Abstract
Study design
Preclinical pharmacology.
Objectives
Our study aims to evaluate the combined effect of Methylprednisolone (MP) and growth factor-rich serum (GFRS) on structural and functional recovery in rats following spinal cord injury (SCI).
Setting
Shiraz University of Medical Sciences, Shiraz, Iran
Methods
Male Sprague-Dawley rats were randomly assigned to five groups: sham group (laminectomy); SCI group (the spinal cord clip compression model); SCI-MP group (30 mg/kg MP was administrated intraperitoneally (IP) immediately after SCI); SCI-GFRS group (GFRS (200 µl, IP) was administrated for six consecutive days); and SCI-MP + GFRS group (the rats received MP (30 mg/kg, IP) immediately after SCI, and GFRS (200 µl, IP) for six consecutive days). Motor function was assessed weekly using the Basso, Beattie, and Bresnahan (BBB) scale. After 4 weeks, we conducted the rotarod test, then removed and prepared the spinal cords (including the epicenter of injury) for stereological and histological estimation, and biochemical assays.
Results
The results showed that MP and GFRS combining treatment enhanced functional recovery, which was associated with a decrement in lesion volume, increased spared white and gray matter volume, reduced neuronal loss, as well as decreased necrosis and hemorrhage after SCI. Moreover, administration of MP and GFRS inhibited lipid peroxidation (malondialdehyde (MDA) content), and increased antioxidant enzymes including glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) after rat SCI.
Conclusions
We suggests that the combination treatment of MP and GFRS may ameliorate the structure and functional changes following SCI by reducing oxidative stress, and increasing the level of antioxidants enzymes.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) induces neurological deficits including motor dysfunction [1, 2]. The pathophysiology of SCI includes two mechanisms: primary injury and secondary injury. The former immediately inflicts damage to the spine, whereas the latter involves oxidative stress, inflammation, neural apoptosis, and other pathological reactions that begin within minutes and continue for days and weeks. Secondary injury is associated with the persistent loss of neuronal and glial cells and extension of the damage, spreading the paralysis to higher segments of the spinal cord [1, 3, 4]. Methylprednisolone (MP) is a synthetic glucocorticoid commonly used in SCI patients’ early stages of secondary injury [5]. Animal studies have also shown that treatment with MP in high doses and for a short time is effective during the early stages of SCI [6,7,8]. However, the effectiveness of MP treatment is limited to a narrow time window after SCI due to the rapid and transient onset of glucocorticoid receptor expression in the spinal cord. Moreover, the use of MP is controversial because its beneficial effects are not reproducible and are associated with severe side effects [9]. Hence, the use of MP in combination with other pharmacological agents seems to be more effective.
Platelet-rich plasma (PRP) is a natural product isolated from whole blood that can secrete different neurotrophic and growth factors when activated, including transforming growth factor-b (TGF-b), fibroblast growth factor (FGF), insulin-like growth factor-1 (IGF-1), brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), platelet-derived angiogenesis factor (PDAF), nerve growth factor (NGF) and neurotrophin-3 (NT3) [10]. Moreover, PRP is known as a non-toxic, biocompatible, autologous material. It should be noted that PRP can be transformed into growth factor-rich serum (GFRS) once the jelly scaffold is separated from the solution, offering a wide range of therapeutic applications [11]. These growth and neurotrophic factors provide a permissive surrounding for neuronal growth, useful for neuronal regeneration after SCI [12,13,14].
However, the neuroprotective potential effects of the combined effect of MP and growth factors remain of continuing interest, but the underlying mechanisms are not yet fully understood in SCI. Moreover, there is much less information about the combination effects of growth factors with MP on structural changes and neurological function improvement following SCI [15]. Hence, the present study was designed to evaluate the impact and possible mechanisms of MP treatment combined with GFRS in a rat SCI model.
Methods
Animals
The male Sprague-Dawley rats (250–280 g) were purchased from the Animal Laboratory Center of Shiraz University of Medical Sciences, Shiraz, Iran. The animals were kept under standard conditions at room temperature (22 ± 2 °C), with normal humidity and 12 h light-dark cycles. The rats had free access to standard food and water. All animal procedures were performed under the standard rules established by the Animal Care and Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.AEC.1401.009).
