Abstract
Introduction
Treatment of recurrent high-grade gliomas (rHGG) has always been challenging. This study aimed to explore the treatment effect of quantitative dynamic susceptibility contrast perfusion-weighted imaging (DSC-PWI)-guided gamma knife radiosurgery (GKRS) on rHGG.
Methods
Between April 2014 and July 2016, 26 consecutive patients were treated by quantitative DSC-PWI-guided GKRS as salvage treatment for rHGG. The gross tumor volume (GTV) was defined as the high perfusion area on absolute cerebral blood volume maps, with a cutoff value of 22 ml/100 g. The clinical target volume (CTV) encompassed the GTV by 3 mm. Overall survival (OS) and progression-free survival (PFS) were calculated by the Kaplan–Meier method. Prognostic factors were tested by the log-rank (Mantel–Cox) test.
Results
With a median follow-up of 32 months, the median PFS after GKRS was 8 months (95% CI [6, 12]); the 1- and 2-year survival rates were 30.8 and 11.5%, respectively. The median OS was 25.5 months (95% CI [18, 40]); the 1- and 2-year survival rates were 96.2 and 57.7%, respectively. Pathology grade and CTV were identified as prognostic factors for PFS. However, none of the parameters tested were independent prognostic factors for OS among these selected patients. No severe radiotoxicity was observed among all patients.
Conclusions
Quantitative DSC-PWI-guided GKRS is feasible for the treatment of rHGG and that these outcomes remain to be validated. Despite this, we think that carefully selected patients can benefit from this treatment method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-grade gliomas (HGG) are the most common malignant primary tumor of the central nervous system in adults and are fatal in that, without effective treatment, most patients die within a few weeks after presentation of symptoms [1, 2]. External beam radiation therapy after the resection of tumors, combined with chemotherapy, is considered to be the standard treatment protocol for high-grade glioma [3, 4]. However, as a result of resistance of the tumors to radiotherapy and chemotherapy, as well as infiltrative growth in normal appearing white matter, aggressive therapy for HGG is not always sufficient [5]. Tumor recurrence will occur within 6 months in most cases [6].
For recurrent high-grade gliomas (rHGG) treated with prior radiotherapy, re-irradiation is an alternative for subsequent treatment. In recent years, gamma knife radiosurgery (GKRS) has become increasingly popular as a salvage treatment modality for patients diagnosed with rHGG because it results in limited treatment-induced morbidity [7]. Treatment planning of GKRS is based on the scope of the enhancing area on contrast T1WI. However, enhancing lesions on contrast T1WI do not always correspond to the real scope of recurrent tumors. On the one hand, in most of the enhancing lesions on contrast T1WI, radionecrosis and tumor recurrence are concurrent [8]. The therapeutic window of gamma knife between effective tumor control and radionecrosis is very narrow, and therefore, a more precise radiation scale is preferred under this condition [9]. On the other hand, some infiltrated parts or solid parts of high-grade glioma demonstrate non-enhancement on contrast T1WI [2, 10], and thus, treatment planning based on contrast T1WI can result in failure of tumor control. To limit the risk of adverse radiation effects while preventing tumor recurrence, the precise location for re-irradiation of the real part of recurrent tumors is crucial for an optimal prognosis. However, contrast T1WI is not able to meet these requirements [7, 10, 11].
Traditional dynamic susceptibility contrast perfusion-weighted imaging (DSC-PWI) is known to be a valid tool for differential diagnosis of radionecrosis and tumor recurrence, with high sensitivity and specificity [12, 13]. However, rCBV is a semi-quantitative parameter with a high subjective bias, which means that an rCBV map is considered to be relatively reliable. In addition, traditional DSC-PWI has the inherent limitation of low resolution and inaccurate inter-modality spatial alignment of the EPI-based image [14]. Therefore, a quantitative DSC-PWI with high resolution is needed to guide the treatment. The bookend perfusion technique, which uses pre- and post-contrast T1 maps to calibrate a conventional DSC sequence, is a quantitative MR technique with high resolution and better inter-modality spatial alignment. It has been validated against PET and demonstrates high test–retest repeatability [15, 16]. Based on previous findings, we believe that an absolute CBV map, derived from the bookend perfusion technique, could help us correctly identify the real part of rHGG and be used to guide the treatment of GKRS in the manner of precision medicine.
To our best knowledge, no articles have focused on the quantitative DSC-PWI-guided GKRS treatment of rHGG until now. This study aimed to investigate the efficiency of quantitative DSC-PWI in guiding GKRS treatment of rHGG.
