Abstract
Purpose
Glioma is the most prevalent primary intracranial tumor globally. WDR34, a member of the WDR superfamily with five WD40 repeats, is involved in the pathogenesis of several tumors. However, the role of WDR34 in glioma progression is unknown.
Methods
The expression and prognostic significance of WDR34 in glioma patients were analyzed using GEPIA. WDR34 expression was detected by qRT-PCR. Western blot was employed to determine the expression of Ki67, proliferating cell nuclear antigen (PCNA), matrix metallopeptidase (MMP)2, MMP9, phosphatase and tensin homolog, protein kinase B (Akt), phosphorylated Akt, β-catenin, and c-Myc. CCK-8, BrdU incorporation assay, Transwell invasion assay, flow cytometry analysis, and measurement of caspase-3 and caspase-9 activities were conducted to examine the effects of WDR34 knockdown on glioma cells.
Results
WDR34 was upregulated in glioma, which predicted a poor prognosis in glioma patients. WDR34 knockdown inhibited cell proliferation and reduced the expression of Ki67 and PCNA in glioma cells. WDR34 knockdown repressed the invasive ability of glioma cells by decreasing MMP-2 and MMP-9 expression. WDR34 knockdown increased the apoptotic rate and caspase-3 and caspase-9 activities in glioma cells. The PI3K/Akt and Wnt/β-catenin pathways were inhibited after WDR34 knockdown in glioma cells. Moreover, overexpression of Akt or β-catenin reversed the function of WDR34 knockdown on proliferation, invasion, and apoptosis. WDR34 knockdown reduced tumor growth in vivo.
Conclusions
WDR34 knockdown inhibited malignant biological behaviors of glioma cells by inactivating the PI3K/Akt and Wnt/β-catenin signaling cascades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma is regarded as the most lethal and aggressive type of primary intracranial tumor globally, occupying an estimated 80% of all malignant brain tumors [1]. Glioma is characterized by poor prognosis, and high morbidity and mortality, seriously endangering human health [2]. In spite of the recent improvements achieved in therapeutic methods including surgical intervention combined with post-operative radiotherapy and chemotherapy, the clinical outcomes of glioma patients are still unfavorable [3,4,5]. Consequently, it is extremely required to develop efficient therapeutic interventions for this devastating malignancy to improve the survival of glioma patients.
WD40-repeat (WDR) proteins represent one of the most abundant regulatory protein superfamily in the human proteome composing of several repetitive WD motifs (also known as the Trp–Asp or WD40 motif) [6]. WDR proteins are proposed to be involved in a seemingly wide range of protein–protein interactions typically as scaffolds that regulate various cellular functions [7, 8]. Members of WDR superfamily have been found to play critical roles in diverse fundamental biological processes, including cell cycle control, DNA damage response, signal transduction, apoptosis, epigenetic regulation of gene expression, as well as chromatin modification [6]. WDR34, located at human chromosome 9q34.11, is a highly conserved protein belonging to the WDR superfamily and consists of five WD40 repeats in the middle and the C terminus, with human and mouse WDR34 sharing 83% identity. It has been demonstrated that missense mutations of WDR34 are associated with short-rib polydactyly syndrome type III or severe asphyxiating thoracic dysplasia [9]. Moreover, WDR34 suppresses transforming growth factor β-activated kinase 1 (TAK1)-associated nuclear factor-kappaB (NF-κB) activation pathway [10]. Despite the close association of WDR34 expression with cancer progression, a direct evidence for its role in the pathogenesis of glioma is still vague.
It is commonly reckoned that phosphatase and tensin homolog (PTEN), a well-characterized tumor suppressor in cancer progression, could inhibit the activation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway [11]. The PTEN/PI3K/Akt pathway has been verified as a crucial oncogenic pathway in glioma [12]. The Wnt/β-catenin pathway, a high conserved cascade, has been demonstrated to be associated with the development of central nervous system and its deregulation contributes to the occurrence and progression of diverse cancers including glioma [13, 14]. In the present study, we initially explored the expression pattern of WDR34 in glioma cells. Then, we determined the effects of WDR34 knockdown on the proliferation, invasion, and apoptosis of glioma cells and further analyzed whether these effects were involved in the PI3K/Akt and Wnt/β-catenin pathways.
