Abstract
Purpose
Cyclin-dependent kinase-retinoblastoma (CDK-RB) pathway is dysregulated in some diffuse intrinsic pontine gliomas (DIPG). We evaluated safety, feasibility, and early efficacy of the CDK4/6-inhibitor ribociclib, administered following radiotherapy in newly-diagnosed DIPG patients.
Methods
Following radiotherapy, eligible patients received ribociclib in 28-day cycles (350 mg/m2; 21 days on/7 days off). Feasibility endpoints included tolerability for at least 6 courses, and a less than 2-week delay in restarting therapy after 1 dose reduction. Early efficacy was measured by 1-year and median overall survival (OS). Patient/parent-by-proxy reported outcomes measurement information system (PROMIS) assessments were completed prospectively.
Results
The study included 10 evaluable patients, 9 DIPG and 1 diffuse midline glioma (DMG)—all 3.7 to 19.8 years of age. The median number of courses was 8 (range 3–14). Three patients required dose reduction for grade-4 neutropenia, and 1 discontinued therapy for hematological toxicity following course 4. The most common grade-3/4 toxicity was myelosuppression. After 2 courses, MRI evaluations in 4 patients revealed increased necrotic volume, associated with new neurological symptoms in 3 patients. The 1-year and median OS for DIPG was 89% and 16.1 months (range 10–30), respectively; the DMG patient died at 6 months post-diagnosis. Five patients donated brain tissue and tumor; 3 were RB+ .
Conclusions
Ribociclib administered following radiotherapy is feasible in DIPG and DMG. Increased tumor necrosis may represent a treatment effect. These data warrant further prospective volumetric analyses of tumors with necrosis. Feasibility and stabilization findings support further investigation of ribociclib in combination therapies.
Trial registration
NCT02607124.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DIPG and DMG with H3K27M mutation are the leading cause of brain cancer death in children and young adults [1,2,3]. The standard of care for DIPG and DMG is radiotherapy. Although radiotherapy can extend survival of DIPG patients by 2 to 3 months, no adjuvant therapy has proven effective. Similarly, patients with DMG, H3K27M-mutant have a poor outcome [3].
CDK4/6 inhibitors have been studied against a variety of neoplasms due to the finding that overexpression of CDK4/6, CCND1 or deletion of CDKN2A are hallmarks of tumorigenesis and invasiveness [4]. Ribociclib (Novartis Pharmaceuticals) is an orally bioavailable inhibitor of cyclin D-CDK4/6, which induces cell-cycle arrest by maintaining the tumor suppressor protein retinoblastoma (RB) in a hypophosphorylated, active state. Therapeutic feasibility of ribociclib for DIPG and DMG is supported by alterations of cell-cycle regulators in some tumors [5,6,7,8,9,10], over 70% of DIPG patients express intact RB [7, 11, 12], tolerable toxicity in adults and children, [13, 14] and prolonged stable disease in a pediatric Phase I study of recurrent rhabdoid tumors, neuroblastoma, and tumors with cell-cycle pathway aberrations [14].
We report the first safety, feasibility, and early response data for ribociclib following radiotherapy in newly diagnosed DIPG and RB+ DMG patients (NCT 02607124) using the pediatric RP2D (350 mg/m2) once daily for 21 days/7 days off [14].
Methods
Study design and objectives
This investigator-sponsored Phase I/II study evaluated ribociclib administered post-radiotherapy in pediatric patients newly diagnosed with DIPG or DMG, K27M-mutant. Primary objectives were safety, feasibility, and 1-year OS. Secondary objectives were median OS and correlative assessments.
Patients
Patients were newly-diagnosed with either imaging confirmed DIPG (aged 1–30 years) or histologically-confirmed DMG. DIPG tumors were defined by neuroimaging (diffuse intrinsic involvement and pontine epicenter) without histological confirmation. Patients with brainstem tumors and atypical imaging were eligible if the tumor was histologically confirmed WHO grade-III/IV glioma according to the 2016 WHO classification [15] and confirmed RB+ . DMG tumors may have extended beyond the brainstem, had to be RB+ and histologically confirmed as DMG, H3K27M-mutant [15]. Patients with primary spinal cord tumors or multi-focal disease within the cerebrum were eligible. Pilocytic astrocytoma, pilomyxoid astrocytoma, pleomorphic xanthoastrocytoma, ganglioglioma, mixed glioma without anaplasia, oligodendroglioma, or oligoastrocytoma were excluded.
