Abstract
Introduction
Diffuse midline gliomas (DMGs) are infiltrative midline gliomas harboring H3K27M mutations and are generally associated with poor outcomes. H3K27M mutations include mutations in HIST1H3B/C (H3.1), HIST2H3B/D (H3.2), or H3F3A (H3.3) genes. It is still unclear whether these mutations each portend a universally poor prognosis, or if there are any factors which modulate outcome. The main objective of this study was to study overall survival (OS) of H3.1 versus H3.3 K27M-mutant DMGs in pediatric and adult patients.
Methods
PubMed and Web of Science were searched, and we included studies if they have individual patient data of DMGs with available H3K27M genotype. Kaplan–Meier analysis and Cox regression models were used to analyze the survival of H3.1 and H3.3 mutations in each subgroup.
Results
We included 26 studies with 102 and 529 H3.1 and H3.3-mutant DMGs, respectively. The H3.1 mutation was more commonly seen in younger age. In pediatric population, H3.3 mutation conferred a shorter survival (median OS of 10.1 vs 14.2 months; p < 0.001) in comparison to H3.1-positive patients, which was further confirmed in the multivariate Cox analysis. Conversely, H3.3 was associated with a prolonged survival in adult patients as compared with H3.1 mutation (median OS of 14.4 vs 1.7 months; p = 0.019).
Conclusion
We demonstrated that the prognosis of H3.1 and H3.3 K27M mutation in DMG patients is modulated by patient age. Routine H3K27M mutation genotyping in newly diagnosed DMGs may further stratify patients with these difficult tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Midline gliomas are mostly seen in children, and typically arise in the pons, thalamus, or spinal cord [1]. The Lysine 27-to-methionine (K27M) mutations, either in the HIST1H3B/C (H3.1), HIST2H3B/D (H3.2), or H3F3A (H3.3) genes, are molecularly defined by mutations in genes encoding the histone H3 [1]. There are different histone H3 proteins including the H3.3, which is expressed constitutively and throughout the cell cycle whereas the H3.1 protein is specifically expressed only during the S-phase [2]. Diffuse midline gliomas (DMG) are infiltrative tumors with a H3 K27M mutation involving the midline structures, These mutations can be found in 80% in pediatric and 15–60% in adult patients [3,4,5,6]. DMGs portend an adverse prognosis with a typical survival of less than 1 year from diagnosis despite decades of clinical trials [7]. These tumors were thus classified as a separate entity in the 2016 and 2021 World Health Organization (WHO) classifications of CNS tumors [8, 9]. On the contrary, only a subset of adult patients harbored H3K27M mutations [6]. Notably, there is evidence that DMGs in adult and pediatric patients are histologically and prognostically similar [10].
Of the H3K27M mutations, H3.3 are the most common, and are seen in about 70% of pediatric DMGs while K27M mutation in H3.1 accounts for the remaining of cases [11, 12]. H3.2 K27M mutations are extremely rare [11]. H3.1 mutations, however, are relatively uncommon in adult DMGs as compared to the pediatric group [13]. Mutations in the H3.1 and H3.3 genes have been shown to drive two distinct oncogenic transformations with different prognoses and phenotypes [11]. However, there is a lack of clarity regarding the difference in prognosis of these mutations in the pediatric and adult populations. Hence, the primary objective of this study was to investigate this difference by integrating individual participant data of published studies on DMGs.
Materials and methods
Search strategy
Our systematic review and meta-analysis of individual participant data was in accordance with the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analysis) guidelines [14]. Two databases (PubMed and Web of Science) were systematically searched using the key words “Glioma” and “H3 K27M OR H3F3A OR HIST1H3B K27M OR H3-K27M OR H3K27M” in July 2021. A hand search of the reference list of the articles was also performed to ensure the identification of all relevant studies pertinent to the topic.
Study selection criteria
Two authors independently reviewed and selected the studies satisfying the following criteria: (i) studies on DMGs with H3K27M genotyping data available, and (ii) studies with individual patient data of H3K27M mutation. All discrepancies were reviewed and resolved by the consensus of all authors. The exclusion criteria were experimental and animal studies, books, review articles, preprints, proceeding papers, conference abstracts, and studies with duplicated data.
