Abstract
Introduction
Alterations in the CDK4/6—RB signaling pathway are common causes of cell cycle dysregulation in many cancers, including glioblastoma. Palbociclib is an oral inhibitor of CDK4/6, which leads to phosphorylation of RB1 and cell-cycle arrest. We conducted a two-arm study evaluating efficacy and tissue pharmacokinetics/pharmacodynamics of palbociclib in patients with recurrent glioblastoma.
Methods
Eligibility criteria included confirmation of RB1 proficiency by IHC; ≤ 3 relapses; KPS ≥ 60; no limit on prior treatments. Arm 1 received palbociclib for 7 days prior to indicated resection followed by adjuvant palbociclib. Arm 2 received palbociclib without resection. Primary objective was PFS6; secondary included toxicity, OS, and ORR. Exploratory aims included biomarker assessment and pharmacokinetic/pharmacodynamic effects in surgical patients.
Results
Total of 22 patients were enrolled; 6 on Arm 1 and 16 on Arm 2. Trial was stopped early secondary to lack of efficacy, with 95% of evaluable patients progressing within 6 months. Median PFS was 5.14 weeks (range 5 days–142 weeks) and median OS was 15.4 weeks (range 2–274 weeks). Two patients (10%) had related grade ≥ 3 AEs. In Arm 1, 5 patients had tissue concentrations of palbociclib felt to be sufficient for biological effect and paired samples available for RB1 IHC. There were no consistent changes in RB1 expression or cell proliferation in the paired tissue.
Conclusion
In this trial, despite adequate tissue PK, palbociclib monotherapy was not an effective treatment for recurrent glioblastoma. However, these were heavily pretreated patients and targeting the CDK4/6 pathway may still deserve further exploration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Patients with glioblastoma have a poor prognosis. Glioblastoma is the second leading cause of cancer mortality in people under the age of 35 and the fourth leading cause in those under the age of 54. Despite treatment including maximal surgical resection, concurrent radiation and temozolomide and adjuvant temozolomide [1], median progression-free survival (PFS) and overall survival (OS) remain 6.9 months and 14.6 months, respectively, and 5-year survival is approximately 10% [2]. Prognosis at recurrence is very poor and treatment options are limited [3, 4], with most targeted agents demonstrating a PFS at 6 months (PFS6) of 10%. There is an urgent need to identify novel therapeutic targets for drug development.
Despite their heterogeneous nature, glioblastomas have common features, among which is the obligate disruption of the cyclin dependent kinases 4 and 6 (CDK4/6)-p16-retinoblastoma protein (Rb) pathway that regulates cell cycling, and dysregulation of which is a hallmark of malignancy [5]. The most common method of dysregulating this pathway in glioblastoma is homozygous deletion of the cyclin-dependent kinase inhibitor 2A and B (CDKN2A/B) genes. CDKN2A encodes for the tumor suppressor p16 (INK4a), which binds to CDK4/6. CDK4 and CDK6 normally function to regulate the cell cycle and stimulate cell cycle progression, but is inhibited by p16 binding Therefore, when CDKN2A is deleted, CDK4/6 remains activated and cell growth continues unregulated [6]. Preclinical work in orthotopic glioblastoma xenografts demonstrated that inhibition of CDK4/6 resulted in reduction of tumor cell proliferation and prolonged survival, indicating the efficacy of blocking these kinases in preclinical models [7].
Palbociclib is an oral highly selective inhibitor of the CDK4/CKD6 protein kinases. CDK4 and CDK6 function to phosphorylate and activate the retinoblastoma (Rb) protein, which allows cell cycle progression from G1 to S phase, creating a redundant mechanism to regulate cell cycle progression. Inhibition of CDK4/6 results in suppression of Rb phosphorylation and apoptosis. Palbociclib received accelerated approval by the FDA for use in combination with aromatase inhibitors for estrogen-receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer in February 2015. This was based on the phase 3, PALOMA-2 study demonstrating an increased PFS when palbociclib was combined with letrozole versus letrozole alone [8] and in March 2017 it received subsequent full approval.
Given the efficacy in breast cancer and pre-clinical data presented above, there was biologic rationale to evaluate palbociclib in patients with glioblastoma. The objective of this phase 2 study was to evaluate the efficacy and safety of palbociclib as a monotherapy in treating recurrent glioblastoma without mutations in RB1.
Patients and methods
Patients
Eligible patients (aged ≥ 18 years) had histologically confirmed glioblastoma or gliosarcoma with radiographic evidence of recurrent disease after prior radiation and temozolomide; ≤ 3 recurrences without limit to the number of prior chemotherapies used; documentation of RB1 positive disease by immunohistochemistry (IHC); Karnofsky Performance Status (KPS) ≥ 60. For Arm 1, patients had surgically resectable disease.
