Abstract
Spinal cord injury (SCI) caused by trauma mainly occurs in two mechanisms as primary and secondary injury. Secondary injury following the primary impact includes various pathophysiological and biochemical events. Methylprednisolone is the only pharmacological agent having clinically proven beneficial effects on SCI. Citicoline has been shown to have clinical and experimental beneficial effects on brain ischemia. This study aims to investigate the neuroprotective effect of citicoline in an experimental SCI model in rats. Sixty adult Wistar albino rats were randomized into five groups. SCI was performed by the weight-drop model. Group 1 underwent laminectomy alone. The Group 2 underwent laminectomy followed by SCI and received no medication. Group3, Group 4 and Group 5 underwent laminectomy followed by SCI and received medication. Group 3 and Group 5 received citicoline and Group 4 and Group 5 received methylprednisolone. The rats were divided into two subgroups for biochemical analysis (sacrificed at 24 h after surgery) and neurobehavioral and histopathological evaluation (sacrificed at 6 weeks after surgery). Malonildialdehyde levels, nitric oxide levels and trauma size ratios were lower and reduced glutathione levels were higher in Group 3, Group 4 and Group 5 as compared to Group 2. Posttraumatic neurological recovery after surgery was significantly better in Group 3, Group 4 and Group 5 compared to Group 2. In conclusion, this study demonstrates that citicoline is as effective as methylprednisolone. The efficacy of citicoline combined with methylprednisolone is not superior to either citicoline or methylprednisolone alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) is still a major health problem. Mechanical impact to the spinal cord is referred to as primary injury, which may cause the death of a number of neurons. Primary injury cannot be avoided. Mechanisms leading to secondary injury are initiated immediately following primary injury. The pathophysiology of secondary injury is complex and not exactly understood. Excitotoxicity plays an important role in the secondary injury processes after SCI. Pathological changes seen after SCI include edema, altered blood flow and changes in microvascular permeability. Secondary damage is determined by a large number of cellular, molecular and biochemical cascades and neuronal death may be caused by substances released from the cells in response to primary injury [1]. The extent of the tissue damage and cell death has been shown to be related to the recovery following SCI [2, 3]. In recent years, much attention has been focused on secondary injury to counteract secondary neurotoxic events or to interrupt the progression of this process. Methylprednisolone (MP) is a potent pharmacological agent that has clinically proven beneficial effects on functional recovery following SCI [4]. Although the underlying mechanism is not fully understood, experimental data point to the protection against membrane peroxidation and edema, the reduction in posttraumatic ischemic area, neurofilament degradation, reversed intracellular calcium accumulation and the improvement in the spinal cord blood flow [5, 6].
Citicoline (CDP-choline) is a compound that is normally present in all cells. It is an endogenous intermediate in membrane phospholipid biosynthesis and, in particular, phosphatidylcholine is essential for membrane integrity and repair [7, 8]. CDP-choline also participates in the biosynthesis of sphingolipids and sphingomyelin [9]. Several studies have shown the beneficial effects of citicoline in a variety of central nervous system (CNS) injury models, brain ischemia and neurodegenerative diseases [7, 10, 11]. It has been suggested that the neuroprotective effect of citicoline is due to its protective effects on cellular membrane integrity with increased phosphatidylcholine synthesis.
The aim of this study was to investigate the neurobehavioral and histopathological recovery and evaluate the biochemical responses to treatment with citicoline, methylprednisolone or both citicoline and methylprednisolone in rats with SCI.