Experimental design
Fifty-five male Sprague-Dawley rats were randomly divided into the following five groups: 1- sham group, laminectomy was performed; 2- SCI group, the spinal cord clip compression model was done; 3- SCI-MP group, the rats were treated intraperitoneally (IP) with MP (30 mg/kg) immediately after SCI; [16] 4- SCI-GFRS group, the rat received GFRS (200 µl, IP) for six consecutive days; and 5- SCI-MP + GFRS group, the rats were treated with MP (30 mg/kg, IP) immediately after SCI, then received GFRS (200 µl, IP) for six consecutive days. Six rats from each group were used for stereological and histological estimation, and five for lipid peroxidation and antioxidant status measurements.
It should be noted that one of the more commonly used routes in rodent studies is the IP route, where a pharmacological agent is administrated into peritoneal cavity. This easy to master technique is quick and minimally stressful for animals.
This route is especially common in chronic studies for which repetitive IV access is challenging. In most cases, IP administration is also preferred over the oral route for biological agents to avoid the GI tract and potential degradation/modification of biopharmaceuticals [17].
Spinal cord surgery procedure
Before induction of SCI model, the rats were administrated with antibiotics (ceftriaxone, 50 mg/kg), and then were anesthetized intraperitoneally with ketamine (80 mg/kg; Pasteur, Romania) and xylazine (10 mg/kg). The animals were placed on a heating pad at 36 °C during surgery, and were subjected to laminectomy at the thoracic level to expose the spinal cord. An aneurysm mini clip (1 N; 103 gms) held in an applicator in the open position, it was rapidly released from the applicator and applied vertically onto the intact T10 level of exposed spinal cord. The clip was then rapidly (1 min) released from the applicator to produce acute impact compression injury [18, 19]. Acute extradural clip compression injury is a reliable model for producing acute experimental spinal cord injury. It should be noted that the rat thoracic cord clip compression model is a reproducible, clinically relevant spinal cord injury model [18]. After surgery, rats were given diclofenac (25 mg/kg) for pain relief. Then, the rats were kept in separate cages and underwent urinary bladder massage at least twice a day until the recovery of spontaneous bladder function [20].
Growth factor-rich serum preparation
Blood was collected from healthy male Sprague-Dawley rats by cardiac punctures under anesthesia. Every 14 ml of blood was added to a tube containing 0.7 ml of 3.8% sodium citrate, then centrifuged at 360 g for 10 min. The plasma was separated above the buffy coat to prevent mixed-up white blood cells. Whole blood cells and the obtained plasma fraction (PRP) were analyzed with a hematologic analyzer (XP-300, Sysmex, Kobe, Japan), and the platelet yield was adjusted to above 60%. To activate the release of biological factors from platelets, 10% calcium chloride (50 µl) was added to every 1 ml of plasma. To complete the clotting and retracting process, the plasma was incubated for 20 min at 37 °C and centrifuged at 1000 g and 4 °C for 20 min. The obtained supernatant, known as growth factor-rich serum, was aliquoted and stored at −80 °C [21].
Behavioral analysis
Basso–Beattie–Bresnahan test
The 21-point Basso–Beattie–Bresnahan (BBB) locomotion scale was used to assess the locomotor behavior recovery after SCI. The BBB scores were documented by observers who were blind to the treatment [22].
Rotarod test
The rats were pre-trained on the rotarod apparatus prior to compression injury at a speed of 5 rpm for at least 5 min on three consecutive days. On the day before SCI induction, the rats were tested by accelerating the speed from 5 to 20 rpm over 5 min. Then, 28 days after SCI, motor performance was examined with the same test conditions [23, 24].
Stereological analysis
Tissue preparation
The animals were anesthetized with ketamine (80 mg/kg) and xylazine (10 mg/kg) on day 28 after SCI. Then, the spinal cords (including the epicenter of injury) were removed (approximately 6 mm) and kept in 10% neutral buffered formalin for 24 h. Subsequently, they were transferred to 30% sucrose in 0.1 M PBS (pH 7.4) for 72 h at 4 °C, flash-frozen in isopentane (2-methlybutane), and stored at 80 °C. Afterward, spinal cord tissues were embedded in optimal cutting temperature compound (OCT, Tissue-Tek) and cut into 40 μm serial transverse sections on a cryostat. Fifteen sections were prepared from each sample (at an interval of 400 μm) and stained with Cresyl Violet (Nissl staining).