Key exclusion criteria included history of other malignancy (except non-melanoma skin cancer or carcinoma in situ of the cervix), unless in complete remission and off of all therapy for at least 3 years; history of acute intracranial hemorrhage; concomitant CYP3A inhibitors or inducers within 14 days; history or evidence of prolonged QTc interval.
The UCSF institutional review board approved the study protocol. All procedures were performed in accordance with the ethical standards of the Helsinki Declaration. All patients provided written informed consent.
Study design
This open-labeled, two-arm, single-agent, study of palbociclib in patients in patients with recurrent glioblastoma was conducted at UCSF (ClinicalTrials.gov: NCT01227434). There were two treatment arms; target enrollment for each arm was 15 patients. Patients enrolled on Arm 1 were candidates for resection, while patients enrolled on Arm 2 did not undergo additional resection. The primary endpoint for the combined cohort was PFS6. Secondary endpoints included toxicity, OS, and objective response rate (ORR) according to the RANO criteria [9]. Exploratory endpoints in the surgical samples from Arm 1 included tumor tissue pharmacokinetics, pharmacodynamics effects such as changes from baseline of RB1 and cell proliferation (Ki-67) by IHC, and correlation with tumor response.
Patients on Arm 1 received palbociclib 125 mg by mouth daily for 7 days prior to surgery. After at least 2 weeks had elapsed post-operatively, patients resumed palbociclib. Post-operative Arm 1 patients and Arm 2 patients both received palbociclib at 125 mg daily for 21 consecutive days followed by a 7 day break, 28-day cycles, until disease progression, intolerability, or withdrawal of consent.
Tumor assessments
For the secondary outcome of ORR, tumor response was assessed by RANO criteria [9]. Patients in both arms had a contrast–enhanced baseline MRI within 14 days of starting palbociclib. In Arm 1, an MRI was conducted within 96 h post-surgery. If treatment was not started within 14 days of surgery, a MRI was repeated. In both arms, tumors were assessed serially with MRI every 8 weeks.
Safety
All AEs from the time of enrollment until the end of study were graded using the Cancer Therapy Evaluation Program (CTEP) Active Version of the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. For both arms, clinical and basic laboratory assessments (liver and renal) were performed at baseline, and day 1 of every cycle. Complete blood counts with differential were also examined on days 1 and 15 of each cycle. In addition, patients on Arm 1 had clinical and laboratory assessments on day 1 pre-surgery and the day of surgery.
Pharmacokinetics and pharmacodynamics
For patients who underwent planned surgical resection, at least 50 mg of fresh, flash-frozen tissue and blood sample were sent to Pfizer for PK analysis.
RB1 IHC was performed on archival formalin-fixed, paraffin-embedded tissue using the Ventana Medical Systems Benchmark XT and the iView detection system and quantified as previously described [10]. Slides were deparaffinized in xylene, and rehydrated through ethanol. Heated Tris buffer pH 8 was used for antigen retrieval and slides were blocked with 3% methanol–hydrogen peroxide at 22 °C for 16 min. Slides were incubated in mouse monoclonal anti-RB1 antibody (G3-245: BD Pharmingen) for 2 h at 37 °C. glioblastoma xenografts of known RB1 status, both positive and negative, served as controls and tumors were scored as either positive (≥ 20% nuclei positive) or negative (< 20% nuclei positive) for RB1 protein.
Ki-67 (MIB-1 epitope), a marker of cell proliferation and indicator of palbociclib activity, should be reduced by palbociclib treatment. Ki-67 IHC was performed as described above except antigen retrieval was performed for 16 min and a rabbit anti-MIB-1 (Ventana, 790–7264) antibody incubated for 32 min at 37 °C. To calculate the nuclear labeling index (LI) for Ki67, 500–2000 nuclei per tissue section were evaluated and the number of Ki-67-positive nuclei per total nuclei counted and multiplied by 100%.
Statistical analysis
The study planned to enroll 30 patients, including 15 surgical cases (Arm 1) and 15 non-surgical cases (Arm 2) and included formal stopping rules for toxicity for each arm independently. The primary endpoint was PFS6, defined as the proportion of patients alive and progression-free at 6 months. PFS was calculated as the time in weeks from the first post-surgical dose of palbociclib [11]. The null hypothesis was PFS6 = 10%, a rate felt to represent an ineffective treatment based upon historical data [12], and alternative hypothesis of PFS6 ≥ 30% based on results of the BRAIN trial [3]. However, recognizing the likelihood that patients may have received prior bevacizumab, even a lower success rate may be of interest. With 30 patients there is 90% power to detect an improvement in the PFS6 from 10 to 30% (assuming the same degree of improvement in efficacy for non-surgical and pre-surgical cases) based on a one-sided exact test with α = 0.1.