Materials and methods
The study was undertaken at the “Laboratory for Experimental Studies of İnönü University” in accordance with the guidelines established in the “Guide for Care and Use of Laboratory Animals” following the approval of the design by the “Animal Ethics Committee of İnönü University”. Sixty male Wistar rats (210–270 g) were used in the study. The rats in each group were kept in separate cages in rooms with controlled light and temperature and were fed with standard chow and water ad libitum. Before surgery, all rats were tested and a normal motor function was found. The animals were anesthetized by an intraperitoneal injection of 10 mg/kg xylasine (Rompun, Bayer Türk Kimya Sanayii Limited Şirketi, İstanbul, Turkey) and 50 mg/kg ketamine hydrochloride (Ketalar, Pfizer İlaçları Limited Şirketi, İstanbul, Turkey). Rats were positioned on a thermistor-controlled heating pad in the prone position and a rectal probe was inserted. Surgical procedures were performed under sterile conditions with the assistance of a surgical microscope. Following T5-12 midline skin incision and paravertebral muscle dissection, spinous processes and laminar arcs of T7-10 were removed. The dura was left intact. Weight-drop model was performed for SCI [12]. The animals were subjected to an impact of 50 g/cm to the dorsal surface of the spinal cord. The force was applied via a stainless steel rod (3-mm diameter tip, weighing 5 g) that was rounded at the surface. The rod was dropped vertically through a 10-cm guide tube that was positioned perpendicular to the center of the spinal cord. Afterwards, the muscles and the incision were sutured with 6-0 vicryl (Vicryl, Ethicon, Johnson & Johnson Intl, Lanneke Marelaan, Belgium).
The rats were randomized into five groups, each having 12 rats. Group 1 underwent laminectomy alone. Group 2 underwent laminectomy followed by SCI and received no medication. Group 3 underwent laminectomy followed by SCI and received citicoline. Group 4 underwent laminectomy followed by SCI and received methylprednisolone. Group 5 underwent laminectomy followed by SCI and received citicoline in combination with methylprednisolone.
For Group 3 and Group 5, citicoline (Cytidine 5′-diphosphocholine sodium salt dihydrate, Sigma Aldrich Chemie Gmbh, Steinheim, Germany) was administered intraperitoneally at a single dose of 400 mg/kg immediately following SCI. For Group 4 and Group 5, methylprednisolone (Prednol-L, Mustafa Nevzat İlaç Sanayii Anonim Şirketi, İstanbul, Turkey) was administered intraperitoneally at a single dose of 30 mg/kg immediately following SCI.
Following the surgical procedure, the rats were placed in a warming chamber and their body temperatures were maintained at approximately 37°C until they were completely awake. In the early postoperative period, the rats received 3 ml of saline intraperitoneally to compensate for the blood loss during the surgical procedure, while the water intake was limited. At this stage, the rats were further allocated into two subgroups for biochemical analysis (sacrificed at 24 h after surgery) and for neurobehavioral and histopathological evaluation (sacrificed at 6 weeks after surgery). The rats in the subgroups for neurobehavioral and histopathological evaluation received gentamycin twice daily as prophylaxis against urinary tract infection and their bladders were emptied manually twice daily during the first 3 days. Posttraumatic neurological recovery was recorded weekly for 6 weeks. All of the animals were killed and 1 cm-long samples of the spinal cord, including the injury site, were removed for biochemical analysis and histopathological evaluation.
Biochemical analysis
Six rats from each group were sacrificed for biochemical analysis at 24 h after SCI. The exposed spinal cord segments and the meninges were removed. The samples were immediately frozen and stored in a −20°C freezer for assays of malondialdehyde (MDA), glutathione (GSH) and nitrite/nitrate levels.
Lipid peroxidation measurements
The level of lipid peroxides in the traumatized spinal cord tissue were measured as thiobarbituric acid-reactive substance and determined using the method of Mihara and Uchiyama [13]. MDA has been identified as the product of lipid peroxidation that reacts with thiobarbituric acid to give a red color, absorbing at 535 nm. The assay for lipid peroxide in the spinal cord tissue was set up as follows. Tissues were homogenized in 10 volumes (w/v) of cold 1.5% KCl. Half a millimeter (0.5 ml) of homogenate was mixed with 3 ml of 1% H3PO4 and 1 ml 0.6% thiobarbituric acid. The mixture was then heated in boiling water for 60 min. After cooling, the color was extracted into 4 ml n-butanol and the absorbance was recorded at 535 nm. Using tetramethoxypropane as the standard, tissue lipid peroxidate levels were calculated as nanomole per gram of wet tissue.