Materials and methods
Cell culture and transfection
Five human glioma cell lines (LN229, U87, T98G, U251, and A172) and normal human astrocyte cells (NHAs) were got from the ATCC (Manassas, VA, USA). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific, Waltham, MA, USA) that consisted of 10% heat-inactivated fetal bovine serum (HyClone, South Logan, UT, USA) and 1% penicillin–streptomycin (HyClone), which were kept at 37 °C in a moist incubator containing 5% CO2. Two small interfering RNAs targeting WDR34 (si-WDR34-1 and si-WDR34-2) and the scrambled control siRNA (si-Con) were synthesized from GeneWiz Technology Co., Ltd. (Suzhou, China). When reaching 70–80% confluence, U87 and A172 cells in logarithmic growth phase were delivered with si-WDR34 or si-Con using an Invitrogen™ Lipofectamine® 3000 Kit (Thermo Fisher Scientific).
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cultured cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The extracted RNA was then revers transcribed into complementary DNA (cDNA) by RevertAid cDNA synthesis kit (Fermentas, Vilnius, Lithuania). WDR34 mRNA expression was determined using SYBR Premix Ex Taq™ reagent (Takara, Dalian, China) on an ABI 7900 Fast System (Applied Biosystems, Foster City, CA, USA), with GAPDH as the normalization. The relative fold changes of target genes were calculated using the 2−ΔΔCt approach. Primer sequences were as follows: WDR34, forward, 5′-TGA TGG CTT CGA GGT GAA C-3′ and reverse, 5′-GGG TAG CCC AGG GTA TAC AGA-3′; GAPDH, forward, 5′-ACC ACA GTC CAT GCC ATC AC-3′ and reverse 5′-TCC ACC ACC CTG TTG CTG TA-3′.
Cell counting kit-8 (CCK-8) assay
CCK-8 assay was conducted to detect the viability of glioma cells. To be more specific, si-WDR34 or si-Con-transfected U87 and A172 cells were placed into 96-well plates at a density of 1 × 104 cells per well. Following this, 10 µl of CCK-8 solution (Dojindo Laboratories, Tokyo, Japan) was supplemented at 48 h after seeding and the cells were incubated for an additional 2 h at 37 °C. A microplate reader (Molecular Device, Sunnyvale, CA, USA) was implemented to measure the optical density at 450 nm.
Transwell invasion assay
Transwell assay inserts (Millipore, Billerica, MA, USA) pre-coated with Matrigel (BD Biosciences, San Diego, CA, USA) were used for the evaluation of cell invasive potential. After transfection, 3 × 103 U87 and A172 cells were subjected to re-suspension in 200 μL serum-free medium and seeded into the upper chamber while 600 µl DMEM medium with 10% FBS was inoculated into the lower chamber. These cells were allowed to invade for 24 h. The cells that had invaded to the lower membranes were fixed with 4% paraformaldehyde prior to staining with 0.5% crystal violet. An inverted microscope (magnification, × 200; Olympus, Tokyo, Japan) was applied to count the number of invaded cells in 3 randomly selected fields.
Western blot analysis
RIPA protein extraction buffer (Beyotime, Beijing, China) supplemented with protease inhibitor cocktail (Roche, Basel, Switzerland) was used to extract total proteins. Equal amount of lysate protein samples (40 μg/lane) were subjected to 10% SDS-PAGE prior to transfer onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). After non-specific binding with 5% non-fat skimmed milk for 1 h, the membrane was probed in a solution containing primary antibodies at 4 °C overnight and then with horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at room temperature for 2 h. An enhanced chemiluminescence kit (Amersham, Arlington Heights, IL, USA) was employed to capture the protein signals. Primary antibodies against Ki67 (Cell Signaling Technology, Danvers, MA, USA), proliferating cell nuclear antigen (PCNA) (Cell Signaling Technology), matrix metallopeptidase (MMP)2 (Cell Signaling Technology), and MMP9 (Cell Signaling Technology), PTEN (Santa Cruz Biotechnology), Akt (Santa Cruz Biotechnology), phosphorylated Akt (p-Akt) (Santa Cruz Biotechnology), lamin B1 (Cell Signaling Technology), β-catenin (Santa Cruz Biotechnology), and c-Myc (Santa Cruz Biotechnology) were employed.
