Abstract
Introduction
Runt-related transcription factor 3 (RUNX3) exerts a tumor suppressor gene associated with gastric and other cancers, including glioma. However, how its anti-tumor mechanism in functions glioma is unclear.
Methods
We assayed expression of RUNX3 with a tissue microarray (TMA), frozen cancer tissues and malignant glioma cell lines using immunohistochemistry, qRT-PCR and Western bolt analysis. Cell proliferation, invasion, cell cycle distribution and apoptosis were also examined to confirm the effect of RUNX3 medicated malignant phenotype. TOP/FOP experiment was used to detect the β-catenin/Tcf-4 transcription activity by RUNX3.
Results
Enforced RUNX3 expression inhibited proliferation and invasion, induced cell cycle arrest and promoted apoptosis in vitro and in vivo, Bim siRNA partically reversed the effect of RUNX3-induced apoptosis in LN229 and U87 cells, suggesting a dependent role of Bim-caspase pathway. Moreover, Mechanism investigations revealed that restoration of RUNX3 suppressed β-catenin/Tcf-4 transcription activity.
Conclusions
RUNX3 plays a pivotal role in glioma initiation and progression as a tumor suppressor via attenuation of Wnt signaling, highlighting it as a potential therapeutic target for glioma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomagenesis is a multifactorial and complicated pathological process involved in multiple genetic alterations and complex signaling pathways. Gene instability, including activation of oncogenes, such as AKT, EGFR and K-ras, inactivation of tumor suppressors containing PTEN and P53 and enhanced signaling pathways, including the Wnt/β-catenin and PI3K/AKT pathways, have been reported to contribute to this type of tumor [1, 2]. Although many studies have confirmed specific genes and signaling pathways, the underlying molecular mechanisms of glioma have not been fully elucidated. Thus, more research is required to elucidate mechanisms related to gliomas to develop novel therapeutic strategies.
The RUNX3 gene is located at 1p36, a region of frequent genomic loss in a variety of human carcinomas, including glioblastoma [3]. The functional relationship between RUNX and TGF-β is supported by a synergism between RUNX3 and Smads to regulate target gene transcription [4]. The gastric epithelium of RUNX3−/− mice were hyperplastic due to increased proliferation and diminished apoptosis arising from insensitivity of gastric epithelia to TGF-β. This was evidence that RUNX3 was a tumor suppressor in gastric cancer [5]. Reports suggest that RUNX3 attenuates β-catenin/T Cell Factors in Intestinal Tumorigenesis, but the function of RUNX3 and its relationship with the Wnt/β-catenin signaling pathway is not well understood in gliomagenesis.
Here, we report that RUNX3 is frequently downregulated in malignant glioma cell lines and tissue specimens. Restoration of RUNX3 inhibits the proliferative, invasive ability of tumor cells and induces cell cycle arrest and the expression of proteins associated with these phenotypes in vitro and in vivo. Additionally, we confirmed that RUNX3 attenuates the Wnt/β-catenin signaling pathway in gliomagenesis, suggesting a molecular mechanism of RUNX3 medicated inhibition of tumorgenesis. RUNX3 may be a biomarker for GBM and may hold promise for therapeutic approaches to GBM treatment.
Materials and methods
Tissue samples and microarray
A tissue microarray was prepared by the Chao Ying Bio-technology Company (Shanxi, China) from archived paraffin-embedded glioma specimens. The tissue microarray slide contained 59 glioma samples of different grades, including 9 grade I samples, 20 grade II samples, 17 grade III samples and 13 grade IV samples. Each patient had three specimens and samples were classified according to the 2007 WHO Classification of Tumors of the Central Nervous System. Then, nine normal brain tissue samples were obtained from areas adjacent to the tumor and identified histopathologically. In addition, three fresh glioblastoma specimens and adjacent noncancerous tissues were prospectively collected from the Department of Neurosurgery at Affiliated Hospital of Taishan Medical University. This study was approved by the hospital institutional review board and written informed consent was obtained from all patients.
Cell culture, adenovirus infection and gene transfection
Human U87, LN229, SNB19, U251, A172, and LN308 glioblastoma cells and low-grade glioma H4 cells were obtained from the China Academia Sinica Cell Repository, Shanghai, China. The TJ905 cell line was established and characterized by the Laboratory of Neuro-Oncology of the Tianjin Neurological Institute. Cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco, Los Angeles, CA) supplemented with 10% fetal bovine serum (Gibco) and were incubated at 37 °C in a 5% CO2 atmosphere.