Within 30 days of radiographic diagnosis or definitive surgery, patients must have initiated radiation therapy dosed within 10% of standard (54 Gy). Other eligibility criteria included the following: Lansky (≤ 16 years) or Karnofsky (> 16 years), performance scores (≥ 50%); no prior therapy other than surgery, radiation, and/or steroids; adequate laboratory data, including bone marrow function (hemoglobin ≥ 9 g/dL, absolute neutrophil count ≥ 1,000 mm3, and platelets ≥ 100,000/mm3 transfusion-independent, defined as no platelet transfusion within a 7-day period prior to enrollment), renal function (age-adjusted normal serum creatinine or glomerular filtration > 70 ml/min/1.73 m2), liver function (total bilirubin < 3X upper limit of normal for age, ALT [SGPT] ≤ 2.5X upper limit of normal for age, albumin ≥ 2 g/dL), and recovered from acute radiation-related toxicities (≤ grade 2). Patients with controlled seizures on non-enzyme inducing anticonvulsants were eligible.
Patients were excluded for the following: pregnancy; disseminated disease to the spine; received a radiosensitizer, investigational agent, or additional adjuvant therapy during radiotherapy; on potent CYP3A4 inducers/inhibitors; significant active cardiac disease, hypertension, uncontrolled heart disease, history of cardiac dysfunction, cardiomyopathy, left ventricular ejection fraction < 50%, QTc > 480 ms; on warfarin or other coumadin-derived anticoagulant; and had major surgery within 14 days of first ribociclib dose.
Treatment and dose modifications
Two-to-4 weeks post-radiotherapy, patients received 350 mg/m2 ribociclib daily for 21 days/7 days off every 28 days for up to 12 courses as capsules or liquid (nasogastric/gastric tube) [13, 14].
One intra-patient dose reduction (280 mg/m2) was permitted for toxicities graded according to the NCI Common Terminology Criteria for Adverse Events (v4.03). Hematological toxicities attributable to ribociclib requiring dose reductions were ≥ grade-3 thrombocytopenia or grade-4 neutropenia during days 1 to 21; reductions were not made for grade-4 neutropenia during the 7-day rest period if patients met study parameters for the subsequent course. Non-hematologic toxicities requiring dose reductions were ≥ grade-2 AST/ALT and/or bilirubin, QTc prolongation, and grade-2 non-hematologic toxicity persisting for ≥ 7 days and considered medically significant or sufficiently intolerable. If a subsequent course was delayed ≥ 14 days, a dose reduction was warranted. Reductions were not made for the following grade-3 non-hematologic toxicities: nausea, vomiting, fever, or infection lasting < 5 days, hypophosphatemia, hypokalemia, hypocalcemia, or hypomagnesemia responsive to oral supplementation, and anorexia.
Assessments
Pretreatment evaluations included history, physical examination, performance status, laboratory, and MRI. Patients were monitored for treatment-related toxicities and disease-related morbidities using neurological examination and performance score (prior to each course), EKG and electrolytes (days 1 and 15 of course 1, and then prior to subsequent courses), complete blood counts (weekly), and echocardiogram (baseline, prior to course 4, and as clinically indicated thereafter). Also monitored were hypothalamic pituitary axis function and pubertal status (baseline, prior to course 7, and yearly) by Tanner staging, FSH, LH, estradiol and ovarian reserve evaluated by anti-Mullerian hormone (AMH; females), and testosterone (males).
PROMIS (Patient-reported outcomes measurement information system) evaluations were administered to eligible patients or parents by proxy (baseline and monthly). PROMIS pediatric measures have validated psychometric characteristics for 8-to-17-year-old children and parent proxy reports for 5-to-17 year-old children [16]. Domains included anxiety, depressive symptoms, fatigue, pain interference, physical function and upper extremity mobility, and social-peer relationships [16]. Each domain utilized a 5-point response scale (higher scores represent greater effect), 7-day recall, and a “T-score” metric using each measure’s item response theory parameters and response-pattern-based scoring (Mplus 8) [17].
Radiographic (MRI) evaluations
MRIs were obtained at baseline (post-radiotherapy and within 2 weeks of starting ribociclib therapy), every 8 weeks during therapy, and upon completion. Images were reviewed by 2 neuroradiologists (J.L. and B.J.), according to Response Assessment in Pediatric Neuro-Oncology [18].