Five-bromo-2′-deoxyuridine (BrdU) incorporation assay
A BrdU cell proliferation assay kit (Roche Diagnostics, Pleasanton, CA, USA) was conducted to examine the in vitro cell proliferative ability. A total of 2 × 103 transfected U87 and A172 cells were inoculated in 96-well plates and allowed to grow overnight at 37 °C. Thereafter, BrdU-labeling solution was mixed into the cell plate at a final concentration of 10 μM, followed by a 4-h incubation at 37 °C. At the end of the incubation period, the U87 and A172 cells were fixed in 4% paraformaldehyde for 20 min and stained with primary anti-BrdU antibody (Sigma-Aldrich, St. Louis, MO, USA) for 1 h prior to incubation with HRP-conjugated secondary antibody (Santa Cruz Biotechnology) for 30 min. After incubating with TMB peroxidase substrate for 30 min in the darkness, the optical density at 450 nm was detected using a microplate reader (Molecular Device).
Flow cytometry analysis
Following transfection, U87 and A172 cells were harvested by trypsinization and washed twice with cold PBS. This is followed by resuspending in 100 µl binding buffer containing 5 μl annexin V-fluorescein isothiocyanate (annexin V-FITC) and 5 μl propidium iodide (PI) using common-used Annexin V-FITC/PI apoptosis detection kit (BioVision, Palo Alto, CA, USA). After incubating for 15 min at 37 °C in the dark, the stained cells were analyzed using a flow cytometer equipped with CellQuest software (BD Biosciences).
Measurement of caspase-3 and caspase-9 activities
U87 and A172 cells were harvested after transfection, lysed, and centrifuged to collect supernatants. Activities of caspase-3 and caspase-9 in the supernatant were determined using respective colorimetric activity kits (R&D Systems, Minneapolis, MA, USA) in terms of the manufacturer’s guides. Absorbance at 405 nm was read using a microplate reader (Molecular Device). The caspase-3 and caspase-9 activities were expressed as a fold of the control group.
Xenograft experiment
The shRNA for WDR34 (sh-WDR34) or negative control (sh-Con) lentivirus vectors were stably transfected into U87 cells. Male BALB/c nude mice (5-week-old) were obtained from Vital River (Beijing, China), and subcutaneously injected with stably transfected U87 cells (1 × 107) (n = 5 per group). Tumor size was measured weekly, and calculated by following formula: volume = 0.5 × length × width2. Four weeks after cell injection, mice were euthanized. The tumors were dissected, weighed, and then harvested for measurement of WDR34 expression by western blot assay. Xenograft experiments were approved by the Animal Ethics Committee of the First Affiliated Hospital of Soochow University.
Statistical analysis
All data are displayed as mean ± standard deviation (SD). All statistical analyses were performed using SPSS version 13.0 statistical software programs (SPSS, Chicago, IL, USA) with one way analysis of variance or Student’s t test. Differences were considered statistically significant when P values were less than 0.05.
