söderberg M, Edlund C, Alafuzoff I, Kristensson K, Dallner G (1992) Lipid composition in different regions of the brain in Alzheimer’s disease/senile dementia of Alzheimer’s type. J Neurochem 59:1646–1653
Svennerholm L, Gottfries CG (1994) Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset (type II). J Neurochem 62:1039–1047
Abad-Rodriguez J, Ledesma MD, Craessaerts K, Perga S, Medina M, Delacourte A, Dingwall C, de Stooper B, Dotti CG (2004) Neuronal membrane cholesterol loss enhances amyloid peptide generation. J Cell Biol 167:953–960
Eckert GP, Cairns NJ, Maras A, Gattaz WF, Müller WE (2000) Cholesterol modulates the membrane-disordering effects of beta-amyloid peptides in the hippocampus: specific changes in Alzheimer’s disease. Dement Geriatr Cogn Disord 11:181–186
Kirsch C, Eckert GP, Mueller WE (2002) Cholesterol attenuates the membrane perturbing properties of β-amyloid peptides. Amyloid 9:149–159
Yanagisawa K, Odaka A, Suzuki N, Ihara Y (1995) GM1 ganglioside-bound amyloid β-protein (Aβ): A possible form of preamyloid in Alzheimer’s disease. Nat Med 1:1062–1066
McLaurin J, Chakrabartty A, (1996) Membrane disruption by Alzheimer β-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. J Biol Chem 271:26482–26489
McLaurin J Franklin T, Fraser PE, Chakrabartty A (1998) Structural transitions associated with the interaction of Alzheimer β-amyloid peptides with gangliosides. J Biol Chem 273:4506–4515
Yanagisawa K, Ihara Y (1998) GM1 ganglioside-bound amyloid β-protein in Alzheimer’s disease brain. Neurobiol Aging 19:S65–S67
Kakio A, Nishimoto S, Yanagisawa K, Kozutsumi Y, Matsuzaki K (2001) Cholesterol-dependent formation of GM1 ganglioside-bound amyloid β-protein, an endogenous seed for Alzheimer amyloid. J Biol Chem 276:24985–24990
Mason RP, Shoemaker WJ, Shajenko L, Chambers TE, Herbette LG (1992) Evidence for changes in the Alzheimer’s disease brain cortical membrane structure mediated by cholesterol. Neurobiol Aging 13:413–419
Distl R, Meske V, Ohm TG (2001) Tangle-bearing neurons contain more free cholesterol than adjacent tangle-free neurons. Acta Neuropathol 101:547–554
Gaudreault SB, Dea D, Poirier J (2004) Increased caveolin-1 expression in Alzheimer’s disease brain. Neurobiol Aging 25:753–759
Ledesma MD, Abad-Rodriguez J, Galvan C, Biondi E, Navarro P, Delacourte A, Dingwall C, Dotti CG (2003) Raft disorganization leads to reduced plasmin activity in Alzheimer’s disease brains. EMBO Rep 4:1190–1196
Molander-Melin M, Blennow K, Bogdanovic N, Dellheden B, Mansson JE, Fredman P (2005) Structural membrane alterations in Alzheimer brains found to be associated with regional disease development; increased density of gangliosides GM1 and GM2 and loss of cholesterol in detergent-resistant membrane domains. J Neurochem 92:171–182
Roher AE, Weiss N Kokjohn TA, Kuo YM, Kalback W, Anthony J, Watson D, Luehrs DC, Sue L, Walker D, Emmerling M, Goux W, Beach T (2002) Increased A beta peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer’s disease. Biochemistry 41:11080–11090
Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncorso JC, Mattson MP (2004) Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci USA 101:2070–2075
Klein J (2000) Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Neural Transm 107:1027–1063
Gattaz WF, Maras A, Cairns NJ, Levy R, Förstl H (1995) Decreased phospholipase A2 activity in Alzheimer brains. Biol Psychiatr 37:13–17. (a) Ross BM, Moszczynska A, Erlich J, Kish SJ (1998) Phospholipid-metabolizing enzymes in Alzheimer’s disease: increased lysophospholipid acyltransferase activity and decreased phospholipase A2 activity. J Neurochem 70:786–793
Launer LJ, White LR, Petrovitch H, Ross GW, Curb JD (2001) Cholesterol and neuropathologic markers of AD. A population-based autopsy study. Neurology 57:1447–1452
Kuo YM, Emmerling MR, Bisgaier CL, Essenburg AD, Lampert HC, Drumm D, Roher AE, (1998) Elevated low-density lipoprotein in Alzheimer’s disease correlates with brain Aβ 1–42 levels. Biochem Biophys Res Commun 252:711–715
Wolf H, Hensel A, Arendt T, Kivipelto M, Winblad B, Gertz HJ (2004) Serum lipids and hippocampal volume: the link to Alzheimer’s diease? Ann. Neurol 56:745–749
Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F (1999) New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther 84:413–428
Hamelin BA, Turgeon J (1998) Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharmacol Sci 19:26–37
Johnson-Anuna LN, Eckert GP, Keller JH, Igbavboa U, Franke C, Fechner T, Schubert-Zsilavecz M, Karas M, Müller W.E., Wood WG (2005) Chronic administration of statins alters multiple gene expression patterns in mouse cerebral cortex. J Pharmacol Exp Ther 312:786–793
Kirsch C, Eckert GP, Mueller WE (2003) Statin effects on cholesterol micro-domains in brain plasma membranes. Biochem Pharmacol 65:843–856
Eckert GP, Kirsch C, Mueller WE (2001) Differential effects of lovastatin treatment on brain cholesterol levels in normal and ApoE-deficient mice. NeuroReport 12:883–887