Tumor response was measured using product of perpendicular dimensions (PPD) analysis. PPD was measured as the largest tumor dimension and its perpendicular from transverse, anterior–posterior, and cranio-caudal planes from T1 enhanced, T2 weighted, or FLAIR images used for serial and 2-dimensional measurements. Necrosis, distinguished as a well-defined non-enhancing signal intensity (hypointense on T1 and hyperintense on T2) with peripheral, rim-like enhancement, was evaluated by position and size: if eccentric and small (< 25% of tumor) or eccentric and large, only the solid portion of the tumor was measured; if central and small, the whole lesion (including necrosis) was measured.
Tumor response was assessed as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). CR was defined by the complete disappearance of tumor and mass effect on MRI, and PR was a 50% or greater reduction in tumor size by PPD (vs baseline). Patients with CR or PR tumors were on stable or decreasing doses of steroids with stable or improving neurologic exams (maintained for 8 weeks). SD was defined as stable neurologic exams, maintenance steroid dosing, and imaging that did not meet PR or PD criteria (maintained for 16 weeks). To qualify as having PD, patients had to meet 1 or more of the following criteria: progressive neurologic symptoms not explained by causes unrelated to tumor progression, > 25% increase in tumor (with smallest PPD as reference), new lesions, or increasing steroid dosing to maintain neurological status.
Patients showing pseudoprogression on MRI in the first 6 months of treatment remained on study at the physician’s discretion—repeating the MRI after 4 to 6 weeks. If this MRI demonstrated tumor regression or SD, and neurological examination remained stable, the patient remained on treatment; if it showed PD, treatment was discontinued. Steroids were allowed as long as the patient maintained on decreasing doses.
Tumor and necrotic volumes As a secondary objective, tumor volume (T2 signal) and necrotic volume (T1 or T2 signal) were measured. Volumetric analyses were performed via MINT lesion (Mint Medical GmbH) to compare against the standard, PPD [19].
Pathology and genomics
Immunohistochemistry for RB and mutation-specific histone H3K27M was performed as previously described [20, 21], in a CLIA-certified laboratory. Tumors were RB+ if 20% or more of nuclei were immunopositive in at least 3 20X fields; RB+ endothelial cells served as an internal positive control. DNA extraction and Sanger sequencing were performed as previously described [22]. Whole-genome sequencing (WGS) was performed on tumor tissue (vs normal), WGS data analyzed by VIVA (CCHMC), somatic mutations identified using GATK and MuTact2, annotated using VEP [23,24,25], and those in coding regions with moderate-to-high impact selected.
Statistical analyses
Feasibility endpoints were toxic death or grade-3/4 toxicities that resulted in discontinuation of ribociclib, delays (> 2 weeks) in starting a treatment course, or discontinuation of therapy after dose reduction. For a patient surviving 6 months after initiation of ribociclib, feasibility was confirmed if 5 of 6 courses were completed and they received at least 80% of therapy administered including 1 dose reduction to 280 mg/m2. If more than 2 of the first 12 enrolled evaluable patients were infeasible, the study stopped and ribociclib was deemed infeasible for this population. In the event of early closure of the study, less than 25% of patients with dose-modifying toxicities after 1 dose reduction would be considered feasible. Patients were evaluable for safety and feasibility if they completed all clinical and laboratory monitoring requirements before ribociclib discontinuation due to toxicity or disease progression. Patients were evaluable for safety if they received any ribociclib therapy and no additional anticancer therapy or supportive care to confound interpretation—even if they were removed for progression or toxicity after dose reduction.
Results
Patients
Between March 31, 2016 and November 8, 2017, 18 patients were screened, and 11 patients (61%) enrolled. Reasons for not enrolling included parental decision, RB− status, delay in starting radiation therapy, insufficient tissue for screening, and disseminated disease to the spine. Upon additional review, 1 of the 11 patients was deemed ineligible due to evidence of disseminated disease discovered after enrollment (but prior to receiving any investigational agents). Of the 10 remaining enrolled patients (4 male, 6 female), the median age at diagnosis was 7.3 years (range 3.7 to 19.8; Table 1); 9 were diagnosed with DIPG, 1 with DMG. Patient 9 (DIPG) had a diagnostic biopsy due to atypical features extending to the left midbrain and thalamus; pathology revealed grade-IV infiltrating glioma in the pons, with intact RB. Patient 1 was enrolled with a midline tumor and what appeared to be a non-contiguous cerebellar lesion, pathology consistent with H3K27M-mutant DMG, and intact RB (later autopsy analysis revealed that the midline and cerebellar tumors were, in fact, contiguous).