Estimation of volumes
The total volumes of the spinal cord, spared white matter, spared gray matter, and lesion were estimated using the Cavalieri method [25]. The slides were labeled with a specific codes and the analysis processes were blinded. A point grid was superimposed on the image of each section on the monitor using stereology software designed by Shiraz University of Medical Sciences, Shiraz, Iran. The total volumes were determined by counting the number of points hitting these areas in the sampled sections, and multiplying the sum of points (∑P) by the area around each point (a/p, 90000 µm2) and the interval between the sampled sections (d). The formula is presented below:
Estimation of cell numbers
A computer linked to a light microscope (Nikon E200, Japan) with an oil immersion lens (100×, numerical aperture = 1.4) was employed to estimate the total number of neurons and other cells in the spared gray matter (the non-neuronal cells which are morphologically different from neuronal cells, characterized by smaller nuclei). According to the optical disector method, the unbiased counting frame with acceptance (upper and right) and forbidden (lower and left) borders was superimposed on the images of the brain sections viewed on the monitor. Then, the microscopic fields were scanned and sampled by moving the microscope stage at equal distances in X and Y directions to ensure systematic uniform random sampling [26, 27]. The nucleus of cells that came into focus within the sampling box (h × a/f) was selected if it was located totally or partially inside the counting frame and did not touch the left and bottom borders of the frame. The total number of cells in the spared gray matter was estimated by multiplying the numerical density (Nv) by the volume of spared gray matter:
where ΣQ is the total number of cells in the spared gray matter coming into focus when scanning the height of the disector; ΣP is the total number of counting frames in most fields; h is the height of the disector; a/f (2500 µm2) is the frame area; t is the mean section thickness calculated in every sampled field using the microcator; BA is the block advance of the microtome set at 40 µm.
Histological evaluation
The Nissl-stained sections were examined under a light microscope (Olympus BX51, Tokyo, Japan) by a pathologist who was blinded to the experimental groups. The groups were graded 0 to 3 (absent, mild, moderate, severe) to assess necrosis, inflammation, and hemorrhage parameters. The number of cavities in the sections was also evaluated [28, 29].
Biochemical analyses
On day 28, the animals were sacrificed after deep anesthesia with ketamine (80 mg/kg) and xylazine (10 mg/kg). Then, the spinal cords (including the epicenter of injury) were immediately removed and weighted. The specimens of spinal cord (each specimen: 0.025 g) homogenized in 1 mL of 0.1 M phosphate buffer solution (PBS; pH 7.4). The homogenates were centrifuged at 3000 rpm for 20 min at 4 °C; then, the supernatants were aliquoted and stored at −80 °C until assessment.
Malondialdehyde assay
The supernatant from each homogenized sample was used for the malondialdehyde (MDA) assay by thiobarbituric acid reactive substances (TBARS) test. Briefly, the solution containing 0.25 N hydrochloric acid (HCl, Sigma, Germany), 20 % trichloroacetic acid (TCA, Sigma, Germany), and 0.8 % thiobarbituric acid (TBA, Sigma, Germany) was added to the supernatants and standards (1,1,3,3-tetra ethoxy propane, Sigma, Germany). Then, they were incubated at 87 °C for 1 h and centrifuged at 12,000 rpm for 5 min and finally read with a microplate reader (Biotek, USA) at the absorbance wavelength 532 nm [30].
Glutathione assay
The glutathione (GSH) concentration was measured by glutathione colorimetric assay kit (ZellBio GmbH, Ulm, Germany). In this assay, DTNB (5,5′- dithio-bis-[2-nitrobenzoic acid]) reacts with reduced glutathione to form a yellow product that absorbs at 412 nm. The optical density, determined at 412 nm, was directly proportional to glutathione concentration in the samples.
Superoxide dismutase and catalase activity
The superoxide dismutase (SOD) and catalase (CAT) enzyme activities were determined by using Assay Kits (ZellBio GmbH, Ulm, Germany) according to the manufacturer’s instructions.
Protein assay
The assessment of protein content of the supernatants was done according to the Bradford method using bovine serum albumin (BSA) as standard [31].
Statistical analysis
Quantitative results are presented as mean ± standard deviation (SD). GraphPad Prism software version 6.0 (Graphpad Software, La Jolla, CA, USA) was used for statistical analysis. After assessing the data normality of distribution, statistical significance for the normal distribution results were evaluated using ANOVA followed by post-hoc Tukey, and to analyze data lacking normal distribution was used Kruskal–Wallis test. P-values below 0.05 were considered statistically significant.