Data collection
The full text of each eligible article was read by two independent reviewers. The following data were extracted: authors, institution, country, year of publication, study period, H3K27M genotypes, demographic information, tumor location, WHO grades, treatments (e.g., extent of resection, radiotherapy, and chemotherapy), overall survival (OS) time, OS status, and accompanying genetic events.
Outcomes of interest
In this study, the primary outcomes of interest were all-cause mortality of H3.1 and H3.3 K27M DMGs in pediatric (age 0–18) and adult populations (age > 18).
Statistical analysis
Categorical data were displayed as frequency, and a comparison between groups was done using the Chi-square test or Fisher’s exact test. Continuous variables are presented as mean ± standard deviation (SD) for normal distributions and median + interquartile range for non-normal distributions. Normality was tested using skewness, kurtosis, visual inspection of the histogram, QQ plot. The t-test and Mann–Whitney U test were performed to compare differences between 2 groups for normally and non-normally distributed variables, respectively. The Kaplan–Meier curve and log-rank test were computed to analyze all-cause mortality differences between two variants (H3.3 vs H3.1). A two-sided p value of less than 0.05 was considered statistically significant. The statistical analyses were performed using the IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, N.Y., USA) and R software, version 4.1.1 (The R Foundation, Vienna, Austria).
Results
We identified 788 articles for the title and abstract screening and 76 articles were selected for full-text review. After reading full-text, we included 26 studies with available H3K27M genotyping information comprising 102 H3.1 and 529 H3.3 K27M DMGs for integrated analyses (Fig. 1) [6, 11,12,13, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. There were two cases with H3.2 K27M mutation which were excluded from the analyses to avoid potential bias.
The differences in patient clinical characteristics between H3.1 and H3.3 K27M-mutant DMGs
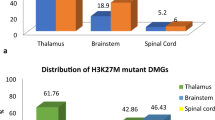
Table 1 presents the clinical characteristics of the H3.1 and H3.3 K27M-mutant DMGs. Compared to the H3.1 K27M, the H3.3 K27 mutation was more commonly identified in adult patients (p < 0.001). No gender difference was observed between the two mutations. Spinal cord more frequently harbored the H3.3 K27M mutations whereas H3.1 K27M-mutant tumors were limited to the brainstem. With respect to treatment modalities, the H3.3 K27M variants were associated with higher rate of tumor resection (p = 0.048) and chemotherapy administration (p = 0.04), but not radiotherapy administration.
The differences in associated genetic events between H3.1 and H3.3 K27M-mutant DMGs
The genetic events associated with these two mutations are shown in Table 2. ACVR1 mutations, EGFR amplification, and PIK3CA mutations were more common in H3.1 K27M-mutant DMGs whereas ATRX and TP53 mutations and PDGFRA amplification were associated with H3.3- mutated tumors.
The survival differences of H3K27M mutation genotypes in pediatric and adult patients
Overall, the median OS was less favorable in the H3.3 K27M- than in the H3.1 K27M-mutant DMGs (11.0 vs. 14.1 months, p < 0.001). Stratified into adult and pediatric groups, the H3.3 K27M mutation was associated with a better survival when compared to patients harboring an H3.1 K27M mutation in adults (median OS of 14.4 vs 1.7 months; p = 0.019) (Fig. 2). Conversely, the H3.3 mutation conferred a poorer outcome in the pediatric DMGs when compared to those with H3.1 K27M (median OS of 10.1 vs 14.2 months; p < 0.001) (Fig. 3). These results were confirmed by multivariate Cox regression analyses with the only exception in the adult population, which was not statistically significant (H3.3 vs H3.1; HR 0.217; 95% CI 0.017–2.736) (Tables S1, S2).
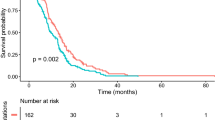
Stratified by H3K27M genotypes and age groups, patient OS were able to be stratified into different subgroups (Fig. 4). Adult DMGs with H3.3 had the best OS, followed by pediatric DMGs harboring H3.1 (p < 0.001), and pediatric patients carrying H3.3 (p = 0.002). The survival of adult cases with H3.1 mutation was only statistically different from H3.3-mutant adult DMGs (p = 0.027) and were not significantly different from the remaining subgroups, probably because of the very limited number of patients in this subgroup.