The primary and secondary endpoints were conducted for all patients in Arm 2 who received ≥ 1 dose of palbociclib and all patients in Arm 1 who received ≥ 1 dose of palbociclib after surgery. Safety analyses were conducted for all patients who received ≥ 1 dose of palbociclib in each cohort. Kaplan–Meier was used to graphically depict the distributions of OS and PFS. Best ORR (complete, response, partial response, stable disease) was calculated as a proportion with 2-sided 95% confidence interval (CI). Cox proportional hazards models were used to assess the relationship between time-to-event end points and baseline variables.
The in vitro and in vivo preclinical data suggested that a biological effect would occur at a tumor concentration of between 0.06 and 1.0 μM (IC50) [13]. The summary statistics (including the mean and standard deviation) of the drug concentration were included in the analysis.
Results
Patients
Between November 30, 2010, and August 2, 2013, 22 patients were enrolled in this study, 6 on Arm 1 and 16 on Arm 2 (Table 1). In the combined cohort, median age was 47.5 years old (range 23–78 years old); 54% (12 patients) were male; and the median KPS was 90 (range 60–100). Palbociclib was started at first recurrence in 50% (11 patients); at second recurrence in 36% (8 patients); and at third recurrence in 14% (3 patients). All patients had received prior radiation and temozolomide, and 77% (17 patients) had previously received bevacizumab—12 received it upfront and 5 at recurrence.
Efficacy
The primary endpoint was PFS6. The trial was stopped early secondary to futility, with 95% (18 of 19) evaluable patients progressing within 6 months of initiating treatment. Median PFS was 5.14 weeks (range 5 days–142 weeks) for all evaluable patients. Median OS was 15.4 weeks (range 2–274 weeks) for all evaluable patients (Fig. 1).
Toxicity
Including both arms, 2 patients (9%) experienced serious adverse events (SAEs) ≥ grade 3 that were thought to be at least possibly related to palbociclib (Table 2). There were 11 SAEs seen in the 6 patients on the Arm 1 surgical arm; 7 SAEs on the Arm 2 nonsurgical arm. There were three deaths on study that were felt to be unrelated to study drug—one related to lung infection and two related to disease progression.
Pharmacokinetic and pharmacodynamic analyses
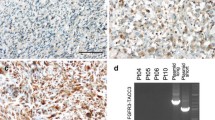
Five paired samples were available for IHC for RB1. While two showed a decrease in RB1 protein expression after therapy, there were no consistent changes in RB1 expression across the five patients (Fig. 2). Cell proliferation, as determined by Ki-67 labeling index, was also not significantly changed in matched samples before and after therapy, (n = 4, data not shown). Concentration of palbociclib in tumor tissue was measured in five samples by high performance liquid chromatography (HPLC), all of which exceeded the 0.06 μM concentration felt to be biologically effective [13] (Table 3). One patient received pre-op treatment but did not undergo surgery due to rapid clinical decline.
Palbociclib exposure had no consistent effects on RB1 expression. a, b Representative of immunohistochemical staining of RB1 expression for patient PD107 (a) at baseline (77%) and b after pre-surgical palbociclib (31%). c Quantification of RB1 expression for five patients who underwent resection demonstrating variable impact on protein expression. Median RB1 at baseline for all patients was 70.9% (range 2.6–97.8%)
Discussion
Although analysis of the surgical samples demonstrated tumor concentrations of palbociclib thought to be biologically effective, there was no consistent reduction of RB1 protein expression or decrease in cell proliferation by Ki-67. Palbociclib also did not appear to have single-agent efficacy in this heavily pre-treated population of patients with recurrent glioblastoma. A significant proportion of patients, 77%, received bevacizumab prior to palbociclib, with a median time off treatment of 10 weeks (range 5–69 weeks). Twelve patients (54%) received bevacizumab upfront as part of a phase 2 trial of erlotinib, bevacizumab, and temozolomide in newly diagnosed glioblastoma. Four of the six (67%) of the patients in the surgical Arm 1 previously received bevacizumab (Table 1). It is now known that bevacizumab does not improve OS in the newly diagnosed setting [14, 15], the data of which was not known at the time of trial conception. Wash-out from bevacizumab and surgical complications may have impacted the outcome of this study as well. The study was stopped early based on futility with 95% (18 of 19) evaluable patients progressing within 6 months, and thus failed to meet its primary efficacy goal of PFS6 ≥ 30%.