Treatment and dose reductions
The median number of courses for all 10 patients was 8 (range 3–14), with 6 receiving at least 6 courses. Dose reductions were required in 3 patients—2 for grade-4 neutropenia following course 4, and 1 for a patient with confounding viral upper-respiratory illness at the time of initial grade-4 neutropenia following course 1 who, despite dose reduction, came off therapy for hematological toxicity following course 4. The primary feasibility endpoint could not be determined due to an amendment investigating ribociclib and everolimus therapy (NCT03355794). Nonetheless, ribociclib post-radiation therapy was deemed feasible, given that fewer than 25% of patients had dose-modifying toxicities after 1 dose reduction, and 6 patients received 6 or more courses. Two of these 6 patients completed therapy (≥ 12 courses) and 4 came off treatment (3 with disease progression, 1 with toxicity). The most common grade-3/4 toxicities were neutropenia (90%), lymphopenia (50%), and leukopenia (70%; Table 2). No patients died due to toxicity.
Outcome
Nine patients developed disease progression, and 1 elected to discontinue therapy after course 14 to pursue another clinical trial. Necrosis was observed in baseline MRIs of 9 patients; 4 exhibited increasing necrosis—3 after cycle 2 and 1 after cycle 3. For 1 of the 4 patients, necrosis, as measured volumetrically, increased steadily through cycle 4, and then decreased, nearly resolving by cycle 12 (Fig. 1, and data not shown). The volumetric increase in necrosis, from baseline to just after cycle 2, ranged from 0.1 to 2.1 ml (0.3–21.9%)—with the largest increases occurring in 2 patients that continued therapy through cycles 10 and 13. The 1-year and median OS for DIPG was 89% and 16.1 months (range 10–30 mo), respectively; 2 DIPG patients survived 28 and 30 months post-diagnosis. OS for the DMG patient was 6 months.
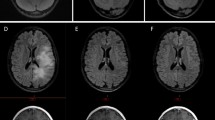
Changes in necrotic volume in patient 3. MRI images obtained at baseline, and after cycles 4 (C4) and 12 (C12)- 113 and 355 days after diagnosis, respectively. Images: T2 weighted (T2), fluid-attenuated inversion recovery image (FLAIR), and T1 + C weighted after contrast administration (T1). Small region of necrosis in left pons at diagnosis had increased markedly by 113 days post- diagnosis (arrow) and was nearly resolved by 355 days. NV necrosis volume, TV tumor volume
Correlative findings
The hypothalamic-pituitary axis and pubertal development were evaluated at baseline for all 10 patients. Of the 6 patients who received at least 6 courses of therapy, 5 had follow-up endocrine function labs prior to course 7; 3 were pre-pubertal (Tanner I). For all 5 patients, endocrine function did not change, and none developed hypothyroidism. AMH assessments were performed in 3 of the 4 patients, and all values were within reference range.
PROMIS assessments were completed for 8 patients: 6 by proxy only, 1 by self-report, and 1 by both. Patients and proxies reported similar patterns. Patients completed a mean of 8 assessments during therapy. Patients did not report elevated anxiety (mean = 44.39), depressive symptoms (mean = 44.39), fatigue (mean = 46.96), pain interference (mean = 44.43), or impaired social relationships (mean = 54.39)—but did report poor physical function mobility (mean = 37.43) and physical function upper extremity (mean = 39.06). While self- and proxy-reports had frequent item non-response across domains, proxy reports had larger proportions of missing data. Questionnaire items presented later were more often incomplete with some exceptions. Although missing data were not used to calculate a score for that measure, poorer functioning was strongly correlated with more missing data. For example, the correlation between proxy report anxiety and the percent of skipped items was 0.81.
Autopsy findings
Of the 5 patients who donated their brain and tumor (Table 3), 3 were RB+; the percentage of RB+ nuclei varied within each of the 3 tumors (Fig. 2). The 2 patients (1 DIPG, 1 DMG) with the lowest RB+ tumor cell numbers (10%) had the shortest OS (< 11 mo) supporting that intact RB is needed for ribociclib to induce cell-cycle arrest [26]. Autopsy also revealed the H3F3A mutation in all 5 patients analyzed. WGS, completed on 4 patients, revealed upregulation of cell-cycle pathways in 2 (patients 4 and 5) (data not shown). The Sanger sequencing data corroborated the IHC data (Table 3).