Glutathione level measurements
GSH levels were measured by the method of Elman [14]. GSH is reacted with 5,5-dithiobis-2-nitrobenzoic acid to result in the formation of a product which has a maximal absorbance at 410 nm. The results are expressed as nanomoles per gram wet tissue.
Nitrite/nitrate measurements
Tissue concentrations of nitrite/nitrate were assayed according to the method described by Ozbek et al. [15]. In the method, the enzymatic conversion of nitrate to nitrite by the enzyme nitrate reductase is followed by the colorimetric detection of nitrite as a colored azo dye product of the Griess reaction. We investigated to determine total NOx production; nitrate was reduced to nitrite by reduced nicotinamide-adenine dinucleotide in the presence of nitrate reductase and reading the optical density at 548 nm. Results are expressed as nmol per gram wet tissue.
Behavioral evaluation
Motor function score
A “motor function scale” [16] that is a slight modification of the “motor score” defined by Gale et al. [17] was used in this study. The test protocol was performed weekly for all of the rats. The animals were allowed to move freely in an open field (0.7 by 0.9 m) and were observed for at least one minute by two observers who were blinded to the study groups. Movements in the hip, knee and ankle joints were recorded (Table 1).
Inclined plane score
An angle board test measuring the maximum angle at which the animal can support its weight on an inclined plane were measured ranging from 0 to 90 degrees to evaluate the inclined plane scores [18]. The animals were placed transversely on the inclined plane and the highest angle that the rats could maintain their position for five seconds were recorded by the blinded observers and described as the “capacity angle” for that animal.
Histopathological evaluation
Six weeks after SCI, the rats were deeply anesthetized using propofol (Propofol, Abbott Laboratuvarı Anonim Şirketi, İstanbul, Turkey) at a dose of 50 mg/kg administered using an intraperitoneal injection. Intracardiac perfusion was performed with isotonic saline for 5 min, followed by 10% formaldehyde for 5 min. After perfusion, the spinal cords were removed immediately and immersed in 10% formaldehyde for 1 week. The spinal cord segments with the contusion epicenter were embedded in paraffin. Each block was serially sectioned horizontally at 5 μm. Sections were stained with hematoxylin and eosin (H×E). The slides were viewed under light microscope to study the structural changes.
Quantitative histopathological evaluation of the spinal lesion was conducted for each sample by light microscopy. Photographs of the spinal cord specimens were obtained under microscopy and these images were exported to a personal computer for analysis. The border of the lesion was drawn and the lesion areas for each sample were measured using Image Analysis System (Leica Micros Imaging Solutions Ltd.; Cambridge, United Kingdom) [19]. The injured area was selected and calculated as a percentage of the whole cross-sectional spinal cord area.
Statistical analysis
Data were analyzed using SPSS 13.0 for Windows software on a personal computer. The results were expressed as means ± S.D. All parameters were tested using Shapiro Wilks test and the distributions were not normal (P > 0.05). Therefore, comparisons among the groups were performed using Kruskal–Wallis test. Mann–Whitney U test was used for dual comparison. P < 0.05 was considered statistically significant.
Results
Biochemical analysis
Lipid peroxidation levels
Figure 1 shows the mean levels of malondialdehyde in all groups. The MDA levels were 240 ± 45 (ranging from 188 to 315 nmol/g) for Group 1; 747 ± 69 (ranging from 677 to 844 nmol/g) for Group 2; 364 ± 49 (ranging from 268 to 409 nmol/mg) for Group 3; 358 ± 34 (ranging from 322 to 400 nmol/g) for Group 4 and 388 ± 35 (ranging from 342 to 429 nmol/mg) for Group 5. MDA levels in Group 2, Group 3, Group 4 and Group 5 revealed a significant elevation compared to Group 1 (P < 0.002, P < 0.004, P < 0.002 and P < 0.002, respectively). Group 3, Group 4 and Group 5 revealed significantly lower MDA levels compared to Group 2 (P < 0.002, P < 0.002 and P < 0.002, respectively). The levels of MDA in Group 3, Group 4 and Group 5 did not reveal significant differences when compared to each other (P > 0.05).