Although our study did not demonstrate efficacy for palbociclib monotherapy in recurrent glioblastoma, the CDK4/6 pathway may remain a rational therapeutic target. Activation of CDK4/6 is very common in glioblastoma. While only five patients had matched tumor tissue before and after therapy, the decrease in RB1 protein expression after therapy in two may suggest heterogeneity in RB1 regulation. Though RB-proficiency is thought to be the predominant determinant of CDK4/6 responsiveness, CDK4/5 amplification and CDKN2A homozygous deletion may also impact responsivity [7, 16, 17]. We did not perform sequencing to confirm wildtype RB1, or clearly delineate the pathway alterations such as deletion in CDKN2A/B, or C, or amplification of CDK4 or CDK6, which may have provided further insight into response.
Abemaciclib, a related drug is under investigation in first recurrent, bevacizumab naïve patients [NCT02981940] and other gliomas [NCT03220646]. Another CDK4/6 inhibitor, ribociclib is also being investigated in high grade glioma [NCT02345824, NCT02933736], meningiomas [NCT02933736], and pediatric gliomas [NCT03355794, NCT03434262]. Combination therapy treatments that include administration of palbociclib, such as was approved for ER-positive, HER2-negative advanced breast cancer, may as well be of interest for clinical evaluation [18]. Pre-clinical work from our group, combining palbociclib with radiation in glioblastoma cell lines and xenografts demonstrated inhibition of DNA double-strand break repair and increased apoptosis [7, 19]. Palbociclib treatment extends the period of unrepaired DNA double-strand breaks caused by tumor cell irradiation, suggesting a possible role for palbociclib in combination with radiation [7, 19]. Pre-clinical work with other CDK4/6 inhibitors, such as abemaciclib, has also identified increased tumor cell antigen presentation coupled with antitumor T-cell responses suggested that immune checkpoint blockade may be synergistic with CDK4/6 inhibition [20].
In summary, our results suggest that palbociclib monotherapy is not associated with antitumor activity in recurrent glioblastoma patients with prior bevacizumab exposure. We confirmed CNS penetration and drug concentration at what would appear to be biologically effective doses based upon pre-clinical data. The sample size of post-treatment specimens limited the ability to generate definitive conclusions regarding target effects. Our lack of efficacy may be in part attributed to the extent of prior treatment and percentage of patients who were refractory to bevacizumab. Targeting the CDK4/6 pathway earlier on in the disease course, potentially in combination with radiation, warrants further investigation.
References
Stupp R et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Stupp R et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466
Friedman HS et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740
Kreisl TN et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745
Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9(3):153–166
Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455(7216):1061–1068
Michaud K et al (2010) Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res 70(8):3228–3238
Finn RS et al (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936
Wen PY et al (2010) Response assessment challenges in clinical trials of gliomas. Curr Oncol Rep 12(1):68–75
Goldhoff P et al (2012) Clinical stratification of glioblastoma based on alterations in retinoblastoma tumor suppressor protein (RB1) and association with the proneural subtype. J Neuropathol Exp Neurol 71(1):83–89
Clarke JL et al (2011) Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro Oncol 13(10): 1118–1124
Lamborn KR et al (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 10(2):162–170
PD-0332991 Investigator’s Brocure. 2010
Chinot OL et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722
Gilbert MR et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708
Hashizume R et al (2016) Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro Oncol 18:1519–1528
Sherr CJ, Beach D, Shapiro GI (2016) Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov 6(4):353–367
Huillard E et al (2012) Cooperative interactions of BRAFV600E kinase and CDKN2A locus deficiency in pediatric malignant astrocytoma as a basis for rational therapy. Proc Natl Acad Sci USA 109(22):8710–8715
Hashizume R et al (2016) Inhibition of DNA damage repair by the CDK4/6 inhibitor palbociclib delays irradiated intracranial atypical teratoid rhabdoid tumor and glioblastoma xenograft regrowth. Neuro Oncol 18(11):1519–1528
Goel S et al (2017) CDK4/6 inhibition triggers anti-tumour immunity. Nature 548(7668):471–475
Funding
Support for this study was provided by Pfizer (M.P.), National Cancer Institute P50CA097257 (M.P. and C.D.J), R01CA159467 (C.D.J.) and the Accelerated Brain Cancer Cure (ABC2) (M.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors report no conflicts of interest relevant to this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Taylor, J.W., Parikh, M., Phillips, J.J. et al. Phase-2 trial of palbociclib in adult patients with recurrent RB1-positive glioblastoma. J Neurooncol 140, 477–483 (2018). https://doi.org/10.1007/s11060-018-2977-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2977-3