Results
Behavioral analysis
Basso–Beattie–Bresnahan (BBB) locomotor score
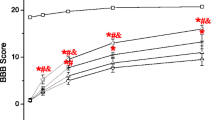
GFRS recovered locomotor function on days 7 to 28 after SCI (Fig. 1A). As a result, a significant difference in improvement was observed in the SCI-GFRS and SCI-MP + GFRS groups compared with SCI and SCI-MP rats. Notably, the results of BBB score on days 21 and 28 showed that treatment with MP + GFRS improved locomotor activity more than the GFRS treatment in SCI rats. The SCI and SCI-MP groups showed locomotor recovery, but there was no significant difference between these groups.
Improvement in locomotor recovery was observed in the growth factor-rich serum (GFRS) and methylprednisolone (MP) + GFRS groups from 1 to 4 weeks after SCI (A). Data are expressed as mean ± SD. 1P < 0.05, 2P < 0.01, 3P < 0.001 and 4P < 0.0001 (a vs. SCI group); (b vs. SCI-MP); (c vs. SCI-GFRS). The latency to fall in the experimental groups was assessed via the rotarod test (B). Data are expressed as the mean ± SD. 1P < 0.05, 2P < 0.01, 3P < 0.001 and 4P < 0.0001 (a vs. sham); (b vs. SCI); (c vs. SCI-MP); (d vs. SCI-GFRS).
Motor performance test
The results of the rotarod test are shown in Fig. 1B. As opposed to the sham group, performance was impaired in all injured groups. Nevertheless, the latency to fall progressively improved in the GFRS treatment groups compared with the SCI group. In addition, the latency to fall was significantly higher in the SCI-MP + GFRS rats relative to the SCI-MP and SCI-GFRS rats.
Stereological analysis
Total volume of the spared tissue
The total volumes of the spinal cord, spared white matter, and spared gray matter were significantly lower in all injured groups relative to the sham group, indicating tissue loss at the site of injury. The spared white matter and spared gray matter volumes in the SCI-GFRS and SCI-MP + GFRS groups significantly improved compared with the SCI and SCI-MP groups, which this difference was more prominent in the SCI-MP + GFRS. No significant difference was found between the SCI-MP and SCI groups (Fig. 2A, C, D).
Mean ± SD of the total spinal cord volume (A), lesion volume (B), spared white matter volume (C), and spared gray matter volume (D). Data are expressed as the mean ± SD. n = 6 rats in each group. 1P < 0.05, 2P < 0.01, 3P < 0.001 and 4P < 0.0001 (a vs. sham); (b vs. SCI); (c vs. SCI-MP); (d vs. SCI-GFRS). SCI spinal cord injury, MP methylprednisolone, GFRS growth factor-rich serum.
Lesion volume
The lesion volume was increased significantly in all experimental groups compared to the sham group. This parameter recovered in the SCI-MP, SCI-GFRS, and SCI-MP + GFRS groups compared to the SCI group. Although, more improvement was seen in the GFRS-treated groups, specifically in the SCI-MP + GFRS group (Fig. 2B).
Total number of cells
The total number of spared gray matter neurons and spared spinal non-neuronal cells declined in all SCI groups as opposed to the sham group, which could imply the loss of volume in the spinal cord. In addition, the numbers of neuronal and non-neuronal cells were increased in the SCI-GFRS and SCI-MP + GFRS groups compared with SCI and SCI-MP rats, which was more prominent in the SCI-MP + GFRS. Also, no significant difference was found in the number of cells of the SCI-MP group compared with the SCI group (Fig. 3).
Normal appearance can be seen in the sham group (A). More pyknotic cells (arrow) and fewer neuronal and non-neuronal cells can be found in the SCI and SCI-MP groups (B, C). Four weeks after SCI induction, the number of cells recovered in the SCI-GFRS and SCI-MP + GFRS groups (D, E). Scale bars = 100 µm. Mean ± SD of the spared gray matter neuron number (F), and spared spinal non-neuronal cells number (G). Data are expressed as the mean ± SD. n = 6 rats in each group. 1P < 0.05, 2P < 0.01, 3P < 0.001 and 4P < 0.0001 (a vs. sham); (b vs. SCI); (c vs. SCI-MP); (d vs. SCI-GFRS). SCI spinal cord injury, MP methylprednisolone, GFRS growth factor-rich serum.