Glutathione levels
Figure 2 shows the mean reduced glutathione levels in all groups. The reduced glutathione levels were 641 ± 146 (ranging from 488 to 744 nmol/g) for Group 1; 58 ± 27 (ranging from 26 to 90 nmol/g) for Group 2; 283 ± 60 (ranging from 204 to 359 nmol/g) for Group 3; 270 ± 40 (ranging from 205 to 315 nmol/g) for Group 4 and 269 ± 43 (ranging from 226 to 342 nmol/g) for Group 5. Levels of reduced glutathione in Group 2, Group 3, Group 4 and Group 5 were significantly lower compared to Group 1 (P < 0.002, P < 0.002, P < 0.002 and P < 0.002, respectively). Group 3, Group 4 and Group 5 resulted in significant elevations of reduced glutathione levels compared to Group 2 (P < 0.002, P < 0.002 and P < 0.002, respectively). The levels of reduced glutathione in Group 3, Group 4 and Group 5 did not reveal significant differences when compared to each other (P > 0.05).
Nitrite/nitrate levels
Figure 3 shows the mean nitrite/nitrate levels in all groups. The nitrite/nitrate levels were 596 ± 39 (ranging from 560 to 664 nmol/g) for Group 1; 1053 ± 39 (ranging from 992 to 1112 nmol/g) for Group 2; 652 ± 57 (ranging from 584 to 728 nmol/g) for Group 3; 637 ± 58 (ranging from 584 to 744 nmol/g) for Group 4 and 661 ± 57 (ranging from 592 to 736 nmol/g) for Group 5. The nitrite/nitrate level was found to be significantly elevated in Group 2 (P < 0.002). Group 3, Group 4, Group 5 resulted in significant reductions of the nitrite/nitrate levels when compared to Group 2 (P < 0.002, P < 0.002 and P < 0.002, respectively). The levels of the nitrite/nitrate in Group 3, Group 4 and Group 5 did not reveal significant differences when compared to each other and Group 1 (P > 0.05).
Behavioral evaluation
Motor function score
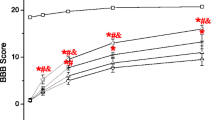
The results of the weekly “motor function score” evaluations are shown in Fig. 4. The “motor function scores” were 6.0 ± 0.0 for Group 1; 2.5 ± 0.5 for Group 2; 5.0 ± 0.0 for Group 3; 4.5 ± 0.5 for Group 4 and 4.0 ± 0.6 for Group 5 at the sixth week. Group 2 revealed a significant change in motor function score as compared to Group 1 at the sixth week (P < 0.002). A significant recovery rate was observed for Group 3, Group 4 and Group 5 at the sixth week when compared to Group 2 (P < 0.002, P < 0.002 and P < 0.002, respectively). The neurobehavioral recovery rates in Group 3, Group 4 and Group 5 did not revealed significant differences when compared to each other at the sixth week after SCI (P > 0.05).
Inclined plane score
The results for the weekly inclined plane evaluations are presented in Fig. 5. The inclined plane scores were 68.8 ± 2.1 for Group 1; 30.1 ± 2.8 for Group 2; 48.0 ± 4.3 for Group 3; 48.3 ± 2.5 for Group 4 and 47.5 ± 4.0 for Group 5 at the sixth week. Group 2 revealed a highly significant decrease in the angle score when compared to Group 1 at the sixth week (P < 0.002). A significant gradual recovery rate was observed for Group 3, Group 4 and Group 5 when compared to Group 2 at the sixth week (P < 0.002, P < 0.002, P < 0.002). In Group 3, Group 4 and Group 5, the capacity on the inclined angle board improved gradually during the observation period, but there was no significant difference when compared to each other (P > 0.05).