Discussion
This is the first study to evaluate safety, feasibility, and early efficacy of ribociclib following radiotherapy in children with newly diagnosed DIPG and DMG. After 19 months, this study was amended to include everolimus. Despite this amendment, 10 patients were treated and 6 received at least 6 courses of ribociclib at the pediatric RP2D, [14] thereby confirming feasibility post-radiotherapy.
The ribociclib therapy was well tolerated with a safety profile similar to prior studies [13, 14]. Grade-3/4 neutropenia and lymphopenia were noted more frequently in our study compared to the pediatric Phase I study [14]. This was expected, given that the median number of courses of the Phase I study was 2 vs 8 in our study, and data suggesting that ribociclib-induced neutropenia appears to be concentration-dependent, transient, and reversible [13, 14]. Grade-3/4 thrombocytopenia was not observed in our chemo-naïve patients vs 27% in the pediatric Phase I study, where the cohort had prior therapy. Our quantitative analysis of necrosis volume revealed that 3 of 4 patients had increased necrosis; 2 correlated with clinical symptoms and subsequently experienced clinical improvement and were able to receive 4 and 10 additional cycles, respectively, suggesting necrosis was a treatment effect rather than a reflection of early tumor progression. Interestingly, the 14 year old DIPG patient developed significantly increased necrosis and received 14 cycles of therapy, raising questions regarding the clinical meaning of necrosis and how to incorporate necrotic areas in future clinical trials.
The median OS was 16.1 months for our DIPG patients, and 2 of our 10 patients survived for 28 and 30 months. In contrast, the International and European Society for Pediatric Oncology DIPG registries, reported 1008 patients with radiographically confirmed DIPG, had a median OS of 11 months and only 10% survived for 2 years or longer [27]. In our study, the ages of the 2 longest surviving patients were less than 3 years and more than 14, corroborating prior reports that long-term DIPG survivors are more commonly diagnosed at the age extremes (age < 3 or > 10) [27] and may have confounded prolonged disease stabilization. Furthermore, these 2 patients underwent subsequent therapies that may have impacted survival; however, this seems less likely given that the therapies were different (Table 1).
Three of the 5 patients’ samples at autopsy had RB+ tumor cells, with heterogeneity. Heterogeneity may be due to autopsy tissue quality or tumor biology. Given the role of the RB pathway in the mechanism of ribociclib, it will be important for future studies to determine whether lack of uniformity of RB+ cells confirmed post-treatment is observed in pre-treatment samples. If observed, it would support not mandating biopsy in DIPG in future clinical trials at this time.
Many studies of pediatric brain tumors have demonstrated endocrinopathies [28,29,30,31,32,33,34,35,36] stemming from the tumor, but also secondary to treatments (e.g., surgical resection, radiotherapy, chemotherapy), patient age, and pre-treatment follicular pool (females) [37, 38]. In our study, ribociclib did not affect pubertal development or AMH, and there were no reports of endocrinopathies as adverse events in the Pediatric Phase I study [14] suggesting that ribociclib does not significantly impact hormonal function.
Our PROMIS assessments were feasible even though significant amounts of missing data for each measure were observed. We found that the poorer a patient’s health, the more likely a respondent was to leave items unanswered. Nevertheless, because PROMIS measures were developed using item-response theory, we were able to estimate scores on the T-score metric for all 8 patients. Non-response did not impede our ability to understand a child’s health-related quality of life. We found that the patients in our study appeared to be functioning relatively well on average across domains but did show impaired physical function mobility and upper extremity most likely due to disease. Our amended trial will build on these findings, by utilizing electronic administration of PROMIS.