Histological evaluation
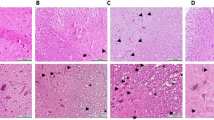
The spinal cord histological analysis has been illustrated in Fig. 4. For necrosis and hemorrhage, significant differences were found in all injured groups compared to the sham group (P < 0.001). However, co-administration of MP and GFRS significantly improved these parameters compared to the SCI group (P < 0.05) (Fig. 4A, C). The inflammation was significantly increased in the SCI and SCI-MP groups compared with the sham group (P < 0.05). Although, no significant difference was found in the SCI-GFRS and SCI-MP + GFRS groups compared to the sham group (Fig. 4B). The number of cavities in all SCI groups were significantly increased compared to the sham group (Fig. 4D). However, this parameter showed more pronounced increase in the SCI and SCI-MP groups as compared with the sham group (P < 0.001).
The images indicate the numerous cavities in various sizes (arrowheads), and extensive necrosis and inflammation (inset). The box plots show the histopathological scores: necrosis (A), inflammation (B), hemorrhage (C), and number of cavities (D) presented as median and interquartile ranges. n = 6 rats in each group. 1P < 0.05, 2P < 0.01, and 3P < 0.001 (a vs. sham); (b vs. SCI). SCI spinal cord injury, MP methylprednisolone, GFRS growth factor-rich serum.
Biochemical analysis
The results indicated that the MDA content was increased significantly in the SCI and SCI-MP compared to the sham group (P < 0.0001). Treatment with GFRS led to a reduction in the MDA level in the SCI-GFRS and specially SCI-MP + GFRS groups compared to the SCI (P < 0.001 and P < 0.0001, respectively) and SCI-MP (P < 0.01 and P < 0.05, respectively) groups. Although, MDA content was still higher in the SCI-GFRS and SCI-MP + GFRS groups compared to the sham group (P < 0.01 and P < 0.05, respectively) (Fig. 5A).
Effects of methylprednisolone (MP) and growth factor-rich serum (GFRS) on the malondialdehyde (MDA) content (A), glutathione (GSH) concentration (B), superoxide dismutase (SOD) activity (C), and catalase (CAT) activity (D) after spinal cord injury (SCI) in rat. Data are expressed as the mean ± SD. n = 5 rats in each group. 1P < 0.05, 2P < 0.01, 3P < 0.001 and 4P < 0.0001 (a vs. sham); (b vs. SCI); (c vs. SCI-MP).
The GSH concentration was decreased significantly in all injured groups compared to the sham group (P < 0.0001). However, the GSH level increased in the SCI-GFRS and SCI-MP + GFRS groups compared to the SCI (P < 0.05) (Fig. 5B).
The activities of SOD and CAT were decreased significantly in the SCI and SCI-MP compared to the sham group (P < 0.01 and P < 0.05, respectively). Although, no significant differences were observed between the GFRS treated groups and the sham group (Fig. 5C, D).
Discussion
The present study examined the effects of MP and GFRS on the structure and functional changes after SCI in rat. We showed that MP and GFRS combining treatment enhanced functional recovery, which was associated with a decrement in lesion volume, increased spared white and gray matter volume, reduced neuronal loss, as well as decreased necrosis and hemorrhage after SCI. Moreover, co-administration of MP and GFRS led to inhibit lipid peroxidation and increase antioxidant enzymes after SCI.
The pathophysiology of SCI is characterized by a primary injury followed by secondary damage. Oxidative stress plays an essential role in the development of primary and secondary injury after SCI, leading to the spread of the lesion, neuronal death, and ultimately neurological deficit [1, 3, 4]. Our findings showed that MDA content, an indicator of lipid peroxidation and oxidative damage, increased significantly in SCI group compared to the sham group. Also, we indicated that the GSH concentration, as well as SOD and CAT activities were significantly decreased in SCI rats, which was also reported by previous studies [32, 33]. GSH and CAT are the most important intracellular antioxidant agents against reactive oxygen species. GSH is involved in the scavenging of both organic and inorganic peroxides [34]. SOD can neutralize oxygen-free radicals and protect cells from oxidation by superoxide toxicity. The accumulation of free radicals in SCI could be related to the reduction of these enzymes in damaged spinal cord, which are highly toxic to neurons and nerve tissues [35]. Therefore, an increase in the lesion volume, neuronal loss, and consequently impaired motor function in SCI rats could be attributed to the disturbance in the oxidant-antioxidant balance.