Histopathological evaluation
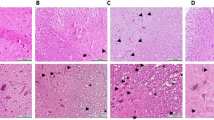
The epicenter of the injured spinal cord obtained from the trauma group showed characteristic necrosis, wide demyelination, and cavitation of white matter in the posteromedial regions of the spinal cord. It also demonstrated complete loss of neurons in the gray matter as well as marked gliosis, infiltration of inflamatory cells and vacuolation. The sections of the injured spinal cord obtained from the treatment groups retained more sparing of the myelin tissue and more neurons in the gray matter than those in the trauma group (Fig. 6A, B, C, D).
Figure 6 presents the quantification of the lesion area in the spinal cord. The ratio of the lesion area was 27.7 ± 2.9% for Group 2, 18.2 ± 2.9% for Group 3, 18.3 ± 2.5% for Group 4 and 18.1 ± 3.4% for Group 5. Group 3, Group 4, and Group 5 had significantly reduced contused areas when compared to Group 2 (P < 0.002, P < 0.002 and P < 0.002, respectively). When Group 3, Group 4 and Group 5 were compared to each other, there was no significant difference (P > 0.05). (Fig. 7)
Discussion
There is still no effective treatment to prevent secondary autodestructive processes. It is well known that one of the most important factors precipitating posttraumatic degeneration in the spinal cord is free oxygen radical-induced lipid peroxidation [20–22]. Numerous investigators have postulated the free-radical-triggered peroxidative events after SCI [21–23]. Lipid peroxidation results in the loss of membrane polyunsaturated fatty acids and oxidized phospholipids that contribute to increased membrane rigidity. Citicoline is an essential precursor of the membrane phosphatidylcholine that has membrane-stabilizing functions and that reduces free fatty acid formation during brain ischemia [7, 8, 11, 24, 25]. Treatment with citicoline reduces the infarct volume and improves neurological outcome after cerebral ischemia. There is only one study that has investigated the neuroprotective effect of citicoline in SCI. In the present study, we investigated the neuroprotective effects of citicoline after SCI and compared these effects to methylprednisolone.
Neuroprotective effects of high-dose methylprednisolone on SCI have been reported previously [5, 20, 26, 27]. The inhibition of posttraumatic lipid peroxidation is the major factor in the improvement of the outcome with methylprednisolone [5, 27]. It suppresses the breakdown of membranes by inhibiting lipid peroxidation and hydrolysis at the site of injury [6, 20]. Experimental studies have shown that a single 30 mg/kg dose of methylprednisolone significantly reduces the lesion volume, decreases injury induced free radical formation and attenuates the associated lipid peroxidative reactions [26, 28]. Since methylprednisolone is the only agent shown to have beneficial effects after SCI, Young et al. recommended that all SCI studies should compare treatment against methylprednisolone [29]. Therefore, methylprednisolone was used to compare the treatment results with citicoline in this study.
Secondary autodestructive processes, such as glutamate excitotoxicity, calcium overload, oxidative stress and ischemia, are related to tissue necrosis, neuronal dysfunction and death [30]. Neurological functional deficit is due to the disruption of cell membranes [31]. It is important to protect cellular membrane integrity after SCI for better neurological recovery. Citicoline is an endogenous intermediate in the biosynthesis of phosphatidylcholine (PC). Previous experimental data pointed that citicoline prevents phospholipase A2 activation [24, 32], decreases lipid peroxidation [33], preserves the arachidonic acid content of PtdCho and phosphatidylethanolamine, restores PtdCho levels [24], stimulates glutathione synthesis and glutathione reductase activity [34] and preserves cardiolipin which is an important component of mitochondrial membrane [24, 25]. Based on these experimental findings, it has been suggested that neuroprotective effect of citicoline might be a result of preserving membrane integrity.