Results
WDR34 was upregulated in glioma
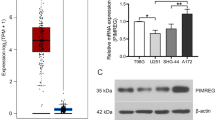
According to the statistical analysis of The Cancer Genome Atlas (TCGA) data from GEPIA (http://gepia.cancer-pku.cn), WDR34 expression was significantly elevated in 518 lower grade glioma (LGG) and 163 glioblastoma (GBM) tissues compared with that in 207 normal brain tissues (Fig. 1A, B). Additionally, TCGA data from GEPIA demonstrated that high expression of WDR34 was correlated with poor overall survival in LGG patients (Fig. 1C), suggesting that high expression of WDR34 predicted a poor prognosis in LGG patients. However, expression of WDR34 was not correlated with overall survival in GBM patients (Fig. 1D). Immunohistochemical data from The Human Protein Atlas project (https://www.proteinatlas.org/) suggested that WDR34 protein level was increased in LGG and GBM tissues compared to that in normal cerebral cortex tissues (Fig. 1E). The expression of WDR34 mRNA and protein was also increased in glioma cell lines (LN229, U87, T98G, U251, and A172) relative to normal human astrocyte cells (NHAs), especially in U87 and A172 cells (Fig. 1F, G). Therefore, we selected U87 and A172 cells for subsequent experiments. To characterize the biological role of MDR34 on the malignant biological behaviors of glioma cells, siRNA-mediated knockdown of WDR34 was performed in U87 and A172 cells by transfecting with si-WDR34. As evidenced by qRT-PCR and western blot analysis, WDR34 mRNA and protein expression was reduced in U87 and A172 cells following delivery with si-WDR34-1 and si-WDR34-2 with respect to si-Con group (Fig. 1H, I).
Expression profile of WDR34 in glioma. A and B Analysis of the expression difference of WDR34 in 518 lower grade glioma (LGG), 163 glioblastoma (GBM), and 207 normal brain tissues based on TCGA database from GEPIA. C and D Analysis of overall survival curves in LGG and GBM based on TCGA data from GEPIA. E Immunohistochemical staining of WDR34 in LGG, GBM, and normal cerebral cortex tissues from The Human Protein Atlas project. F and G qRT-PCR and western blot analysis of WDR34 mRNA and protein expression in glioma cell lines (LN229, U87, T98G, U251, and A172) and NHAs. P < 0.05 was considered statistically significant when compared to NHAs. (I) qRT-PCR and western blot analysis of WDR34 mRNA and protein expression in U87 and A172 cells 48 h after transfection with si-WDR34-1, si-WDR34-2, or si-Con. P < 0.05 was considered statistically significant when compared to si-Con
WDR34 knockdown inhibited the proliferation of glioma cells
As shown in Fig. 2A, knockdown of WDR34 caused a reduction of the viability of U87 and A172 cells versus control group. As demonstrated by BrdU incorporation assay, MDR34 silencing significantly decreased the BrdU incorporation of U87 and A172 cells when compared with control group (Fig. 2B). Western blot analysis proved that si-WDR34-introduced U87 (Fig. 2C, D) and A172 (Fig. 2E, F) cells exhibited a decreased expression of Ki67 and PCNA compared to si-Con-transfected cells. These data suggested that the proliferation of glioma cells was inhibited in response to knockdown of WDR34.
Effect of WDR34 knockdown on the proliferation of glioma cells. The proliferation of U87 and A172 cells 48 h after transfection with si-WDR34-1, si-WDR34-2, or si-Con was estimated by CCK-8 (A) and BrdU incorporation assays (B). Western blot analysis of Ki67 and PCNA expression in U87 (C and D) and A172 cells (E and F) 48 h after transfection with si-WDR34-1, si-WDR34-2, or si-Con
WDR34 knockdown suppressed the invasive ability of glioma cells
Transwell invasion assay revealed that the invasive ability of U87 and A172 cells transfected with si-WDR34 was dampened in comparison to control group (Fig. 3A). MMPs including MMP2 and MMP9 are well-known to be involved in cancer invasion [15]. Depletion of WDR34 led to a reduction of MMP2 and MMP9 expression in U87 (Fig. 3B, C) and A172 (Fig. 3D, E) cells versus control group. Collectively, these findings suggested that WDR34 knockdown retarded the invasive ability of glioma cells.