MP is an agent used during the early stages of secondary SCI [5]. However, its usage is controversial because its beneficial effects are not reproducible and are associated with severe side effects [9]. In addition, MP is effective only in a relatively narrow time window after SCI. One possible cause of the very limited therapeutic window of MP may be the rapid and transient onset of glucocorticoid receptor expression in the spinal cord after traumatic injury [36]. MP reduces neuroinflammation, lipid peroxidation and relieves to some extent complications related to secondary SCI mechanisms such as blood-spinal cord barrier alterations and edema formation [37]. Various experimental results indicate that high-dose MP treatment in the acute stage can reduce the infiltration of inflammatory cells and prevent the inflammatory reaction [38, 39]. Nevertheless, we did not find any improvement in tissue structure and motor performance as well as oxidative stress markers following the administration of MP. Similar results have previously been reported [40]. Hence, some clinical and experimental studies have recommended using MP as an initial neuroprotective agent in combination with other regenerative treatments [41,42,43].
PRP is a natural, non-toxic, biocompatible, autologous product isolated from whole blood that can secrete different neurotrophic and growth factors when activated [10]. The lack of a suitable substrate, the absence of growth factors, and the accumulation of inflammatory factors can prevent neuronal regeneration and axonal attachment and extension through the lesion site after SCI [44, 45], whereas providing a permissive environment for neuronal growth is useful for neuronal regeneration [46]. PRP contains growth and neurotrophic factors like IGF, EGF, VEGF, and PDGF, which offer neurotrophic effects in neurodegenerative diseases [47]. The neuroprotective and neuroregenerative effects of PRP and its growth factors have been established in SCIs [12,13,14].
Our results indicated that treatment of SCI rats with GFRS ameliorated the structure and motor function and improved biochemical parameters. However, the combination treatment of GFRS with MP led to a greater improvement of structural and functional outcomes compared with single treatment, which could be explained by the synergistic or additive effects of MP and GFRS. The combined treatment had a more pronounced effect on attenuation of necrosis, inflammation, and hemorrhage as well as reduction of lesion volume and neuronal loss. In addition, biochemical results showed that co-administration of MP and GFRS could restore the GSH concentration, as well as SOD and CAT activities close to normal levels, and reduced MDA after SCI. In agreement with our study, it has been demonstrated that EGF treatment could significantly lead to alteration on catalase, GPx and SOD activities in spinal cord after SCI [48]. Furthermore, growth factors have been shown to exert antioxidant properties, and can scavenge toxic oxidation products, thereby preventing neuronal injury [48].
Hence, it seems that the combination treatment of MP and GFRS ameliorates the structural changes following SCI by reducing oxidative stress, and increasing the level of antioxidants enzymes, which can underlie motor function improvements. However, further research is needed to elucidate detailed mechanisms of the observed effects. One future direction for this line of research could be the immunohistochemical evaluations of inflammatory markers and the potential molecular indices that could justify the involved mechanisms.
Conclusion
Our study suggests that the combination treatment of MP and GFRS may ameliorate the structure and functional changes following SCI by reducing oxidative stress and increasing the level of antioxidant enzymes, which could be a potential therapeutic strategy for SCI patients.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Anjum A, Yazid MDI, Fauzi Daud M, Idris J, Ng AMH, Selvi Naicker A, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020;21:7533.
Schuld C, Franz S, Brüggemann K, Heutehaus L, Weidner N, Kirshblum SC, et al. International standards for neurological classification of spinal cord injury: impact of the revised worksheet (revision 02/13) on classification performance. J Spinal Cord Med. 2016;39:504–12.
Hausmann OB. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–78.
Okada S. The pathophysiological role of acute inflammation after spinal cord injury. Inflamm Regeneration. 2016;36:1–7.
Sayer FT, Kronvall E, Nilsson OG. Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 2006;6:335–43.
Bartholdi D, Schwab ME. Methylprednisolone inhibits early inflammatory processes but not ischemic cell death after experimental spinal cord lesion in the rat. Brain Res. 1995;672:177–86.
Fu ES, Saporta S. Methylprednisolone inhibits production of interleukin-1β and interleukin-6 in the spinal cord following compression injury in rats. J Neurosurgical Anesthesiol. 2005;17:82–5.
Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY. Methylprednisolone inhibition of TNF-α expression and NF-kB activation after spinal cord injury in rats. Mol Brain Res. 1998;59:135–42.
Chikuda H, Yasunaga H, Takeshita K, Horiguchi H, Kawaguchi H, Ohe K, et al. Mortality and morbidity after high-dose methylprednisolone treatment in patients with acute cervical spinal cord injury: a propensity-matched analysis using a nationwide administrative database. Emerg Med J. 2014;31:201–6.