Materials and methods
Study population
Between April 2014 and July 2016, 187 patients were treated as high grade glioma in our neurosurgery team and 96 of them were diagnosed as recurrent high glioma after resection and adjuvant treatments. Twenty-six patients were treated by gamma knife radiotherapy, the rest of them were treated by EBRT. Therefore, these 26 consecutive patients (median age: 53 years old, range 10–69, 16 male/10 female) were treated by quantitative DSC-PWI-guided GKRS as a salvage treatment for rHGG. The inclusion criteria were as follows: (1) patients underwent a surgical resection or stereotactic needle biopsy, and a pathological diagnosis of malignant glioma as World Health Organization (WHO) grade 3 or GBM was made; (2) patients underwent subsequent fractionated brain irradiation; and (3) patients demonstrated the development of new or increasing contrast-enhanced lesions at the margin of the initial lesion at least 6 months after fractionated radiotherapy, indicating tumor recurrence or progression. In this study, MRI perfusion and magnetic resonance spectroscopy (MRS) had been used to identify pseudo-progression, patients in this study had examinations of MRI perfusion and MRS during each following-up. Pseudo-progression, corresponding to the enlarged enhancing area in post-T1WI, would show a decrease perfusion (in comparison with last absolute CBV value). In addition, pseudo-progression would show a decreased ratio of Cho/Cr and Cho/NAA (in comparison with last value).
Written informed consent was obtained from each volunteer prior to the study, after the approval of the local ethical committee. All experiments were performed in compliance with the Helsinki Declaration.
MRI protocol
All of the patients were examined in a supine position with a 3.0 T MRI machine (Magnetom Skyra, Siemens Healthcare, Erlangen, Germany) using a transmit/receive quadrature 16-channel head and neck combined coil. A prototype quantitative DSC-PWI sequence named ScalePWI was used in this study. The ScalePWI sequence merged the pre- and post-contrast T1 mapping onto the GRE-EPI sequence for DSC-PWI and added the same “gradient noise” between T1 mapping and the DSC-PWI scan to avoid head motion. The imaging parameters of ScalePWI were as follows: TR/TE 1600/30 ms, bandwidth 1748 Hz/pixel, 21 axial slices, field of view (FOV) 220 × 220 mm, voxel size 1.8 × 1.8 × 4 mm3, slice thickness 4.0 mm, and flip angle (FA) 90°. For each slice, 50 measurements were acquired for each DSC-PWI analysis. After 46 s of injector delay, 0.2 mmol/kg bodyweight of contrast agent (Gd-DTPA, Magnevist; Schering, Berlin, Germany), followed by a 20 ml saline flush, was administered. An injection velocity of 4.5 ml/s was used in this study. The details of the mathematical methods of quantitative DSC-PWI were described previously [16]. The quantification of CBV was based on the bookend technique, which determines CBV from T1 changes in normal white matter in relation to the changes in the blood pool. This approach relies on careful modeling of the effects of intravascular to extravascular water exchange, which is a well-known confounding effect in determining CBV from pre- and post-gadolinium T1 changes [17].
After the DSC sequence, an axial three-dimensional T1 weighted image (3D-T1WI) was also acquired. The detailed parameters were as following: TR/TE 2300/3 ms, TI 900 ms, FOV 256 × 256 mm, slice thickness 1 mm, and FA 9°. All sequences were taken during the same session, and the imaging protocol was the same for all patients.
Gamma knife re-irradiation
All patients were initially placed in a stereotactic head frame. A radio-oncologist and a neurosurgeon were involved in treatment planning and target volume determinations for all patients.
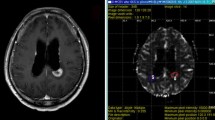
All treatment plans were performed using the ELEKTA’s GAMMA Knife Treatment System. Gross tumor volume (GTV) was delineated on the absolute CBV map, while the tumor recurrence scale was defined as the area of high perfusion. High perfusion was defined using a threshold value for the lesions of at least 22 ml/100 g. This value was based on the experience in our institution; the CBV value of normal-appearing white matter (NAWM) was approximately 12 ml/100 g, and the best cutoff value for distinguishing recurrence from radionecrosis was 22 ml/100 g for application of our protocol. Finally, the clinical target volume (CTV) was acquired by expanding 3 mm on the basis of the GTV. The prescribed dose for re-irradiation was dependent on tumor volumes, initial radiation dose, time since adjuvant postoperative radiotherapy, and location of the lesion with proximity to eloquent brain or radiosensitive structures, including the brainstem, optic chiasm, lens, optic nerves, and cerebral cortex (see Fig. 1).