Lipid peroxidation products increase immediately after SCI and the peak concentrations of reactive oxygen and nitrogen species occur within the first 24 h [1, 22, 35]. In this study, tissue lipid peroxidation was evaluated by measuring the thiobarbituric acid reactive substances. Malondialdehyde is also a well-known secondary product of lipid peroxidation in spinal myelin, glial and neural membranes. Previous studies have shown that citicoline decreases lipid peroxidation following cerebral ischemia [33] and SCI [36]. Cakir et al. used 300 mg/kg of citicoline immediately after trauma and evaluated MDA level in the spinal cord at 48 h. In the present study, 400 mg/kg of citicoline was used and MDA levels were evaluated at 24 h, since a dose-dose dependent study on citicoline after traumatic brain injury demonstrated that 400 mg/kg of citicoline significantly decreases brain edema and blood-brain barrier breakdown [10, 37]. In the present study, we demonstrated that citicoline treatment immediately after trauma decreased MDA level. Combined treatment with methylprednisolone did not reveal additional neuroprotective effects. Although neuroprotective effects of citicoline and methylprednisolone follow different routes, stabilization of the cellular membrane is the final result for both drugs. Therefore, it can be claimed that citicoline is effective at least methylprednisolone in stabilizing cellular membrane.
Antioxidant defense mechanisms are important to prevent the potential harmful effects of oxidative stress. In neural tissues, one of the important cellular antioxidant defense mechanisms is reduced glutathione. Glutathione, an essential tripeptide, is an endogenous antioxidant found in all animal cells. It reacts with the free radicals and can protect from single oxygen, hydroxide radical and superoxide radical damage. It has been shown that citicoline increases GSH level and glutathione reductase activity [34]. In the present study, reduced glutathione level increased after SCI. It is well known that the attenuation of glutathione level after trauma is secondary to oxidative stress. Treatment with citicoline, methylprednisolone or both citicoline and methylprednisolone resulted in significantly higher glutathione levels when compared to the trauma group.
Nitric oxide (NO) is a free radical gas molecule produced from l-arginine by the catalytic action of nitric oxide synthases (eNOS, iNOS, and nNOS) [38]. Although NO in physiological levels participate in a variety of physiological processes consisting of neurotransmission and regulation of blood vessel wall [39, 40], increased NO is especially associated with oxidative stress. This is the first study to show that citicoline reduces NO level after SCI. Our results show that treatment with citicoline after SCI is as effective as methylprednisolone considering NO levels and combined treatment does not lead to additional reduction.
Much experimental and clinical research have focused on the secondary injury mechanisms in an effort to improve neurological outcome following SCI. A reliable test protocol suitable for the injury model used is essential to evaluate functional recovery after SCI [17, 41, 42]. Behavioral tests are important tools to assess the outcome of experimental SCI, including spontaneous functional improvement over time and the effects of different treatments. The inclined plane is a test known to be sensitive and reliable in evaluating different degrees of SCI. This test has been validated for rats with compression injuries as well as contusive injuries [4, 17, 18]. The present study showed that an impact of 50 g/cm to the dorsal surface of the spinal cord caused reversible paraplegia and gradual improvement has been observed within 6 weeks after the trauma. A significant gradual recovery rate was observed in all of the injured rats treated with citicoline, methylprednisolone or combined treatment regarding motor function score and inclined angle board capacity. At the end of the observation, the effects of citicoline and methylprednisolone on neurological recovery were found to be similar and that of the combined treatment with both drugs did not contribute to an increased rate of recovery.
Previous studies have shown that treatment with citicoline after middle cerebral artery occlusion reduced the size of infarction area in the brain [11, 37]. The percentage of the whole spinal cord area to the injured spinal cord area was used in this study to evaluate histopathological recovery. The histopathological findings support that citicoline attenuates the lesion area in the spinal cord. Treatment with citicoline revealed similar protective effects on the lesion area when compared to treatment with methylprednisolone whereas combined treatment did not result in any additional histopathological recovery. Taken together with neurobehavioral recovery, we have shown that citicoline treatment after SCI provides an equal neuroprotection with methylprednisolone. The outcome of combined treatment has been similar to the single treatments.