The data that we report here, have already led to 2 further studies. First, our findings support a combination study, specifically with everolimus (NCT03355794), given that alterations in the PI3K signaling pathway are frequently observed in DIPG [5]; this combination is likely to be tolerable, given the non-overlapping single-agent toxicities and clinical experience in adults [39]. The second study, which is open to enrollment (NCT03387020), is evaluating adequacy of ribociclib concentrations reaching target tissue in pediatric brain tumor patients.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Hargrave D, Bartels U, Bouffet E (2006) Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7:241–248. https://doi.org/10.1016/S1470-2045(06)70615-5
Jansen MH, van Vuurden DG, Vandertop WP, Kaspers GJ (2012) Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev 38:27–35. https://doi.org/10.1016/j.ctrv.2011.06.007
Karremann M, Gielen GH, Hoffmann M, Wiese M, Colditz N, Warmuth-Metz M, Bison B, Claviez A, van Vuurden DG, von Bueren AO, Gessi M, Kuhnle I, Hans VH, Benesch M, Sturm D, Kortmann RD, Waha A, Pietsch T, Kramm CM (2018) Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro-Oncology 20:123–131. https://doi.org/10.1093/neuonc/nox149
Shapiro GI (2006) Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol 24:1770–1783. https://doi.org/10.1200/JCO.2005.03.7689
Wu G, Broniscer A, McEachron TA, Lu C, Paugh BS, Becksfort J, Qu C, Ding L, Huether R, Parker M, Zhang J, Gajjar A, Dyer MA, Mullighan CG, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Ellison DW, Baker SJ, Project SJCsRHWUPCG (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44:251–253. https://doi.org/10.1038/ng.1102
Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tönjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jäger N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Frühwald MC, Roggendorf W, Kramm C, Dürken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482:226–231. https://doi.org/10.1038/nature10833
Buczkowicz P, Hoeman C, Rakopoulos P, Pajovic S, Letourneau L, Dzamba M, Morrison A, Lewis P, Bouffet E, Bartels U, Zuccaro J, Agnihotri S, Ryall S, Barszczyk M, Chornenkyy Y, Bourgey M, Bourque G, Montpetit A, Cordero F, Castelo-Branco P, Mangerel J, Tabori U, Ho KC, Huang A, Taylor KR, Mackay A, Bendel AE, Nazarian J, Fangusaro JR, Karajannis MA, Zagzag D, Foreman NK, Donson A, Hegert JV, Smith A, Chan J, Lafay-Cousin L, Dunn S, Hukin J, Dunham C, Scheinemann K, Michaud J, Zelcer S, Ramsay D, Cain J, Brennan C, Souweidane MM, Jones C, Allis CD, Brudno M, Becher O, Hawkins C (2014) Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. https://doi.org/10.1038/ng.2936
Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, Nikbakht H, Gerges N, Fiset PO, Bechet D, Faury D, De Jay N, Ramkissoon LA, Corcoran A, Jones DT, Sturm D, Johann P, Tomita T, Goldman S, Nagib M, Bendel A, Goumnerova L, Bowers DC, Leonard JR, Rubin JB, Alden T, Browd S, Geyer JR, Leary S, Jallo G, Cohen K, Gupta N, Prados MD, Carret AS, Ellezam B, Crevier L, Klekner A, Bognar L, Hauser P, Garami M, Myseros J, Dong Z, Siegel PM, Malkin H, Ligon AH, Albrecht S, Pfister SM, Ligon KL, Majewski J, Jabado N, Kieran MW (2014) Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet. https://doi.org/10.1038/ng.2950
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O'Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, Network TR (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477. https://doi.org/10.1016/j.cell.2013.09.034
Paugh BS, Broniscer A, Qu C, Miller CP, Zhang J, Tatevossian RG, Olson JM, Geyer JR, Chi SN, da Silva NS, Onar-Thomas A, Baker JN, Gajjar A, Ellison DW, Baker SJ (2011) Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol 29:3999–4006. https://doi.org/10.1200/JCO.2011.35.5677
Taylor KR, Mackay A, Truffaux N, Butterfield YS, Morozova O, Philippe C, Castel D, Grasso CS, Vinci M, Carvalho D, Carcaboso AM, de Torres C, Cruz O, Mora J, Entz-Werle N, Ingram WJ, Monje M, Hargrave D, Bullock AN, Puget S, Yip S, Jones C, Grill J (2014) Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet. https://doi.org/10.1038/ng.2925
Grill J, Puget S, Andreiuolo F, Philippe C, MacConaill L, Kieran MW (2012) Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr Blood Cancer 58:489–491. https://doi.org/10.1002/pbc.24060