Discussion
Infiltrative midline gliomas affecting the brainstem, thalamus, or spinal cord are high-grade tumors with a dismal outcome and are mostly seen in children [8]. Nearly 70–80% of these tumors carry mutations in the histone H3 gene, which are termed as DMGs and are associated with a worse outcome in comparison with H3-wild-type tumors [3,4,5, 37]. Among DMGs, the H3.3 K27M mutation is predominant, surprising because only two genes encode H3.3 while the H3.1 protein is encoded by 12 genes [38]. Interestingly, recent evidence indicates that DMG represents a heterogeneous disease with distinct genetic and molecular profiles in adults in comparison to children [3, 11, 39]. Several studies showed that H3.1 K27M mutation is more commonly detected in pediatric patients and rarely seen in adults [6, 11, 40, 41]. Additionally, H3.3 K27M mutation has been reported to affect all anatomical structures of the midline [6, 13, 28] whereas H3.1-mutated DMGs were mainly restricted in the brainstem region [11, 13]. Our integrated analyses confirmed these discrepancies between the two H3 genotypes and revealed the differences in treatment patterns between them. Because H3.1-mutant DMGs are primarily located in the pons and thalamus, the rate of tumor resection of these tumors is lower as compared to H3.3-mutated DMGs.
Although histone H3.1 and H3.3 proteins are very similar in their amino acid sequences, they are deposited into the chromatin through different pathways and associated with a distinct molecular entity [11]. The H3.1 and H3.2 proteins—also referred to as “canonical” H3—are linked with an earlier onset of gliomagenesis, primarily produced during the DNA synthesis phase of the cell cycle, and deposited throughout the genome [42]. On the other hand, histone H3.3 not only functions as canonical H3 in the nucleosome, but also is expressed throughout the cell cycle and accumulates at sites of histone turnover [43]. It has also been suggested that H3.1 and H3.3 K27M-mutant tumors may derive from distinct progenitor cells or at different steps in the differentiation of cell lineage [44]. However, the differences regarding how H3.1 and H3.3 K27M mutations involve in the glioma-genesis of DMGs, and how they affect the patient outcomes remained investigational. A multicenter study demonstrated that long-term survivors of diffuse intrinsic pontine glioma are more likely to carry H3.1 mutation whereas majority of patients with short-term prognosis have H3.3 mutation [45].
Our results showed that the H3.1 K27M mutation confers a favorable prognosis as compared to the H3.3 K27M mutation in pediatric DMGs; however, this was not the case in the adult population. It is still poorly understood as to why these H3K27M mutations have different prognoses in pediatric and adult patients. There are several plausible explanations for a better prognosis of H3.1 K27M mutation in pediatric patients. First, it has been well established that the cooccurrence of ACVR1 mutation in H3K27M DMGs is associated with a longer survival [35, 46] and ACVR1 mutations are only seen in pediatric H3.1 K27M-mutated DMGs [35, 47, 48] as compared to H3.3-mutant tumors. Additionally, DMG patients harboring H3.1 K27M mutations are more clinically responsive to radiotherapy than those with H3.3 mutation [11]. Conversely, in the adult population, H3.3-mutated tumors were associated with better survival in comparison with H3.1-mutated tumors. The mutational differences highlight that these are entities with distinct biological processes. Although we failed to establish a significant association of H3.3 mutation with prolonged survival in the multivariate model, it should be noted that the result might be skewed by the rarity of the H3.1 mutation in the adult population.
Our results are of clinical interest as they may provide insights into the survival difference between H3.1 and H3.3 K27M mutations and may have therapeutic applications in pediatric and adult populations. Therefore, genotyping of H3K27M mutations in midline gliomas should be routinely recommended for patients in clinical practice. Currently, published guidelines favor the use of immunohistochemistry for the detection of H3K27M mutations in midline gliomas because of its low cost, rapid turnaround time, and easy access [1, 9, 49]. A major drawback of this method is strong immunoreactivity of existing antibodies to both H3.1 and H3.3 proteins.