Adenovirus containing a RUNX3 cDNA (rAD-RUNX3) and negative control (rAD-vector) were obtained from Genesil (Wuhan, China). TS-RUNX3 and goal vector PEC-IRES-EGFP were doubly digestion with BamHI and EcoRI and subcloned into PEC-IRES-EGFP. The RUNX3 expression box was transferred to the pGSadeno adenovirus by in vitro homologous recombination of LR. The recombinant adenoviral plasmid was identified by restriction analysis with PacI and PCR examination. The recombinant products were plague purified and expanded by transducing HEK293 cells. GBM cells were infected with adenoviruses at multiplicities of infection (MOI) of 5.
Human U87 and LN229 glioma cell lines were infected with viral suspension and then transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Bim siRNA (Cell Signaling Technology, USA) was used to knock down the expression of Bim.
Real-time PCR
Total RNA was extracted using Trizol Reagent (Invitrogen) according to a standard protocol. Total RNA (1 µg) was used for cDNA synthesis by reverse transcription with MMLV reverse transcriptase (Promega) according to the manufacturer’s protocol in a total volume of 10 µL. RT-PCR products were separated in a 2% agarose gel which was then stained with ethidium bromide, digitally photographed, and scanned with an UVI Gel Analyzing System (UVI Tech, Cambridge, UK). Quantitative analysis of expression was calculated using qRT-PCR (7500 ABI, USA). The TaqMan assay kit (Applied Biosystems) was used to measure gene expression. Relative quantification was conducted using amplification efficiencies derived from cDNA standard curves and relative gene expression. Data represented as fold change s(2 − ΔΔCt) and were initially analyzed using Opticon Monitor Analysis Software V2.02 software (MJ Research, Waltham, MA). Specific RT-PCR primers were obtained from Fulen Gene BiolEngineering Inc., Guangdong, China.
Western blot analysis
Total protein was extracted from cells, and 40 µg of protein lysate from each sample were resolved by SDS-PAGE and transferred to a PVDF membrane. Membranes were incubated with primary antibodies against RUNX3, Bim, cyclinD1, C-myc, β-catenin (1:1000, Abcam), caspases 3 and 9, cleaved caspase 9, MMP2, MMP9, TIMP1 and TIMP3 (1:500, Santa Cruz), followed by an HRP-conjugated secondary protein (1:1000 Zymed, San Diego, 40 µg CA). Protein was quantified using a SuperSignal protein kit (Pierce). β-actin or GADPH primary antibody (1:1000, Santa Cruz) was used as the control.
Colony formation assay
Cells were seeded in 6-well plates (0.5 × 103 cells/well) and cultured for 2 weeks. Colonies were fixed with methanol for 10 min and stained with 1% crystal violet (Sigma) for 1 min. Each group was measured in triplicate.
Proliferation assay
U87 and LN229 cells were seeded into 96-well plates (4000 cells/well). After transfection, on each day for 7 consecutive days, 20 mL MTT (5 mg/mL) was added to each well, and the cells were incubated at 37 °C for an additional 4 h. Then, the supernatant was discarded. The reaction was terminated by lysing the cells with 200 mL of DMSO. The optical density was measured at 570 nm and expressed as a percentage of the control. Data are presented as means ± SEM derived from triplicate samples of at least three independent experiments.
Cell-cycle analysis
For cell-cycle analysis by flow cytometry, transfected and control cells in the log phase of growth were harvested by trypsinization, washed with PBS, fixed with 75% ethanol overnight at 4 °C and then incubated with RNase at 37 °C for 30 min. A total of 104 nuclei were assessed with a FACS Calibur Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ), and DNA histograms were analyzed using Modifit software (Becton Dickinson). Experiments were performed in triplicate.
Cell invasion assays
Transwell membranes coated with Matrigel (BD Biosciences, San Jose, CA) were used to quantify glioma cell invasion. Transfected cells were plated at 5 × 104 per well in the upper chamber in serum-free medium. U87 and LN229 cells (200 µL) was used as chemoattractant and placed in the bottom chamber. After a 24 h incubation, the filters were gently taken out, the medium was removed from the upper chamber. The noninvaded cells on the upper surface of the inserted filter were scraped off with a cotton swab. The cells that had migrated into the lower surface of the inserted filter were fixed with methanol. Data are presented as means ± SE of three independent experiments.
Apoptosis assays
Apoptosis was quantified 48 h after transfection, using Annexin-V labeling with an an Annexin-V-FITC-labeled Apoptosis Detection Kit (Abcam) according to the manufacturer’s protocol and a TUNEL assay was used to measure apoptosis in tumor specimens [6].
Luciferase reporter assay
To evaluate β-catenin/Tcf-4 transcriptional activity, we used TOP-FLASH and FOP-FLASH (Upstate) luciferase reporter constructs. TOP-FLASH (with three repeats of the Tcf-binding site) or FOP-FLASH (with three repeats of a mutated Tcf-binding site) plasmids were transfected into cells treated with Ad-RUNX3. After a 48-h incubation, luciferase activity was measured using a dual luciferase reporter system (Promega). Luciferase activity was measured 48 h after transfection. Renilla luciferase activity was used as an internal control.