Effect of WDR34 silencing on the invasive ability of glioma cells. A Transwell invasion assay was employed to detect the invasive ability of U87 and A172 cells 24 h after transfection with si-WDR34-1, si-WDR34-2, or si-Con. Western blot analysis was conducted to examine the protein levels of MMP2 and MMP9 in U87 (B and C) and A172 cells (D and E) 48 h after transfection with si-WDR34-1, si-WDR34-2, or si-Con
WDR34 knockdown increased apoptosis rate of glioma cells
Apoptosis analysis by Annexin V-FITC/PI double staining manifested that transfection with si-WDR34 increased the apoptotic rate in U87 (Fig. 4A) and A172 (Fig. 4B) cells relative to the corresponding control group. Moreover, we found that caspase-3 (Fig. 4C) and caspase-9 (Fig. 4D) activities were enhanced following WDR34 downregulation in U87 and A172 cells. Therefore, we concluded that WDR34 knockdown increased apoptosis rate of glioma cells.
Effect of WDR34 knockdown on the apoptosis of glioma cells. U87 and A172 cells were transfected with si-WDR34-1, si-WDR34-2, or si-Con for 48 h, followed by the determination of apoptosis (A and B), and activities of caspase-3 (C) and caspase-9 (D) by flow cytometry analysis, caspase-3 and caspase-9 activity assays, respectively
Knockdown of WDR34 inhibited the PI3K/Akt and Wnt/β-catenin pathways in glioma cells
It was demonstrated that PTEN expression was boosted and p-Akt protein level was repressed in U87 (Fig. 5A) and A172 (Fig. 5B) cells transfected with si-WDR34 relative to si-Con-transfected group. However, WDR34 knockdown failed to influence the protein expression of Akt in both U87 and A172 cells. The western blot analysis also uncovered that the protein levels of β-catenin in nucleus and c-Myc in cell lysate of U87 (Fig. 5C) and A172 (Fig. 5D) cells were lower in the si-WDR34 group than those in si-Con group. These results suggested that knockdown of WDR34 blocked the PI3K/Akt and Wnt/β-catenin pathways in glioma cells.
Effect of WDR34 knockdown on the PI3K/Akt and Wnt/β-catenin pathways in glioma cells. A and B The protein levels of PTEN, p-Akt (Ser473) and Akt in U87 and A172 cells 48 h after transfection with si-WDR34-1, si-WDR34-2, or si-Con were measured by western blot analysis. C and D Western blot analysis was implemented to analyze the protein levels of β-catenin in nucleus and c-Myc in cell lysate of U87 and A172 cells 48 h after transfection with si-WDR34-1, si-WDR34-2, or si-Con
Overexpression of Akt or β-catenin reversed the effect of WDR34 knockdown on proliferation, invasion, and apoptosis
To verify whether the PI3K/Akt and Wnt/β-catenin pathways were involved in the effect of WDR34 knockdown, glioma cells were co-transfected with si-WDR34-2 and Akt/β-catenin overexpression vector. As shown in Fig. 6A and B, WDR34 knockdown inhibited U87 and A172 cell viability, but Akt or β-catenin overexpression reversed it. WDR34 knockdown decreased the BrdU incorporation of U87 and A172 cells, while this effect was attenuated after Akt or β-catenin overexpression (Fig. 6C, D). The number of invaded U87 and A172 cells were decreased after WDR34 knockdown, which was resisted by Akt or β-catenin overexpression (Fig. 6E, F). Transfection with si-WDR34-2 increased the apoptotic rate in U87 and A172 cells, while Akt or β-catenin overexpression attenuated this effect (Fig. 6G, H). Taken together, these findings suggested that WDR34 regulates malignant biological behaviors of glioma cells by affecting the PI3K/Akt and Wnt/β-catenin pathways.
Effect of Akt or β-catenin overexpression on proliferation, invasion, and apoptosis in WDR34 knockdown cells. U87 and A172 cells were co-transfected with si-Con/si-WDR34-2 and pcDNA-Akt/pcDNA–catenin. The proliferation of U87 and A172 cells was estimated by CCK-8 (A and B) and BrdU incorporation assays (C and D) 48 h after transfection. E and F Transwell invasion assay was employed to detect the invasive ability of U87 and A172 cells 24 h after transfection. G and H Apoptosis of U87 and A172 cells was evaluated by flow cytometry analysis 48 h after transfection
WDR34 knockdown reduced glioma cell growth in an animal model
To further explore the anti-growth role of WDR34 silencing in glioma cells, the animal model was established using U87 cells harboring sh-WDR34 or sh-Con. As shown in Fig. 7A–C, tumor volume and weight were decreased in sh-WDR34 group compared with sh-Con group. Moreover, WDR34 protein level was reduced in sh-WDR34 group compared with sh-Con group (Fig. 7D). These results showed that WDR34 knockdown decreased glioma cell growth in vivo.