This is the first study to show that the prognostic outcome of H3K27M mutation genotypes is modulated by patient age. However, it has several limitations that need to be discussed. First, we could not avoid selection bias and unmeasured confounders because most included studies were retrospective. Second, our results are likely influenced by the rarity of DMG with H3K27M mutation in adults so the survival differences between H3.1 and H3,3 in adults should be interpreted with caution. Hopefully these findings and others inspire multiple centers to pool their data in this rare patient population. Future multicenter studies and/or meta-analyses with additional data for H3.1-mutant DMG in adult population are needed to confirm the prognostic differences of H3.1 − vs H3.3-mutant DMGs in adults.
In conclusion, the present study demonstrated that when compared to the H3.3 variant, the H3.1 K27M mutation is associated with a favorable prognosis in the pediatric DMGs but might portend an adverse outcome in the adult population. Routine genotyping of H3K27M mutations in DMGs in newly diagnosed DMGs may further refine our understanding and prediction of patient survival.
Data availability
Further data inquiries can be directed to the corresponding author.
References
Louis DN et al (2018) cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol 135(4):639–642
Hake SB, Allis CD (2006) Histone H3 variants and their potential role in indexing mammalian genomes: the “H3 barcode hypothesis.” Proc Natl Acad Sci 103(17):6428
Schulte JD et al (2020) Clinical, radiologic, and genetic characteristics of histone H3 K27M-mutant diffuse midline gliomas in adults. Neurooncol Adv 2(1):vdaa142
Schreck KC et al (2019) Incidence and clinicopathologic features of H3 K27M mutations in adults with radiographically-determined midline gliomas. J Neurooncol 143(1):87–93
Broniscer A, Gajjar A (2004) Supratentorial high-grade astrocytoma and diffuse brainstem glioma: two challenges for the pediatric oncologist. Oncologist 9(2):197–206
Picca A et al (2018) FGFR1 actionable mutations, molecular specificities, and outcome of adult midline gliomas. Neurology 90(23):e2086–e2094
Lynes J et al (2020) Variations in attitudes towards stereotactic biopsy of adult diffuse midline glioma patients: a survey of members of the AANS/CNS Tumor Section. J Neurooncol 149(1):161–170
Louis DN et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820
Louis DN et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23(8):1231–1251
Kleinschmidt-DeMasters BK, Mulcahy Levy JM (2018) H3 K27M-mutant gliomas in adults vs. children share similar histological features and adverse prognosis. Clin Neuropathol 37(2):53–63
Castel D et al (2015) Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol 130(6):815–827
Sturm D et al (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22(4):425–437
Ebrahimi A et al (2019) High frequency of H3 K27M mutations in adult midline gliomas. J Cancer Res Clin Oncol 145(4):839–850
Moher D et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Aihara K et al (2014) H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro Oncol 16(1):140–146
Bruzek AK et al (2020) Electronic DNA analysis of CSF cell-free tumor DNA to quantify multi-gene molecular response in pediatric high-grade glioma. Clin Cancer Res 26(23):6266–6276
Buczkowicz P et al (2014) Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet 46(5):451–456
Chiba K et al (2020) Pulvinar locus is highly relevant to patients’ outcomes in surgically resected thalamic gliomas in children. World Neurosurg 134:e530–e539
Crotty EE et al (2020) Children with DIPG and high-grade glioma treated with temozolomide, irinotecan, and bevacizumab: the Seattle Children’s Hospital experience. J Neurooncol 148(3):607–617
Dono A et al (2020) Adult diffuse midline gliomas: clinical, radiological, and genetic characteristics. J Clin Neurosci 82(Pt A):1–8
Eschbacher KL et al (2021) Diffuse gliomas of the brainstem and cerebellum in adults show molecular heterogeneity. Am J Surg Pathol 45(8):1082–1090
Fukami S et al (2018) Pathologic findings and clinical course of midline paraventricular gliomas diagnosed using a neuroendoscope. World Neurosurg 114:e366–e377
Garibotto F et al (2020) Pediatric diffuse midline gliomas H3 K27M-mutant and non-histone mutant midline high-grade gliomas in neurofibromatosis type 1 in comparison with non-syndromic children: a single-center pilot study. Front Oncol 10:795
Giagnacovo M et al (2020) Retrospective analysis on the consistency of MRI features with histological and molecular markers in diffuse intrinsic pontine glioma (DIPG). Childs Nerv Syst 36(4):697–704