Immunofluorescence and immunohistochemical staining
Immunofluorescence and IHC assays were performed as previously described. Immunofluorescence was measured using an antibody against β-catenin (1:1000 dilution; Abcam). IHC scores were performed using a semi-quantitative 5-category grading system [7, 8].
Intracranial nude mouse models
ALL animal protocols were performed in line with an approved Institutional Animal care and Committee protocol. BALB/c-A nude mice at 4 weeks old were purchased from the Animal Center at the Cancer Institute at the Chinese Academy of Medical Science. To establish intracranial gliomas, 5 × 105 U87 glioblastoma cells pretreated with Ad-RUNX3 or vector were implanted stereotactically [9]. Bioluminescent imaging was used to confirm intracranial tumor growth or tumorigenicity using an IVIS Imaging System (caliper Life Sciences) for determining the integrated flux of photons within each region of interest. The overall survival was analyzed according to the Kaplan–Meier method.
Statistical analysis
Statistics were performed using SPSS Graduate Pack, statistical software, version 16.0. Descriptive statistics, including means and SEs and 1-way analysis of variance, were used to determine statistically significant differences. Overall survival curves were plotted according to a Kaplan–Meier method, with the log-rank test applied for comparisons (P < 0.05 was considered to be statistically significant).
Results
RUNX3 is frequently downregulated in glioma and is correlated with tumor grade
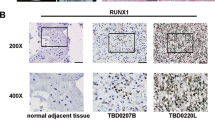
To further study the role of RUNX3 related with the development and progression in glioma, we first examine its expression in seven Glioblastoma derived cell lines. Real-time PCR analysis and Western blot showed that all seven malignant cell lines had significantly less RUNX3 gene expression compared with low-grade cell lines H4 (p < 0.001; Fig. 1a). The protein levels were consistent with the mRNA expression and significantly evident in LN229 and U87 cells (Fig. 1b). In addition, three human glioblastoma tissues and matched adjacent noncancerous tissues, western blot results showed RUNX3 was significantly loss or lower expressed compared with adjacent noncancerous tissues (Fig. 1c). Immunohistochemical staining scores used to study the tissue microarray (TMA, Normal, grades I–IV) confirmed that RUNX3 is significantly overexpressed in normal brain tissues and inversely associated with WHO grade by staining score evaluation (p < 0.01; Fig. 1d). Moreover, RUNX3 expression decreased markedly in high-grade gliomas (grade III or IV) compared with low-grade gliomas (grade I or II). The clinicopathological characteristics of 58 glioma specimens are summarized in Table 1 and there was no correlation between RUNX3 expression with other clinicopathologic variables, including gender and age except for the relationship with WHO grade.
RUNX3 is downregulated in GBM cell lines and human glioma tissues with various grades according to WHO. a and b Real-time PCR and Western blot analysis from the low grade H4 cell line and GBM derived cell lines (A172, SNB19, U251, U87, TJ905, LN229, LN308). c Expression protein levels of RUNX3 in three GBM and the matched adjacent noncancerous tissues by western blot. d Immunohistochemical staining of 9 normal brain tissues and 59 primary human gliomas tissues, WHO grades I, II, III, IV (TMA) using anti-REUNX3 antibody. RUNX3 protein expression reduced markedly in high grade gliomas (WHO III and IV) compared to low grade gliomas (WHO I and II). Heat map representing its expression level is determined in a linear scale with a maximum value of 6 immunoreactive score to highlight differences within this range. Staining score described in “Materials and methods”
Restoration of RUNX3 expression inhibits glioma cell proliferation and induces cell cycle G0/G1 arrest
To know the effect of RUNX3 restoration expression on glioma, we stably transfected LN229 and U87 cells with recombinant adenoviruses harboring RUNX3 (Ad-RUNX3) and control vectors. The mRNA and protein expression identification of RUNX3 were determined by Real-time PCR, western blot. RUNX3 was significantly up-regulated in these cell lines that showed evidently lower expression (Fig. 2a, b). To evaluate the effect of the reintroduction of RUNX3 on LN229 and U87 cell proliferation, we used an MTT assay. The growth curve showed re-introduction of RUNX3 gene inhibited cell growth. A evident difference was observed after 3 days of culture in these two cells (Fig. 2c). Moreover, overexpression of RUNX3 caused decreased colony formation (Fig. 2d, 21 days after infection). Cell-cycle analysis data showed that a significant increase in the proportion of cells in G0/G1 transfected with Ad-RUNX3 in comparison with control and control vectors (Fig. 2e). Thus, restoration of RUNX3 expression induces cell arrest at the G0/G1 phase, delays cell cycle progression and suppresses cell proliferation.