This graph shows the process of our method performed using a gamma knife planning system. First, the GTV were defined as the high perfusion area on an absolute CBV map, with a cutoff value of 22 ml/100 g. Then, the CTV was acquired by expanding 3 mm on the basis of the GTV. Precise adjustment was performed using three dimensional views. Lastly, the final planning was transferred to post-contrast T1WI. Precise adjustment was performed again. GTV gross tumor volume, CBV cerebral blood volume, CTV planning target volume, T1WI T1 weighted image
In principle, if patients with recurrent high glioma had a CTV < 15 cm3, gamma knife radiotherapy was greatly suggested in our neurosurgery team. To our practical experience, if the CTV was over 15 cm3 or the marginal dose was over 22 Gy, the patients would have a great possibility to develop into radiation-induced toxicity. For those with a CTV over 15 cm3, GKRS would be performed if following criteria were all reached: (1) KPS score is over 85; (2) patients have a good neurological condition; (3) patients refused to have a craniotomy.
Chemotherapy
After re-irradiation treatment, a dose of 200 mg/m2/day for 5 days of temozolomide (TMZ) chemotherapy was administered. Cycles were repeated every 28 days with 3 or more cycles. TMZ was not administered to patients who either refused treatment or who did not meet the treatment criteria. The treatment criteria for TMZ were as follows: KPS core was over 50; major organ function (liver, kidney, etc.) appeared normal; and the predicted survival time was longer than 3 months.
Follow-up after gamma knife re-irradiation
The quantitative perfusion before re-irradiation was treated as the baseline perfusion. All patients underwent follow-up MRI examination every 2 months, except when the patients’ neural condition deteriorated, using a quantitative DSC perfusion MRI sequence. Local progression was defined as a higher or equal perfusion (in comparison with baseline perfusion), corresponding to the re-irradiated area, appearing in the absolute CBV map; however, the size of enhancing lesions changed. A diagnosis of “distant failure” was defined as the appearance of a new enhanced lesion distant from the original tumor site. The day on which local progression or distant failure appeared was defined as the date of progression. Radionecrosis was defined as lower perfusion (in comparison with baseline perfusion), corresponding to the re-irradiated area, appearing on the absolute CBV map; however, the size of the enhancing lesions changed.
Grades for radiation-induced damage
In this article, we used toxicity criteria of the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer (RTOG/EORTC) late radiation morbidity to grade toxicity. Only grade 4 were treated as serious toxicity.
Statistical analysis
Descriptive statistics (median and other measures) were determined for each parameter. Intergroup differences were tested for significance using the two-tailed Mann–Whitney U test. The Wilcoxon paired test was used to determine the statistical difference before and after GKRS treatment. Survival events were defined as death from any cause for overall survival (OS) and as disease progression for progression-free survival (PFS). OS was calculated from the time of histologic diagnosis of the tumor, and PFS was calculated from the time of re-irradiation to tumor progression using the Kaplan–Meier method. Radiographic and clinical variables were tested for a possible correlation with survival. The medians of variables were selected as the cutoff values for separation. Prognostic factors were tested by the log-rank (Mantel–Cox) test. A P value of < 0.05 was considered as statistically significant. All tests were performed using SPSS software (version 12.0; SPSS Inc., Chicago).
Results
Clinical characteristics
Eleven patients (42.3%) were diagnosed with anaplastic astrocytoma (WHO III), and six patients (23.1%) were diagnosed with anaplastic oligodendroglioma (WHO III). Patients with either diagnosis were assigned to the group WHO III. Nine patients (34.6%) were diagnosed with glioblastoma (GBM, WHO IV), and they were assigned to the group GBM. Their clinical characteristics are summarized in Table 1. The detailed information of each participant is supplied in the Supplementary material. Twenty-one (80.8%) cases of progression after GKRS were marginal, two (7.7%) cases of progression were central, and three (11.5%) cases of progression were distant failure.
Age and absolute CBV were significantly different between the two groups (p = 0.022 and p = 0.029, respectively), while other parameters were not. KPS before and after GKRS was significantly different (p = 0.004).
Radiation-induced damage
No patient demonstrated serious radiation-induced toxicity based on the principle of RTOG/EORTC late radiation morbidity, and they all completed the gamma knife radiosurgery treatment without any adverse events. In the later period of follow-up, two patients (7.7%) experienced grade 2 and one patient (3.8%) experienced grade 3 radiation necrosis, which were confirmed by quantitative DSC perfusion. Their symptoms were slowly resolved after administering corticosteroid treatment.