Effect of WDR34 knockdown on the growth of glioma cells in vivo. U87 cells stably transfected with sh-WDR34 or sh-Con were subcutaneously injected into mice to establish a xenograft model. A Images of removed tumors in each group. B Tumor volume was detected weekly. C Tumor weight was measured in each group. D WDR34 protein level in tumor tissues was detected using western bolt analysis. n = 5
Discussion
Glioma is one of the most frequently occurring types of malignant tumors in the central nervous system with an incidence rate of 3–8/100,000, thus seriously affecting the quality of life of patients suffered from glioma [16]. Therefore, there is increased interest in searching for new reliable therapeutic methods for glioma and further deciphering the molecular mechanism responsible for glioma development. Over the last decades, a number of studies have focused on the molecularly targeted therapy including key genes and downstream regulatory mechanisms in glioma therapies [17].
It has been well-documented that WDR proteins participate in the regulation of a variety of cellular processes, and perturbations in these proteins are involved in human malignancies [18]. WDR54 was demonstrated to be highly expressed in colorectal cancer (CRC) patients, which was an independent risk factor for disease-specific survival [19]. Knockdown of WDR54 significantly inhibited the growth and aggressiveness of CRC cells and reduced tumor growth in a xenograft model by regulating Akt and extracellular signal-regulated kinase (ERK) signaling [19]. DCAF4L2, a member of WDR proteins, was reported to be elevated in CRC patients and cells, and its overexpression promoted cell migration, invasion and epithelial-mesenchymal transition (EMT) through activating NF-κB pathway [20]. More importantly, the involvement of WDR34, a member of the WDR family, in several tumors has been well-documented. For instance, a significant upregulation of WDR34 expression was observed in the bladder cancer without recurrence compared with that in the patients with recurrence [21]. High expression of WDR34 was associated with poor overall survival and shorter relapse-free survival in patients with breast cancer [22]. A study showed that upregulation of WDR34 was found in hepatocellular carcinoma (HCC) tissues and negatively correlated with the survival of HCC patients [23]. Knockdown of WDR34 inhibited the growth, colony formation and migration of HCC cells [23]. Conversely, it was demonstrated that WDR34 was downregulated in oral squamous cell carcinoma (OSCC) tissues and functioned as a potential tumor suppressor in OSCC progression [24]. However, the detailed role and mechanism of WDR34 in glioma progression remain largely undefined. In this research, our analysis of TCGA data demonstrated that WDR34 expression was increased in glioma, which predicted poor clinical outcomes in glioma patients. We also demonstrated a high expression of WDR34 in 5 different glioma cell lines compared with that in NHAs. Functional experiments demonstrated that WDR34 knockdown inhibited the proliferation, invasive ability and apoptosis in glioma cells. Furthermore, we established a mouse xenograft model to further validate the anti-tumor growth role of WDR34 knockdown. Collectively, these data suggested the oncogenic role of WDR34 in glioma development.
It is well known that glioma is molecularly heterogeneous, resulting in dysregulation and over-activation of multiple signaling pathways including PI3K/Akt pathway [25, 26]. The PI3K/Akt pathway is an intracellular signal transduction pathway targeted by PTEN that participates in the regulation of various cellular functions, such as cell growth, apoptosis, differentiation, and metastasis in glioma [27, 28]. Inactivation of the Wnt/β-catenin signaling pathway has been shown to effectively suppress the invasion, survival and tumorigenesis of glioma cells [29, 30]. Therefore, the Wnt/β-catenin signaling has been proposed to be a potential valuable therapeutic target for glioma [29]. To elucidate the molecular mechanism underlying the oncogenic role of WDR34 in glioma progression, we explored the effect of WDR34 silencing on the PI3K/Akt and Wnt/β-catenin signaling pathways. As a result, we demonstrated that WDR34 knockdown suppressed the PI3K/Akt and Wnt/β-catenin signaling cascades. Accordingly, it is reasonable to infer from these results that WDR34 knockdown inhibited malignant biological behaviors of glioma cells by inactivating the PI3K/Akt and Wnt/β-catenin signaling cascades.