RUNX3 suppresses glioma proliferation and induces cell cycle arrest at the G0/G1. a and b Restoration expression identification of RUNX3 after transfacted with recombinant adenoviruses harboring RUNX3 by real-time PCR and Western blot. c Proliferation rate of LN229 and U87 cells transfected with Ad-RUNX3 determined by MTT assay. d Colony formation assay of LN229 and U87 cells expressing Control vector, Ad-RUNX3 after 21 days. Cells stained with 0.005% crystal violet solution. e Flow cytometry data represents more cells were arrested in G0/G1 phase of cell cycle in the Ad-RUNX3 group compared with control and vector groups
RUNX3 induces glioma apoptosis via the Bim-caspase pathway
Previous reports implicated Bim in medicating apoptotic effects of RUNX3 in the TGF-β signaling pathway. Re-introduction of RUNX3 gene led to overexpression of Bim according to real-time PCR and Western blot in the absence of TGF-β (Fig. 3a, c). In cell lines, average apoptotic fractions (early apoptotic + apoptotic) were significantly increased with Ad-RUNX3 upregulation compared with control and control vectors (p < 0.05) as detected by Annexin-V assay (Fig. 3b).
Upregulation of RUNX3 increase the expression of Bim and induce cell apoptosis dependent on Bim/caspase pathway. a Bim expression level detection after transfected with Ad-RUNX3 for 48 h in LN229 and U87 cells. Compared with control and vector group, Bim expression was increased markedly in Ad-RUNX3 group. b After transfected Ad-RUNX3 and co-transfected Bim siRNA, Annexin V-PI apoptosis detection assay were performed. Compared with control and vector group, RUNX3 resulted in a significant increase of apoptotic cells in both cell lines, while siRNA against Bim reduced RUNX3-induced apoptosis in co-transfected group. All data represented mean ± SD of three individual experiments performed in triplicate for each treatment. P < 0.05 compared with control and vector groups. c Western blot assay was performed to analyse the expression apoptosis-related signaling molecules using antibodies against Bim, Bcl2, caspase 3, 9, cleaved caspase 3 and GAPDH
In LN229 and U87 cell lines, co-transfected with Ad-RUNX3 and Bim siRNA, Bim disruption reduced RUNX3-induced apoptosis in RUNX3-overexpressing cell lines. Western blotting confirmed that the pro-apoptotic factors caspase 9 and 3 and cleaved-caspase 3 expression were associated with reintroduction of RUNX3 and downregulation of Bim. Apoptotic-inhibitory factor BCL2 exhibited the contrast change compared to the Bim and caspase 9 (Fig. 3c). Thus, RUNX3 overexpression promotes apoptosis in the absence of TGF-β in glioma cells and the effect was achieved by Bim-caspase signaling at least in LN229 and U87 cell lines.
RUNX3 restoration inhibits glioma cell invasion in vitro
To assess the role of RUNX3 in invasion, we took transwell assay. The transfection of RUNX3 reduced the number of invasion U87 and LN229 cells, compared with control and vector group (Fig. 4a). In addition, western blot analysis showed that the invasive relative factor MMP2, MMP9 expression decreased and TIMP1, TIMP3 expression increased after RUNX3 restoration (Fig. 4b). Thus, these data demonstrate that RUNX3 plays a role in glioma cell invasion.
Effects of RUNX3 on the abilities of invasion in vitro. a Cell invasion assays using transwell coated with Matrigel. Significant reduction of invasion was observed after increased RUNX3 expression in LN229 and U87 cells. b Western blot analysis of MMP2/9, TIMP1/3 expression associated with invasion ability
Restoration of RUNX3 attenuates the β-catenin/Tcf-4 signaling pathway
To investigate the downstream effectors of RUNX3 and the effective mechanism of β-catenin transcriptional activity, TOP/FOP FLASH luciferase assay was used after RUNX3 gene re-introduction. In LN229 and U87 cells, restoration of RUNX3 reduced Top with no change in FOP-FLASH luciferase activity (Fig. 5a). To determine whether decreased transcriptional activity was accompanied by reduced expression, Western blot and immunofluorescence assay recealed that ectopic expression of RUNX3 reduced the expression of β-catenin in the cytoplasm and nucleus (Fig. 5b, c). This confirmed a role for the β-catenin/Tcf-4 pathway as a mediator of RUNX3 activity. Western blot analysis indicated reduced relative expression of downstream targets cyclinD1 and c-Myc in cells with high RUNX3 expression (Fig. 5d). Therefore, RUNX3 regulates β-catenin/Tcf-4 transcriptional activity.