Gojo J et al (2019) Personalized treatment of H3K27M-mutant pediatric diffuse gliomas provides improved therapeutic opportunities. Front Oncol 9:1436
Hoffman LM et al (2016) Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: implications for diagnostic biopsy and targeted therapeutics. Acta Neuropathol Commun 4:1
Mackay A et al (2018) Molecular, pathological, radiological, and immune profiling of non-brainstem pediatric high-grade glioma from the HERBY phase II randomized trial. Cancer Cell 33(5):829-842.e5
Meyronet D et al (2017) Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol 19(8):1127–1134
Mueller S et al (2019) A pilot precision medicine trial for children with diffuse intrinsic pontine glioma-PNOC003: a report from the Pacific Pediatric Neuro-Oncology Consortium. Int J Cancer 145(7):1889–1901
Nomura M et al (2017) Distinct molecular profile of diffuse cerebellar gliomas. Acta Neuropathol 134(6):941–956
Pan C et al (2019) Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol 137(2):297–306
Reinhardt A et al (2019) Tumors diagnosed as cerebellar glioblastoma comprise distinct molecular entities. Acta Neuropathol Commun 7(1):1–12
Reuss DE et al (2015) Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol 130(3):407–417
Sievers P et al (2021) A subset of pediatric-type thalamic gliomas share a distinct DNA methylation profile, H3K27me3 loss and frequent alteration of EGFR. Neuro Oncol 23(1):34–43
Taylor KR et al (2014) Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet 46(5):457–461
Yi S et al (2019) Impact of H3.3 K27M mutation on prognosis and survival of grade IV spinal cord glioma on the basis of new 2016 World Health Organization classification of the central nervous system. Neurosurgery 84(5):1072–1081
Vuong HG et al (2019) Prognostic significance of genetic biomarkers in isocitrate dehydrogenase-wild-type lower-grade glioma: the need to further stratify this tumor entity - a meta-analysis. Eur J Neurol 26(3):379–387
Tang MC et al (2015) Contribution of the two genes encoding histone variant H3.3 to viability and fertility in mice. PLoS Genet 11(2):e1004964
Vuong HG et al (2022) Risk stratification of H3 K27M–mutant diffuse midline gliomas based on anatomical locations: an integrated systematic review of individual participant data. J Neurosurg Pediatr. https://doi.org/10.3171/2022.3.PEDS2250
Khuong-Quang DA et al (2012) K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 124(3):439–447
Vuong HG et al (2022) Prognostic implication of patient age in H3K27M-mutant midline gliomas. Front Oncol 12:1–8
Sarthy JF et al (2020) Histone deposition pathways determine the chromatin landscapes of H3.1 and H3.3 K27M oncohistones. Elife 9:e61090
Tagami H et al (2004) Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116(1):51–61
Castel D et al (2018) Transcriptomic and epigenetic profiling of “diffuse midline gliomas, H3 K27M-mutant” discriminate two subgroups based on the type of histone H3 mutated and not supratentorial or infratentorial location. Acta Neuropathol Commun 6(1):117
Hoffman LM et al (2018) Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol 36(19):1963–1972
Vuong HG et al (2021) H3K27M-mutant diffuse midline gliomas should be further molecularly stratified: an integrated analysis of 669 patients. J Neurooncol 155(3):225–234
Fortin J et al (2020) Mutant ACVR1 arrests glial cell differentiation to drive tumorigenesis in pediatric gliomas. Cancer Cell 37(3):308-323.e12
Wu G et al (2014) The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 46(5):444–450
Weller M et al (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18(3):170–186
Acknowledgements
Not applicable
Funding
This study receives no funding support.
Author information
Authors and Affiliations
Contributions
HGV: conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, validation, writing original, review, and editing. TNNM: data curation, formal analysis, investigation, methodology, writing original, review, and editing. HTL: data curation, formal analysis, investigation, methodology, review, and editing. IFD: data curation, formal analysis, investigation, methodology, project administration, validation, review, editing, and supervision. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vuong, H.G., Ngo, T.N.M., Le, H.T. et al. The prognostic significance of HIST1H3B/C and H3F3A K27M mutations in diffuse midline gliomas is influenced by patient age. J Neurooncol 158, 405–412 (2022). https://doi.org/10.1007/s11060-022-04027-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04027-2