Taken together, our study provided the first evidence that WDR34 was reinforced in glioma and predicted poor clinical outcomes in glioma patients. WDR34 exerted its oncogenic role in glioma through activating the PI3K/Akt and Wnt/β-catenin signaling cascades (Fig. 8), contributing to our understanding of the glioma pathogenesis. Our research suggested that WDR34 may be a promising therapeutic target for glioma. However, it is still unclear how WDR34 regulates the Akt or Wnt signaling pathways. This deserves further study.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Jansson MRN, von Heymann-Horan A (2018) Risk for use of antidepressants, anxiolytics, and hypnotics in partners of glioma patients-A nationwide study covering 19 years of prescriptions. Psychooncology 27:1930–1936
Miyauchi JT, Tsirka SE (2018) Advances in immunotherapeutic research for glioma therapy. J Neurol 265:741–756
Tykocki T, Eltayeb M (2018) Ten-year survival in glioblastoma. A systematic review. J Clin Neurosci 54:7–13
Nakada M, Nakada S, Demuth T, Tran NL, Hoelzinger DB, Berens ME (2007) Molecular targets of glioma invasion. Cell Mol Life Sci 64:458–478
Kalpathy-Cramer J, Gerstner ER, Emblem KE, Andronesi O, Rosen B (2014) Advanced magnetic resonance imaging of the physical processes in human glioblastoma. Cancer Res 74:4622–4637
van Nocker S, Ludwig P (2003) The WD-repeat protein superfamily in Arabidopsis: conservation and divergence in structure and function. BMC Genomics 4:50
Tao N, Zhu W, Gan M, Chen M, Li T, Tendu A, Jiao D, Wang M, Xue C, Lin Y, Yang Q (2019) Genome-wide identification and functional analysis of the WDR protein family in potato. 3 Biotech 9:432
Stirnimann CU, Petsalaki E, Russell RB, Muller CW (2010) WD40 proteins propel cellular networks. Trends Biochem Sci 35:565–574
Huber C, Wu S, Kim AS, Sigaudy S, Sarukhanov A, Serre V, Baujat G, Le Quan Sang KH, Rimoin DL, Cohn DH, Munnich A, Krakow D, Cormier-Daire V (2013) WDR34 mutations that cause short-rib polydactyly syndrome type III/severe asphyxiating thoracic dysplasia reveal a role for the NF-κB pathway in cilia. Am J Hum Genet 93:926–931
Gao D, Wang R, Li B, Yang Y, Zhai Z, Chen DY (2009) WDR34 is a novel TAK1-associated suppressor of the IL-1R/TLR3/TLR4-induced NF-κB activation pathway. Cell Mol Life Sci 66:2573–2584
Wu H, Goel V, Haluska FG (2003) PTEN signaling pathways in melanoma. Oncogene 22:3113–3122
Liao YX, Zhang ZP, Zhao J, Liu JP (2018) Effects of fibronectin 1 on cell proliferation, senescence and apoptosis of human glioma cells through the PI3K/AKT signaling pathway. Cell Physiol Biochem 48:1382–1396
Zuccarini M, Giuliani P, Ziberi S, Carluccio M, Iorio PD, Caciagli F, Ciccarelli R (2018) The role of Wnt signal in glioblastoma development and progression: a possible new pharmacological target for the therapy of this tumor. Genes (Basel) 9:105
Liu C, Tu Y, Sun X, Jiang J, Jin X, Bo X, Li Z, Bian A, Wang X, Liu D, Wang Z, Ding L (2011) Wnt/β-Catenin pathway in human glioma: expression pattern and clinical/prognostic correlations. Clin Exp Med 11:105–112
Rao JS (2003) Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer 3:489–501
Chen R, Smith-Cohn M, Cohen AL, Colman H (2017) Glioma subclassifications and their clinical significance. Neurotherapeutics 14:284–297