RUNX3 restoration suppresses β-catenin/TCF-4 transcriptional activity. a U87 and LN229 cells were cotransfected with Top/Fop, Ad-RUNX3. And luciferase reporter assays were performed. b and c Immunofluorescence and Western blot detection of β-catenin 48 h following transfection of U87 and LN229 cells with Ad-RUNX3. d Western blot detection of cyclinD1, c-myc 48 h following transfection of U87 and LN229 cells with Ad-RUNX3
RUNX3 inhibits tumor growth in vivo
Since re-introduction of RUNX3 represses the proliferation and invasion of gliomas cells and pro-apoptotic effect in vitro, we further assess its effect on tumor growth in vivo. The effect of RUNX3 on an intracranial U87 xenograft model was studied by implanting U87 cells conditionally expressing luciferase lentiviruses and Ad-RUNX3 or control vectors, xenograft growth was monitored by bioluminescence imaging. Results from Kaplan–Meier analyses showed that re-introduction of RUNX3 correlated with significantly longer survival (Fig. 6a). U87 cells stably transfected with Ad-RUNX3 had slight ability for tumorigenesis. Figure 6b demonstrated representative animal BLI images of RUNX3-expressing xenografts and vector-expressing xenograft indicating a markedly reduction of the tumor (Fig. 6c). Tumor samples were dissected from mice, and paraffin-embedded sections were prepared for immunohistopathological examination and TUNEL assay. The overexpression of RUNX3 in U87 cells transfected with Ad-RUNX3 was confirmed by immunohistopathological assay in tumor (Fig. 6d). Consistent with the in vitro results, expression of Bim, TIMP1, caspase 3 were significantly increased and the expression of β-catenin, CyclinD1, MMP2 were decreased in specimens from Ad-RUNX3 samples (Fig. 6f). Compared with vector controls, the Ad-RUNX3 group had more apoptotic nuclei, as verified by TUNEL analysis (Fig. 6e). Therefore, restoration of RUNX3 in vivo had functions similar to those in vitro.
Increased RUNX3 expression in U87 cells in vivo significantly inhibited intracranial tumor growth in a mice xenograft model. a Improved survival was observed in mice that were treated with Ad-RUNX3 transfection. b Luminescence images from Ad-RUNX3-treated U87 animals at 10, 17 after implantation compared with vector-treated group. c Tumor growth curves were analyzed. The data are described as mean ± SD. *p < 0.05. d and f Representative photomicrographs of immunohistochemistry of tumor sections for RUNX3, β-catenin, cyclinD1, Bim, Timp1, MMP2 and caspase 3. e TUNEL analysis on xenograft tumor sections
Discussion
Glioblastoma multiforme (GBM) is the most common malignant primary brain tumor of adults, accounting for > 50% of all primary brain gliomas. Because GBM is complex and heterogeneous, current therapies, including surgery, radiotherapy, and chemotherapy are only modestly effective and represent poor prognosis. Thus, personalized treatment may be used to target aberrant genes and oncogeneic signaling pathways, such as P53, PTEN, Rb, PI3K/AKT/mTOR, Wnt/β-catenin and the Notch pathway involved in the molecular pathogenesis of malignant gliomas [1]. To this end, we studied the anti-tumor gene RUNX3 on proliferation, invasion, apoptosis, cell cycle distribution and the correlation with the Wnt signaling pathway. We hypothesize RUNX3 may be a tumor suppressor at least partly through inactivation of the Wnt pathway in gliomas.
Evidences suggest that RUNX3 expression is the lost or downregulated in numerous malignant tumors, including gastric, breast and colorectal cancers, renal cell carcinoma and hepatocellular cancer. Previous work suggests that RUNX3 frequently is inactivated due to hemizygous deletion of the Runx3 gene, hypermethylation of the promoter, or cytoplasmic sequestration of the RUNX3 protein. RUNX3 downregulation reported to be medicated by microRNA such as microRNA130b and microRNA-148a [10, 11]. We verified reduced expression or loss of RUNX3 in malignant glioma cell lines and tissue specimens, suggesting that it has potential anti-tumor activity. Then, we used functional analyses to examine glioma malignancy and underlying anti-tumor mechanisms induced by inhibition of the Wnt/β-catenin pathway.