Infante JR, Cassier PA, Gerecitano JF, Witteveen PO, Chugh R, Ribrag V, Chakraborty A, Matano A, Dobson JR, Crystal AS, Parasuraman S, Shapiro GI (2016) A phase I study of the cyclin-dependent kinase 4/6 Inhibitor Ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin Cancer Res 22:5696–5705. https://doi.org/10.1158/1078-0432.CCR-16-1248
Geoerger B, Bourdeaut F, DuBois SG, Fischer M, Geller JI, Gottardo NG, Marabelle A, Pearson ADJ, Modak S, Cash T, Robinson GW, Motta M, Matano A, Bhansali SG, Dobson JR, Parasuraman S, Chi SN (2017) A phase I study of the CDK4/6 inhibitor ribociclib (LEE011) in pediatric patients with malignant rhabdoid tumors, neuroblastoma, and other solid tumors. Clin Cancer Res 23:2433–2441. https://doi.org/10.1158/1078-0432.CCR-16-2898
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Irwin DE, Gross HE, Stucky BD, Thissen D, DeWitt EM, Lai JS, Amtmann D, Khastou L, Varni JW, DeWalt DA (2012) Development of six PROMIS pediatrics proxy-report item banks. Health Qual Life Outcomes 10:22. https://doi.org/10.1186/1477-7525-10-22
Muthén LKaM, B.O. (1998–2017) Mplus user’s guide
Warren KE, Patronas N, Aikin AA, Albert PS, Balis FM (2001) Comparison of one-, two-, and three-dimensional measurements of childhood brain tumors. J Natl Cancer Inst 93:1401–1405
Gilligan LA, DeWire-Schottmiller MD, Fouladi M, DeBlank P, Leach JL (2020) Tumor response assessment in diffuse intrinsic pontine glioma: comparison of semiautomated volumetric, semiautomated linear, and manual linear tumor measurement strategies. AJNR Am J Neuroradiol 41:866–873. https://doi.org/10.3174/ajnr.A6555
Goldhoff P, Clarke J, Smirnov I, Berger MS, Prados MD, James CD, Perry A, Phillips JJ (2012) Clinical stratification of glioblastoma based on alterations in retinoblastoma tumor suppressor protein (RB1) and association with the proneural subtype. J Neuropathol Exp Neurol 71:83–89. https://doi.org/10.1097/NEN.0b013e31823fe8f1
Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N, Phillips JJ, Perry A (2016) Diffuse midline gliomas with histone H3–K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol 26:569–580. https://doi.org/10.1111/bpa.12336
Hoffman LM, DeWire M, Ryall S, Buczkowicz P, Leach J, Miles L, Ramani A, Brudno M, Kumar SS, Drissi R, Dexheimer P, Salloum R, Chow L, Hummel T, Stevenson C, Lu QR, Jones B, Witte D, Aronow B, Hawkins CE, Fouladi M (2016) Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: implications for diagnostic biopsy and targeted therapeutics. Acta Neuropathol Commun 4:1. https://doi.org/10.1186/s40478-015-0269-0
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The Genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. https://doi.org/10.1101/gr.107524.110
Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES, Getz G (2013) Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 31:213–219. https://doi.org/10.1038/nbt.2514
McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, Flicek P, Cunningham F (2016) The ensembl variant effect predictor. Genome Biol 17:122. https://doi.org/10.1186/s13059-016-0974-4
Sherr CJ, Beach D, Shapiro GI (2016) Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov 6:353–367. https://doi.org/10.1158/2159-8290.CD-15-0894
Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, Baugh J, Chaney B, Hoffmann M, Lane A, Fuller C, Miles L, Hawkins C, Bartels U, Bouffet E, Goldman S, Leary S, Foreman NK, Packer R, Warren KE, Broniscer A, Kieran MW, Minturn J, Comito M, Broxson E, Shih CS, Khatua S, Chintagumpala M, Carret AS, Escorza NY, Hassall T, Ziegler DS, Gottardo N, Dholaria H, Doughman R, Benesch M, Drissi R, Nazarian J, Jabado N, Boddaert N, Varlet P, Giraud G, Castel D, Puget S, Jones C, Hulleman E, Modena P, Giagnacovo M, Antonelli M, Pietsch T, Gielen GH, Jones DTW, Sturm D, Pfister SM, Gerber NU, Grotzer MA, Pfaff E, von Bueren AO, Hargrave D, Solanki GA, Jadrijevic Cvrlje F, Kaspers GJL, Vandertop WP, Grill J, Bailey S, Biassoni V, Massimino M, Calmon R, Sanchez E, Bison B, Warmuth-Metz M, Leach J, Jones B, van Vuurden DG, Kramm CM, Fouladi M (2018) Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of Diffuse Intrinsic Pontine Glioma (DIPG): a Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol. https://doi.org/10.1200/JCO.2017.75.9308
Stevens MC, Mahler H, Parkes S (1998) The health status of adult survivors of cancer in childhood. Eur J Cancer 34:694–698