Bastien JI, McNeill KA, Fine HA (2015) Molecular characterizations of glioblastoma, targeted therapy, and clinical results to date. Cancer 121:502–516
Schapira M, Tyers M, Torrent M, Arrowsmith CH (2017) WD40 repeat domain proteins: a novel target class? Nat Rev Drug Discov 16:773–786
Yuan Y, Qi G, Shen H, Guo A, Cao F, Zhu Y, Xiao C, Chang W, Zheng S (2019) Clinical significance and biological function of WD repeat domain 54 as an oncogene in colorectal cancer. Int J Cancer 144:1584–1595
Wang H, Chen Y, Han J, Meng Q, Xi Q, Wu G, Zhang B (2016) DCAF4L2 promotes colorectal cancer invasion and metastasis via mediating degradation of NFκb negative regulator PPM1B. Am J Transl Res 8:405–418
Mares J, Szakacsova M, Soukup V, Duskova J, Horinek A, Babjuk M (2013) Prediction of recurrence in low and intermediate risk non-muscle invasive bladder cancer by real-time quantitative PCR analysis: cDNA microarray results. Neoplasma 60:295–301
Hu DJ, Shi WJ, Yu M, Zhang L (2019) High WDR34 mRNA expression as a potential prognostic biomarker in patients with breast cancer as determined by integrated bioinformatics analysis. Oncol Lett 18:3177–3187
Luo X, Liu Y, Ma S, Liu L, Xie R, Wang S (2019) WDR34 Activates Wnt/β-catenin signaling in hepatocellular carcinoma. Dig Dis Sci 64:2591–2599
Yamamoto JI, Kasamatsu A, Okubo Y, Nakashima D, Fushimi K, Minakawa Y, Kasama H, Shiiba M, Tanzawa H, Uzawa K (2018) Evaluation of tryptophan-aspartic acid repeat-containing protein 34 as a novel tumor-suppressor molecule in human oral cancer. Biochem Biophys Res Commun 495:2469–2474
Huang TT, Sarkaria SM, Cloughesy TF, Mischel PS (2009) Targeted therapy for malignant glioma patients: lessons learned and the road ahead. Neurotherapeutics 6:500–512
Li X, Wu C, Chen N, Gu H, Yen A, Cao L, Wang E, Wang L (2016) PI3K/Akt/mTOR signaling pathway and targeted therapy for glioblastoma. Oncotarget 7:33440–33450
Zhao HF, Wang J, Shao W, Wu CP, Chen ZP, To ST, Li WP (2017) Recent advances in the use of PI3K inhibitors for glioblastoma multiforme: current preclinical and clinical development. Mol Cancer 16:100
Manning BD, Toker A (2017) AKT/PKB signaling: navigating the Network. Cell 169:381–405
Gao L, Chen B, Li J, Yang F, Cen X, Liao Z, Long X (2017) Wnt/β-catenin signaling pathway inhibits the proliferation and apoptosis of U87 glioma cells via different mechanisms. PLoS ONE 12:e0181346
Liu HW, Su YK, Bamodu OA (2018) The disruption of the β-Catenin/TCF-1/STAT3 signaling axis by 4-acetylantroquinonol b inhibits the tumorigenesis and cancer stem-cell-like properties of glioblastoma cells, in vitro and in vivo. Cancers 10:491
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
JZ conducted the experiments and wrote the manuscript. CL collected and analyzed the data. HN analyzed the data. ZY conceived the experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
This study approved by the Animal Ethics Committee of the First Affiliated Hospital of Soochow University.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zuo, J., Liu, C., Ni, H. et al. WDR34 affects PI3K/Akt and Wnt/β-catenin pathways to regulates malignant biological behaviors of glioma cells. J Neurooncol 156, 281–293 (2022). https://doi.org/10.1007/s11060-021-03932-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03932-2