The Wnt/β-catenin signaling pathway is a crucial oncogenic pathway essential for glioma cell survival and nuclear accumulation of β-catenin for malignant progression and poor patient prognosis [8]. Our work confirmed that overexpression of RUNX3 repressed the nuclear transcription of β-catenin and downstream targets, such as cyclinD1 and c-myc, indicating that RUNX3-mediated tumor inhibitory effects occur via suppression of Wnt/β-catenin signaling pathway. Moreover, MTT and flow cytometry data show that reintroduction of RUNX3 inhibited glioma cell proliferation and induced cell cycle arrest at the GO/G1 phase. CyclinD1 is a downstream effector of the Wnt/β-catenin signaling pathway and preferentially binds to and activates CDK4 and CDK6 at the G1 phase to initiate cell cycle progression. Lin’s group reported that RUNX3, acts as a key transcriptional factor, directly binds to the AKT1 promoter and inhibits oncogenic factor AKT1 expression, subsequent β-catenin protein degradation and cyclinD1 downregulation [12]. This, and our data, suggest cyclinD1 is a target of RUNX3-induced cell proliferation and cell cycle arrest. However, RUNX3-mediated suppression of the Akt1/GSK-3β/β-catenin/cyclinD1 pathway reported by Lin suggest that RUNX3 forms a complex with β-catenin/TCFs by preventingβ-catenin/TCF DNA-binding to targets but does not change the β-catenin protein in intestinal tumorigenesis [13]. Thus, RUNX3 regulation of β-catenin differs with tissue type. Our data show that β-catenin expression was reduced in the nucleus and cytoplasm, indicating that RUNX3-mediated inhibition of the Wnt/β-catenin signaling pathway is complex. More work is required to investigate correlations between RUNX3 and the Wnt/β-catenin pathway in glioma initiation and progression.
The TGF-β signaling pathway plays a role in tumor suppressor mechanisms and has been studied in colon, gastric and pancreatic cancers [14]. RUNX3 is considered to be a key component of the TGF-β signaling pathway and has been shown to interact with TGF-β-activated Smads to regulate relevant target gene expression and the subsequent inhibition of tumor progression. Bim is a BCL2 family member that induces apoptosis via counteracting functions of anti-apoptotic members of BCL2 family [15]. Previous studies show that Bim is a target of RUNX3 in TGF-β-induced apoptosis in gastric epithelial cells [16]. Therefore, increased RUNX3 expression induced apoptosis in the presence of TGF-β [5]. In esophageal cancer cell lines, overexpression of RUNX3 sensitized the cancer cells to TGF-β-induced apoptosis, but RUNX3 did not induce apoptosis in the absence of TGF-β stimulation [17]. These data suggest that TGF-β is responsible for RUNX3-mediated apoptosis. However, Nakanishi reported that ectopic RUNX3 expression enhanced serum starvation-induced hepatocellular carcinoma cell apoptosis via the Bim-caspase pathway in the absence of TGF-β [18]. Moreover, RUNX3 cooperates with FOXO3A/FKHRL1 to activate Bim expression and induces apoptosis independently of TGF-β [19]. We found restoration of RUNX3 induced Bim expression and promoted apoptosis in the absence of TGF-β in glioma cell lines. Bim siRNA was used to evaluate whether Bim expression regulates RUNX3-induced apoptosis, and Bim siRNA partly reversed the effect of RUNX3-induced apoptosis in LN229 and U87 cells co-transfected with AD-RUNX3 and Bim siRNA. Thus, RUNX3 may induce glioma apoptosis via the Bim-caspase pathway in the absence of TGF-β.
MMPs and tissue inhibitors such as TIMPs participate in tumor metastasis [20] but few reports are available to describe relationship between RUNX3 and MMPs or TIMPs. A study of invasive mechanisms of gliomas by RUNX3 showed that RUNX3 inhibits glioma invasion and migration by regulating MMP2 protein expression and enzyme activity [21]. Consistent with this study, He’ group confirmed that RUNX3 repressed renal cancer cells metastasis by upregulation of TIMP1 rather than MMP2 and MMP9 [22]. However, Chen et al. confirmed that RUNX3 directly binds to the TIMP1 promoter and enhances its transcription activity to inactivate MMP9 in gastric cancer cells [23]. This suggests RUNX3 inhibits tumors in a tissue specific manner.
We verified that restoration of RUNX3 causes re-upregulation of TIMP1 and reduced expression of MMP2 and MMP9 but no significant change in TIMP3 expression in LN229 and U87 cells. This indicates a potential regulatory correlation between RUNX3 and TIMPS and MMPs in glioma cell lines. Previous data suggest that MMPs are key to tumor invasion and angiogenesis by cleaving extracellular matrix components [24]. VEGF, a major promoter of angiogenesis, is directly regulated by RUNX3 in gastric cancer cells via repression of VEGF promoter activity [25]. Additionally, because VEGF is a downstream target of the Wnt/β-catenin pathway, which is regulated by RUNX3, the invasive mechanism is complex and how RUNX3 modulates TIMP1, VEGF and their signaling pathways is unclear.
Our in vitro study showed that reintroduction of RUNX3 was therapeutically beneficial inhibited glioma cell proliferation and induced apoptosis. Expression of relevant molecules related to malignancy confirmed that the Wnt/β-catenin pathway was inhibited by RUNX3. Chi’ group reported that RUNX3 synergistically activates the p21 promoter and suppresses Gastric Epithelial Cell Growth in Cooperation with Transforming Growth Factor -Activated SMAD [26]. MIN’ group identified RUNX3 as a component of the MST/Hpo signaling pathway which deregulation leads to tumorigenesis [27]. These data and our work suggest that RUNX3 may be a promising diagnostic and therapeutic marker for glioblastoma.