Papahadjopoulos D, Cowden M, Kimelberg H (1973) Role of cholesterol in membranes. Effects on phospholipids-protein interaction, membrane permeability and enzyme activity. Biochim Biophys Acta 330:8–26
Farias RN, Bloj B, Morero RD, Sineriz F, Trucco RE (1975) Regulation of allosteric membrane-bound enzymes through changes in membrane lipid composition. Biochim Biophys Acta 415:231–251
Meske V, Albert F, Richter D, Schwarze J, Ohm TG (2003) Blockade of HMG-CoA reductase activity causes changes in microtubule-stabilizing protein tau via suppression of geranylgeranylphosphate formation: implications for Alzheimer’s disease. Eur J Neurosci 17:93–102
Fan QW, Yu W, Senda T, Yanagisawa J, Michikawa M (2001) Cholesterol-dependent modulation of tau phosphorylation in cultured neurons. J Neurochem 76:391–400
Fan QW, Yu W, Gong JS, Zou K, Sawamura N, Senda T, Yanagisawa K, Michikawa M (2002) Cholesterol-dependent modulation of dendrite outgrowth and microtubule stability in cultured neurons. J Neurochem 80:178–190
Kilsdonk EPC, Yancey PG, Stoudt WG, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GJ (1995) Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem 270:17250–17256
Neufeld EB, Cooney AM,Pitha J, Dawidowicz EA, Dwyer NK, Pentchev PG, Blanchette-Mackie EJ (1996) Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem 271:21604–21613
Kojro E, Gimpl G, Lammich S, März W, Fahrenholz F (2001) Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α-secretase ADAM 10. Proc Natl Acad Sci USA 98:5815–5820
Bodovitz S, Klein WL (1996) Cholesterol modulates α-secretase cleavage of amyloid precursor protein. J Biol Chem 271:4436–4440
Simons M, Keller P, de Strooper B, Beyreuther K, Dotti CG, Simons K (1998) Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc Natl Acad Sci USA 95:6460–6464
Frears ER, Stephens DJ, Walters CE, Davies H, Austen BM (1999) The role of cholesterol in the biosynthesis of beta-amyloid. NeuroReport 10:1699–1705. (a) Hao M, Mukherjee S, Maxfield FR (2001) Cholesterol depletion induces large scale domain segregation in living cell membranes. Proc Natl Acad Sci USA 98:13072–13077
Eckert GP, Wood WG, Müller WE (2005) Statins: drugs for Alzheimer’s disease? J Neural Transm 112:1057–1071
Fassbender K, Simons M, Bergmann C, Stroick M, Lütjohann D., Keller P, Runz H, Kühl S, Bertsch T, von Bergmann K, Hennerici M, Beyreuther K, Hartmann T (2001) Simvastatin strongly reduces levels of Alzheimer’s disease β-amyloid peptides Aβ42 and Aβ40 in vitro and in vivo. Proc Natl Acad Sci USA 98:5856–5861
Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G (2000) Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arch Neurol 57:1439–1443
Jick H, Zornberg GL, Jick SS, Seshradi S, Drachman DA (2000) Statins in the risk of dementia. Lancet 350:1627–1631
Hajjar I, Schumpert J, Hirth V, Wieland D, Eleazer GP (2002) The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J Gerontol 57A: M414-M418
Simons M, Schwärzler F., Lütjohann D., von Bergmann K, Beyreuther K, Dichgans J, Wormstall H, Hartmann T, Schulz JB (2002) Treatment with simvastatin in normocholesterolemic patients with Alzheimer’s disease: A26-week randomized, placebo-controlled, double-blind trial. Ann Neurol 52:346–350
Sjögren M, Gustafsson K, Syversen S, Olsson A, Edman A, Davidsson P, WAllin A, Blennow K (2003) Treatment with simvastatin in patients with Alzheimer’s disease lowers both α- and β-cleaved amyloid precursor protein. Dement Geriatr Cogn Disord 16:25–30
Hoglund K, Thelen KM, Syversen S, Sjogren M, von Bergmann K, Wallin A, Vanmechelen E, Vanderstichele H, Lütjohann D, Blennow K (2005) The effect of simvastatin treatment on the amyloid precursor protein and brain cholesterol metabolism in patients with Alzheimer’s disease. Dement Geriatr Cogn Dis 19:256–265
Sparks DL, Sabbagh MN, Connor DJ, Lopez K, Launer LJ, Browne P, Wasser D, Johnson-Traver S, Lochhead J, Ziolwolski C (2005) Atorvastatin for the treatment of mild to moderate Alzheimer disease. Preliminary results. Arch Neurol 62:753–757
Kivipelto M, Solomon A, Winblad B (2005) Statin therapy in Alzheimer’s disease. Neurol Lancet 4:521–522
Wolozin B (2004) Cholesterol, statins and dementia. Curr Opin Lipidol 15:667–672
Hartmann T (2005) Cholesterol and Alzheimer’ disease: statins, cholesterol depletion in APP processing and Abeta generation. Subcell Biochem 38:365–380
Suribhatla S, Dennis MS, Potter JF (2005) A study of statin use in the prevention of cognitive impairment of vascular origin in the UK. J Neurol Sci 229–230:147–150
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor John P. Blass.
Rights and permissions
About this article
Cite this article
Hoyer, S., Riederer, P. Alzheimer Disease—No Target for Statin Treatment. A Mini Review. Neurochem Res 32, 695–706 (2007). https://doi.org/10.1007/s11064-006-9168-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9168-x