Landier W, Bhatia S (2008) Cancer survivorship: a pediatric perspective. Oncologist 13:1181–1192. https://doi.org/10.1634/theoncologist.2008-0104
Nandagopal R, Laverdière C, Mulrooney D, Hudson MM, Meacham L (2008) Endocrine late effects of childhood cancer therapy: a report from the Children's Oncology Group. Horm Res 69:65–74. https://doi.org/10.1159/000111809
Armstrong GT, Liu Q, Yasui Y, Huang S, Ness KK, Leisenring W, Hudson MM, Donaldson SS, King AA, Stovall M, Krull KR, Robison LL, Packer RJ (2009) Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst 101:946–958. https://doi.org/10.1093/jnci/djp148
Merchant TE, Pollack IF, Loeffler JS (2010) Brain tumors across the age spectrum: biology, therapy, and late effects. Semin Radiat Oncol 20:58–66. https://doi.org/10.1016/j.semradonc.2009.09.005
Rose SR, Schreiber RE, Kearney NS, Lustig RH, Danish RK, Burghen GA, Hudson MM (2004) Hypothalamic dysfunction after chemotherapy. J Pediatr Endocrinol Metab 17:55–66
Merchant TE, Williams T, Smith JM, Rose SR, Danish RK, Burghen GA, Kun LE, Lustig RH (2002) Preirradiation endocrinopathies in pediatric brain tumor patients determined by dynamic tests of endocrine function. Int J Radiat Oncol Biol Phys 54:45–50
Spoudeas HA (2002) Growth and endocrine function after chemotherapy and radiotherapy in childhood. Eur J Cancer 38:1748–1759 (discussion 1760-1741)
Green DM, Sklar CA, Boice JD, Mulvihill JJ, Whitton JA, Stovall M, Yasui Y (2009) Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol 27:2374–2381. https://doi.org/10.1200/JCO.2008.21.1839
Wallace WH, Anderson RA, Irvine DS (2005) Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol 6:209–218. https://doi.org/10.1016/S1470-2045(05)70092-9
DeWire M, Green DM, Sklar CA, Merchant TE, Wallace D, Lin T, Vern-Gross T, Kun LE, Krasin MJ, Boyett JM, Wright KD, Wetmore C, Broniscer A, Gajjar A (2015) Pubertal development and primary ovarian insufficiency in female survivors of embryonal brain tumors following risk-adapted craniospinal irradiation and adjuvant chemotherapy. Pediatr Blood Cancer 62:329–334. https://doi.org/10.1002/pbc.25274
Bardia A (2015) Triplet therapy with ribociclib, everolimus, and exemestane in postmenopausal women with HR+/HER2- advanced breast cancer. Clin Cancer Res 24(21):5206–5218
Acknowledgements
We gratefully acknowledge the excellent regulatory support of Dr. Renee Doughman, data management by Nicole O’Connell, and the generosity of the patients and their families for supporting the study.
Funding
This work was financially supported by Novartis Pharmaceuticals, The Cure Starts Now Foundation, Hope for Caroline Foundation, Julian Boivin Courage for Cures Foundation, Abbie's Army, Michael Mosier Defeat DIPG Foundation, Reflections of Grace Foundation, The Cure Starts Now Australia, Brooke Healey Foundation, Soar With Grace Foundation, Jeffrey Thomas Hayden Foundation, Cure Brain Cancer Foundation, The Jones Family Foundation, Musella Foundation, Pray, Hope Believe Foundation, Smiles for Sophie Foundation, Benny's World, Love Chloe Foundation, Aiden's Avengers, A Cure from Caleb Society, The Operation Grace White Foundation, Ryan's Hope, Wayland Villars DIPG Foundation, American Childhood Cancer Organization, Juliana Rose Donnelly Trust, Sheila Jones & Friends, The Ellie Kavalieros DIPG Research Fund, Voices Against Brain Cancer, and The DIPG Collaborative.
Author information
Authors and Affiliations
Contributions
Conception and design: MD, MF, and AL. Data collection and assembly: NOC. Data analyses and interpretation: all authors with survival analyses by AL, molecular analyses by XZ, and pathologic analyses by CF. Manuscript writing and editing: MD with critical feedback from all authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The institutional review board approved the protocol and continuing approval was maintained throughout the study.
Informed consent
Informed consent and assent were obtained according to institutional guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
DeWire, M., Fuller, C., Hummel, T.R. et al. A phase I/II study of ribociclib following radiation therapy in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG). J Neurooncol 149, 511–522 (2020). https://doi.org/10.1007/s11060-020-03641-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03641-2