In conclusion, this is the first study that reveals the neuroprotective effect of citicoline on neurobehavioral and histopathological recovery after SCI. Although the neuroprotective mechanisms of methylprednisolone and citicoline are through different routes, experimental data shows that both agents provide cellular membrane stability. In view of our findings, we claim that citicoline is an effective agent on secondary damage after SCI. Citicoline is a well-known neuroprotective agent with rarely observed side effects. However, combination therapy using methylprednisolone and citicoline have not revealed synergistic effects on SCI. Further studies are warrented of citicoline on the effect of SCI for the evaluation of dose-response relationship and efficacy of combination therapies.
References
Tator CH, Fehlings MG (1991) Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 75:15–26
Blight AR, Young W (1989) Central axons in injured cat spinal cord recover electrophysiological function following remyelination by Schwann cells. J Neurol Sci 91:15–34
Basso DM, Beattie MS, Bresnahan JC (1996) Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139:244–256
Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS (1990) Eisenberg HM, Flamm E, Leo-Summers L, Maroon J, et al, A randomized, controlled trial of MP or naloxone in the treatment of acute spinal-cord injury, Result of the Second National Acute Spinal Cord Injury Study. N Engl J Med 322:1405–1411
Braughler JM, Hall ED (1982) Correlation of methylprednisolone levels in cat spinal cord with its effects on (Na+K)-ATP-ase, lipid peroxidation, and alpha motor neuron function. J Neurosurg 56:838–844
Young W, Flamm ES (1982) Effects of high-dose corticosteroid therapy on blood flow, evoked potentials and extracellular calcium in experimental spinal cord injury. J Neurosurg 57:667–673
Schabitz WR, Li F, Irie K, Sandage BW, Locke KW, Fisher M (1999) Synergistic effects of a combination of low dose basic fibroblast growth factor and citicoline after temporary experimental focal ischemia. Stroke 30:427–432
Adibhatla RM, Hatcher JF (2002) Citicoline mechanisms and clinical efficacy in cerebral ischemia. Journal of Neurosci Res 70:133–139
Secades JJ, Frontera G (1995) CDP-choline: pharmacological and clinical review. Meth Find Exp Clin Pharmacol 17:2–54
Baskaya MK, Dogan A, Rao AM, Dempsey RJ (2000) Neuroprotective effects of citicoline on brain edema and blood-brain barrier breakdown after traumatic brain injury. J Neurosurg 92:448–452
Shuaib A, Yang Y, Li Q (2000) Evaluating the efficacy of citicoline in embolic ischemic stroke in rats: neuroprotective effects when used alone or in combination with urokinase. Exp Neurol 161:733–739
Allen AR (1911) Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column. A preliminary report. JAMA 57:878–880
Mihara S, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Elman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77
Ozbek E, Turkoz Y, Gokdeniz R, Davarcı M, Ozugurlu F (2000) Increased nitric oxide production in the spermatic vein of patients with varicocele. Eur Urol 37:172–175
Farooque M, Isaksson J, Jackson D, Olsson Y (1999) Clomethiazole improves hind limb function and reduces neuronal damage after severe spinal cord injury in rat. Acta Neuropathol 98:22–30
Gale K, Kerasidis H, Wrathall JR (1985) Spinal cord contusions in the rat: behavioral analysis of functional neurologic impairment. Exp Neurol 88:123–134
Rivlin AS, Tator CH (1977) Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg 47:577–581
Huang SS, Tsai MC, Chih CL, Hung LM, Tsai SK (2001) Resveratrol reduction of infarct size in Long-Evans rats subjected to focal cerebral ischemia. Life Sci 69:1057–1065