Pavlovic V, Ciric M, Jovanovic V, Stojanovic P. Platelet rich plasma: a short overview of certain bioactive components. Open Med. 2016;11:242–7.
Anitua E, Prado R, Sánchez M, Orive G. Platelet-rich plasma: preparation and formulation. Operative Tech Orthop. 2012;22:25–32.
Kojima A, Tator CH. Intrathecal administration of epidermal growth factor and fibroblast growth factor 2 promotes ependymal proliferation and functional recovery after spinal cord injury in adult rats. J Neurotrauma. 2002;19:223–38.
Chen N-F, Sung C-S, Wen Z-H, Chen C-H, Feng C-W, Hung H-C, et al. Therapeutic effect of platelet-rich plasma in rat spinal cord injuries. Front Neurosci. 2018;12:252.
White RE, Yin FQ, Jakeman LB. TGF-α increases astrocyte invasion and promotes axonal growth into the lesion following spinal cord injury in mice. Exp Neurol. 2008;214:10–24.
Baffour R, Achanta K, Kaufman J, Berman J, Garb JL, Rhee S, et al. Synergistic effect of basic fibroblast growth factor and methylprednisolone on neurological function after experimental spinal cord injury. J Neurosurg. 1995;83:105–10.
Bi J, Feng E, Sun P, Shen J, Chen C, Tan H, et al. Melatonin synergizes with methylprednisolone to ameliorate acute spinal cord injury. Front Pharmacol. 2022;12:3764.
Al Shoyaib A, Archie SR, Karamyan VT. Intraperitoneal route of drug administration: should it be used in experimental animal studies? Pharm Res. 2020;37:1–17.
Poon PC, Gupta D, Shoichet MS, Tator CH. Clip compression model is useful for thoracic spinal cord injuries: histologic and functional correlates. Spine. 2007;32:2853–9.
Kang B-J, Yu S-H, Cho D-C, Sung J-K, Park J-Y, Cho H-J, et al. Neurologic and histological study of clip compression model for rat thoracic spinal cord injuries. Korean J Spine. 2011;8:24–30.
Rivlin A, Tator C. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surgical Neurol. 1978;10:38–43.
Khorsand Ghaffari M. A modified method for Preparation of growth factor rich serum (GFRS) from human blood. 2021. https://doi.org/10.13140/RG.2.2.16164.81289.
Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21.
Avila-Martin G, Mata-Roig M, Galan-Arriero I, Taylor JS, Busquets X, Escriba PV. Treatment with albumin-hydroxyoleic acid complex restores sensorimotor function in rats with spinal cord injury: efficacy and gene expression regulation. Plos one. 2017;12:e0189151.
Erfanizadeh M, Noorafshan A, Naseh M, Karbalay-Doust S. The effects of copper sulfate on the structure and function of the rat cerebellum: a stereological and behavioral study. IBRO Neurosci Rep. 2021;11:119–27.
Asadi-Golshan R, Razban V, Mirzaei E, Rahmanian A, Khajeh S, Mostafavi-Pour Z, et al. Efficacy of dental pulp-derived stem cells conditioned medium loaded in collagen hydrogel in spinal cord injury in rats: stereological evidence. J Chem Neuroanat. 2021;116:101978.
Gundersen H, BENDTSEN TF, KORBO L, MARCUSSEN N, Møller A, Nielsen K, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Apmis. 1988;96:379–94.
Kristiansen SLB, Nyengaard JR. Digital stereology in neuropathology. Apmis. 2012;120:327–40.
Aziz I, Che Ramli MD, Mohd Zain NS, Sanusi J. Behavioral and histopathological study of changes in spinal cord injured rats supplemented with Spirulina platensis. Evid Based Complement Alternat Med. 2014;2014:871657.
de Mesquita Coutinho P, Cristante A, de Barros Filho T, Ferreira R, Dos Santos G. Effects of tacrolimus and erythropoietin in experimental spinal cord lesion in rats: functional and histological evaluation. Spinal Cord. 2016;54:439–44.
Naseh M, Dehghanian A, Ketabchi F. Vagotomy improves hypoxic pulmonary vasoconstriction in rats subjected to brain ischemia-reperfusion injury. Iran J Med Sci. 2020;45:250.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal cord. 2012;50:264–74.
Lucas JH, Wheeler DG, Guan Z, Suntres Z, Stokes BT. Effect of glutathione augmentation on lipid peroxidation after spinal cord injury. J neurotrauma. 2002;19:763–75.