In summary, in vitro and in vivo data show that restoration of RUNX3 suppresses tumor growth and tumorigenic potential and prolonged survival in a xenograft model, also RUNX3 inhibits Wnt/β-catenin signaling pathway, but the underlying mechanisms require additional study.
References
Kanu OO, Hughes B, Di C, Lin N, Fu J, Bigner DD, Yan H, Adamson C (2009) Glioblastoma multiforme oncogenomics and signaling pathways. Clin Med Oncol 3:39–52
Zhang K, Zhang J, Han L, Pu P, Kang C (2012) Wnt/beta-catenin signaling in glioma. J Neuroimmune Pharmacol 7:740–749. https://doi.org/10.1007/s11481-012-9359-y
Mueller W, Nutt CL, Ehrich M, Riemenschneider MJ, von Deimling A, van den Boom D, Louis DN (2007) Downregulation of RUNX3 and TES by hypermethylation in glioblastoma. Oncogene 26:583–593. https://doi.org/10.1038/sj.onc.1209805
Ito Y, Miyazono K (2003) RUNX transcription factors as key targets of TGF-beta superfamily signaling. Curr Opin Genet Dev 13:43–47
Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, Kim HM, Kim WJ, Yamamoto H, Yamashita N, Yano T, Ikeda T, Itohara S, Inazawa J, Abe T, Hagiwara A, Yamagishi H, Ooe A, Kaneda A, Sugimura T, Ushijima T, Bae SC, Ito Y (2002) Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 109:113–124
Zhang CZ, Zhang JX, Zhang AL, Shi ZD, Han L, Jia ZF, Yang WD, Wang GX, Jiang T, You YP, Pu PY, Cheng JQ, Kang CS (2010) MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol Cancer 9:229. https://doi.org/10.1186/1476-4598-9-229
Zhou X, Ren Y, Moore L, Mei M, You Y, Xu P, Wang B, Wang G, Jia Z, Pu P, Zhang W, Kang C (2010) Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab Investig: J Tech Methods Pathol 90:144–155. https://doi.org/10.1038/labinvest.2009.126
Shi Z, Qian X, Li L, Zhang J, Zhu S, Zhu J, Chen L, Zhang K, Han L, Yu S, Pu P, Jiang T, Kang C (2012) Nuclear translocation of beta-catenin is essential for glioma cell survival. J Neuroimmune Pharmacol 7:892–903. https://doi.org/10.1007/s11481-012-9354-3
Bagci-Onder T, Wakimoto H, Anderegg M, Cameron C, Shah K (2011) A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell-delivered TRAIL in experimental glioma models. Cancer Res 71:154–163. https://doi.org/10.1158/0008-5472.CAN-10-1601
Lai KW, Koh KX, Loh M, Tada K, Subramaniam MM, Lim XY, Vaithilingam A, Salto-Tellez M, Iacopetta B, Ito Y, Soong R, Singapore Gastric Cancer C (2010) MicroRNA-130b regulates the tumour suppressor RUNX3 in gastric cancer. Eur J Cancer 46:1456–1463. https://doi.org/10.1016/j.ejca.2010.01.036
Zuo J, Xia J, Ju F, Yan J, Zhu A, Jin S, Shan T, Zhou H (2013) MicroRNA-148a can regulate runt-related transcription factor 3 gene expression via modulation of DNA methyltransferase 1 in gastric cancer. Mol Cells 35:313–319. https://doi.org/10.1007/s10059-013-2314-9
Lin FC, Liu YP, Lai CH, Shan YS, Cheng HC, Hsu PI, Lee CH, Lee YC, Wang HY, Wang CH, Cheng JQ, Hsiao M, Lu PJ (2012) RUNX3-mediated transcriptional inhibition of Akt suppresses tumorigenesis of human gastric cancer cells. Oncogene 31:4302–4316. https://doi.org/10.1038/onc.2011.596
Ito K, Lim AC, Salto-Tellez M, Motoda L, Osato M, Chuang LS, Lee CW, Voon DC, Koo JK, Wang H, Fukamachi H, Ito Y (2008) RUNX3 attenuates beta-catenin/T cell factors in intestinal tumorigenesis. Cancer Cell 14:226–237. https://doi.org/10.1016/j.ccr.2008.08.004
Markowitz SD, Roberts AB (1996) Tumor suppressor activity of the TGF-beta pathway in human cancers. Cytokine Growth Factor Rev 7:93–102
Ramjaun AR, Tomlinson S, Eddaoudi A, Downward J (2007) Upregulation of two BH3-only proteins, Bmf and Bim, during TGF beta-induced apoptosis. Oncogene 26:970–981. https://doi.org/10.1038/sj.onc.1209852
Wei D, Gong W, Oh SC, Li Q, Kim WD, Wang L, Le X, Yao J, Wu TT, Huang S, Xie K (2005) Loss of RUNX3 expression significantly affects the clinical outcome of gastric cancer patients and its restoration causes drastic suppression of tumor growth and metastasis. Cancer Res 65:4809–4816. https://doi.org/10.1158/0008-5472.CAN-04-3741