Hall ED (1992) The neuroprotective pharmacology of methylprednisolone. J Neurosurg 76:13–22
Hall ED (1993) Lipid peroxidants in acute central nervous system injury. Ann Emerg Med 22:1022–1027
Anderson DK, Hall ED (1993) Pathophysiology of spinal cord trauma. Ann Emerg Med 22:987–992
Fercakova A, Halat G, Marsala M, Lukacova N, Marsala J (1992) Graded postischemic reoxigenation reduces lipid peroxidation and reperfusion injury in the rabbit spinal cord. Brain Res 593:159–167
Rao AM, Hatcher JF, Dempsey RJ (2000) Lipid alterations in transient forebrain ischemia: possible new mechanisms of CDP-choline neuroprotection. J Neurochem 75:2528–2535
Rao AM, Hatcher JF, Dempsey RJ (1999) CDP-choline; neuroprotection in transient forebrain ischemia of gerbils. J Neurosci Res 58:697–705
Constantini S, Young W (1994) The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J Neurosurg 80:97–111
Braughler JM, Hall ED (1985) Current application of “high dose” steroid therapy for CNS injury. A pharmacological perspective. J Neurosurg 62:806–810
Hall ED, Braughler JM (1982) Effects of intravenous methylprednisolone on spinal cord lipid peroxidation and (Na+-K+ ) ATPase activity. Dose-response analysis during the 1st hour after contusion injury in the cat. J Neurosurg 57:247–253
Young W (1992) Medical treatments of acute spinal cord injury. J Neurol Neurosurg Psychiatry 55:635–639
Amar AP, Levy ML (1999) Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery 44:1027–1039
Demediuk P, Saunders RD, Clendenon NR, Means ED, Anderson DK, Horrocks LA (1985) Changes in lipid metabolism in traumatized spinal cord. Prog Brain Res 63:1–16
Rao AM, Hatcher JF, Dempsey RJ (2001) Does CDP-choline modulate phospholipase activities after transient forebrain ischemia? Brain Res 893:268–272
Adibhatla RM, Hatcher JF, Dempsey RJ (2003) Phospholipase A2, hydroxyl radicals and lipid peroxidation in transient cerebral ischemia. Antioxid Redox Signal 5:647–654
Adibhatla RM, Hatcher JF, Dempsey RJ (2001) Effects of citicoline on phospholilipid and glutathione levels in transient cerebral ischemia. Stroke 32:2376–2381
Barut S, Canbolat A, Bilge T, Aydin Y, Cokneseli B, Kaya U (1993) Lipid peroxidation in experimental spinal cord injury: time–level relationship. Neurosurg Rev 16:53–59
Cakir E, Ulusul H, Peksoylu B, Sayin OC, Alver A, Topbas M, Baykal S, Kuzeyli K (2005) Effects of citicoline on experimental spinal cord injury. J Clin Neurosci 12:923–926
Schabitz WR, Weber J, Tokano K, Sandage BW, Locke KW, Fisher M (1996) The effects of prolonged treatment with citicoline in temporary experimental focal ischemia. J Neurol Sci 138:21–25
Moncada S, Higgs A (1993) The L-arginine-nitric oxide pathway. N Engl J Med 329:2002–2012
Peunova N, Enikolopov G (1993) Amplification of calcium-induced gene transcription by nitric oxide in neuronal cells. Nature 364:450–453
Ignarro LJ, Buga GM, Wood KS, Byrns RE (1987) Chaudhuri G, Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84:9265–9269
Goldberger ME, Bregman BS, Vierck CJ Jr, Brown M (1990) Criteria for assesing recovery of function after spinal cord injury: behavioral methods. Exp Neurol 107:113–117
Von Euler M, Akesson E, Samuelsson EB, Seiger A, Sundström E (1996) Motor performance score: a new algorithm for accurate behavioral testing of spinal cord injury in rats. Exp Neurol 137:242–245
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yücel, N., Çaylı, S.R., Ateş, Ö. et al. Evaluation of the Neuroprotective Effects of Citicoline after Experimental Spinal Cord Injury: Improved Behavioral and Neuroanatomical Recovery. Neurochem Res 31, 767–775 (2006). https://doi.org/10.1007/s11064-006-9075-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9075-1