Wang W, Ballatori N. Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol Rev. 1998;50:335–56.
Jiang ZS, Pu ZC, Hao ZH. Carvacrol protects against spinal cord injury in rats via suppressing oxidative stress and the endothelial nitric oxide synthase pathway. Mol Med Rep. 2015;12:5349–54.
Yan P, Xu J, Li Q, Chen S, Kim G-M, Hsu CY, et al. Glucocorticoid receptor expression in the spinal cord after traumatic injury in adult rats. J Neurosci. 1999;19:9355–63.
Cabrera-Aldana EE, Ruelas F, Aranda C, Rincon-Heredia R, Martínez-Cruz A, Reyes-Sánchez A, et al. Methylprednisolone administration following spinal cord injury reduces aquaporin 4 expression and exacerbates edema. Mediators of inflammation. 2017;1–7. https://doi.org/10.1155/2017/4792932.
Zou H-j, Guo S-W, Zhu L, Xu X, Liu J-b. Methylprednisolone induces neuro-protective effects via the inhibition of A1 astrocyte activation in traumatic spinal cord injury mouse models. Front Neurosci. 2021;15. https://doi.org/10.3389/fnins.2021.628917.
Liu J-t, Zhang S, Gu B, Li H-n, Wang S-y, Zhang S-y. Methotrexate combined with methylprednisolone for the recovery of motor function and differential gene expression in rats with spinal cord injury. Neural Regeneration Res. 2017;12:1507.
Pereira JE, Costa LM, Cabrita AM, Couto PA, Filipe VM, Magalhães LG, et al. Methylprednisolone fails to improve functional and histological outcome following spinal cord injury in rats. Exp Neurol. 2009;220:71–81.
Li X-G, Lin X-J, Du J-H, Xu S-Z, Lou X-F, Chen Z. Combination of methylprednisolone and rosiglitazone promotes recovery of neurological function after spinal cord injury. Neural Regeneration Res. 2016;11:1678.
Mu X, Azbill RD, Springer JE. Riluzole and methylprednisolone combined treatment improves functional recovery in traumatic spinal cord injury. J Neurotrauma. 2000;17:773–80.
Ji B, Li M, Budel S, Pepinsky RB, Walus L, Engber TM, et al. Effect of combined treatment with methylprednisolone and soluble Nogo‐66 receptor after rat spinal cord injury. Eur J Neurosci. 2005;22:587–94.
Jones T, McDaniel E, Popovich P. Inflammatory-mediated injury and repair in the traumatically injured spinal cord. Curr Pharm Des. 2005;11:1223–36.
Jones LL, Oudega M, Bunge MB, Tuszynski MH. Neurotrophic factors, cellular bridges and gene therapy for spinal cord injury. J Physiol. 2001;533:83–9.
Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–73.
Shen Y-X, Fan Z-H, Zhao J-G, Zhang P. The application of platelet-rich plasma may be a novel treatment for central nervous system diseases. Med Hypotheses. 2009;73:1038–40.
Ozturk AM, Sozbilen MC, Sevgili E, Dagci T, Özyalcin H, Armagan G. Epidermal growth factor regulates apoptosis and oxidative stress in a rat model of spinal cord injury. Injury. 2018;49:1038–45.
Acknowledgements
This work was performed at the Histomorphometry and Stereology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. The authors would like to thank Dr. Seyed Ali Hosseini (Native-Speaking Language Editor) for improving the use of English in the manuscript.
Funding
This work was performed at the Histomorphometry and Stereology Research Center and was financially supported by grant No. 1401.009 from Shiraz University of Medical Sciences, Shiraz, Iran.
Author information
Authors and Affiliations
Contributions
SRM: Designing the study, supervising laboratory works. MRF: Conceptualization, Methodology. MKH: Performing laboratory works, collecting the data and Analysis of the data. FK: Conceptualization, Methodology. SK: Conceptualization, Methodology. ARD: Conceptualization, Methodology. MN: Analysis of the data, writing, editing and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All experimental procedures in the current study were done in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978) and were approved by the Medical and Research Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (Approval No. IR.SUMS.AEC.1401.009).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mousavi, S.R., Farrokhi, M.R., Ghaffari, M.K. et al. The combination treatment of methylprednisolone and growth factor-rich serum ameliorates the structural and functional changes after spinal cord injury in rat. Spinal Cord 62, 17–25 (2024). https://doi.org/10.1038/s41393-023-00942-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-023-00942-x
- Springer Nature Limited