Torquati A, O’Rear L, Longobardi L, Spagnoli A, Richards WO, Daniel Beauchamp R (2004) RUNX3 inhibits cell proliferation and induces apoptosis by reinstating transforming growth factor beta responsiveness in esophageal adenocarcinoma cells. Surgery 136:310–316. https://doi.org/10.1016/j.surg.2004.05.005
Nakanishi Y, Shiraha H, Nishina S, Tanaka S, Matsubara M, Horiguchi S, Iwamuro M, Takaoka N, Uemura M, Kuwaki K, Hagihara H, Toshimori J, Ohnishi H, Takaki A, Nakamura S, Kobayashi Y, Nouso K, Yagi T, Yamamoto K (2011) Loss of runt-related transcription factor 3 expression leads hepatocellular carcinoma cells to escape apoptosis. BMC Cancer 11:3. https://doi.org/10.1186/1471-2407-11-3
Yamamura Y, Lee WL, Inoue K, Ida H, Ito Y (2006) RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J Biol Chem 281:5267–5276. https://doi.org/10.1074/jbc.M512151200
Sampieri CL, Leon-Cordoba K, Remes-Troche JM (2013) Matrix metalloproteinases and their tissue inhibitors in gastric cancer as molecular markers. J Cancer Res Ther 9:356–363. https://doi.org/10.4103/0973-1482.119302
Mei PJ, Bai J, Liu H, Li C, Wu YP, Yu ZQ, Zheng JN (2011) RUNX3 expression is lost in glioma and its restoration causes drastic suppression of tumor invasion and migration. J Cancer Res Clin Oncol 137:1823–1830. https://doi.org/10.1007/s00432-011-1063-4
He L, Zhao X, Wang H, Zhang P, Guo C, Huang C, Liu X, Yao F, Chen Y, Lou W, Sun S, Fan D (2012) RUNX3 mediates suppression of tumor growth and metastasis of human CCRCC by regulating cyclin related proteins and TIMP-1. PLoS ONE 7:e32961. https://doi.org/10.1371/journal.pone.0032961
Chen Y, Wei X, Guo C, Jin H, Han Z, Han Y, Qiao T, Wu K, Fan D (2011) Runx3 suppresses gastric cancer metastasis through inactivation of MMP9 by upregulation of TIMP-1. Int J Cancer 129:1586–1598. https://doi.org/10.1002/ijc.25831
Rundhaug JE (2005) Matrix metalloproteinases and angiogenesis. J Cell Mol Med 9:267–285
Peng Z, Wei D, Wang L, Tang H, Zhang J, Le X, Jia Z, Li Q, Xie K (2006) RUNX3 inhibits the expression of vascular endothelial growth factor and reduces the angiogenesis, growth, and metastasis of human gastric cancer. Clin Cancer Res: Off J Am Assoc Cancer Res 12:6386–6394. https://doi.org/10.1158/1078-0432.CCR-05-2359
Chi XZ, Yang JO, Lee KY, Ito K, Sakakura C, Li QL, Kim HR, Cha EJ, Lee YH, Kaneda A, Ushijima T, Kim WJ, Ito Y, Bae SC (2005) RUNX3 suppresses gastric epithelial cell growth by inducing p21 (WAF1/Cip1) expression in cooperation with transforming growth factor {beta}-activated SMAD. Mol Cell Biol 25:8097–8107. https://doi.org/10.1128/MCB.25.18.8097-8107.2005
Min B, Kim MK, Zhang JW, Kim J, Chung KC, Oh BC, Stein GS, Lee YH, van Wijnen AJ, Bae SC (2012) Identification of RUNX3 as a component of the MST/Hpo signaling pathway. J Cell Physiol 227:839–849. https://doi.org/10.1002/jcp.22887
Acknowledgements
This study was partially supported by National High Technology Research, the China National Natural Scientific Fund (81101915), Tai’an City Technology Development Project (2017NS0117), High Level Training Program of Taishan Medicine University (GCC16) and the China Scholarship Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We confirm that there are potential conflict of interest associated with this publication. We also confirm that order of authors listed in the manuscript has been approved by all of us.
Rights and permissions
About this article
Cite this article
Sun, J., Li, B., Jia, Z. et al. RUNX3 inhibits glioma survival and invasion via suppression of the β-catenin/TCF-4 signaling pathway. J Neurooncol 140, 15–26 (2018). https://doi.org/10.1007/s11060-018-2927-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2927-0