Survival rate analysis
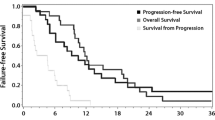
Because there was only a small group of patients with GBM in this study, their survival analysis was not analyzed separately according to the WHO grade. They were analyzed together as high-grade glioma. With a median follow-up of 32 months, the median PFS after GKRS was 8 months (95% CI [6, 12]); the 1- and 2-year survival rates were 30.8 and 11.5%, respectively. The median OS was 25.5 months (95% CI [18, 40]); the 1- and 2-year survival rates were 96.2 and 57.7%, respectively (see Fig. 2). The results of log-rank (Mantel–Cox) tests for PFS and OS are summarized in Table 2. Pathology grade and re-irradiation volume (CTV) were identified as prognostic factors for PFS (see Fig. 2). However, none of the parameters were validated as independent prognostic factors for OS.
Representative cases are shown in Fig. 3, in which the absolute CBV map derived from quantitative DSC-PWI guided the re-irradiation of rHGG using the ELEKTA’s GAMMA Knife Treatment System, and the follow-up results are presented.
Illustrations of the treatment effect of our method. A 69-year-old man with recurrent GBM was re-irradiated by GKRS using our method. The CTV was defined on an absolute CBV map (a). The CTV was relatively smaller than the enhancing area on post-T1WI (b). Five months later, the high perfusion and enhancement had almost disappeared (c, d). A 72-year-old man with distant failure of GBM was also treated by GKRS using our method. The CTV was defined on an absolute CBV map (e). The CTV was larger than the enhancing area on post-T1WI (f). Four months later, the high perfusion and enhancement had almost disappeared (g, h). GBM glioblastoma multiforme, GKRS gamma knife radiosurgery, CTV planning target volume, T1WI T1 weighted image
Discussion
Re-irradiation of recurrent high-grade glioma by GKRS is becoming the alternative choice for this complicated problem. Precise treatment with GKRS is still challenging because the enhancing lesions in post-T1WI after first irradiation therapy are often complicated, where recurrent tumors and radionecrosis usually exist together [8]. On the other hand, some infiltrated parts of high-grade glioma demonstrate non-enhancement on post-T1WI, but relative CBV maps demonstrate an obvious neovascularization [2, 10]. Therefore, precise detection of the contour of the target volume for radiosurgery is vital for the irradiation of rHGG because it could be possible to deliver an effective therapy that may improve clinical outcomes and the life quality of patients with minimal radiotoxicity. Fortunately, absolute CBV maps, derived from quantitative DSC-PWI with high resolution and better inter-modality spatial alignment, could solve this problem [16]. This new technique enables us to develop individualized, patient-tailored therapy strategies for those with rHGG.
This prospective study aimed to explore the treatment effect using absolute CBV maps to guide GKRS for rHGG. We found that the median OS was 25.5 months (95% CI [18, 40]) and that the 1- and 2-year survival rates were 96.2 and 57.7%, respectively. Our primary results were in a reasonable interval compared with prior studies of GKRS for rHGG [7, 18].
No obvious treatment benefits in terms of OS were demonstrated using the method in this study. Even though traditional GKRS treatment of rHGG can slightly improve OS [7, 18], this precise method of GKRS did not further improve it. Previous studies have established that the survival time of patients with glioma is greatly dependent on its genotype [19, 20], and this may explain why no benefit in terms of OS was demonstrated, even in the application of this precise therapeutic method. It seems that the 1-year survival rate was good, but we cannot ignore the fact that patients with glioma of grade III were the primary participants in this study, which may have mendaciously improved the 1-year survival. Prognostic factors were not discovered for OS by log-rank (Mantel–Cox) tests among these selected patients. Age, KPS and chemotherapy were considered to be prognostic factors for recurrent GBM in some articles [21, 22]. Because most of the tumors in this study were WHO III, these factors lost their ability to predict the outcomes in this population. In addition, differing medical histories of study populations among different studies may also lead to this divergence.
We found that the median PFS after GKRS was 8 months (95% CI [6, 12]); the 1- and 2-year survival rates were 30.8 and 11.5%, respectively. The progression-free survival results of our study were favorable in comparison with prior studies that did not use absolute CBV maps [7, 18]. In other words, it is possible that our method could improve the PFS after GKRS and that patients with rHGG could benefit from this because an absolute CBV map can precisely detect the location and scope of recurrent tumors, which prevents patients from suffering from either unnecessary radiotoxicity or residual tumors. Log-rank (Mantel–Cox) tests revealed that pathology grade and CTV were prognostic factors for PFS. This means that patients with a tumor of grade III, or with a small re-irradiation volume could obtain more benefit from this method.
Three patients suffered from mild to moderate radiotoxicity in this study. The high perfusion area was larger than the enhancing area in these patients. To control tumor development, a larger marginal dose was used for these three patients, which may have damaged the normal brain tissue adjacent to the boundary of the recurrent tumors. The KPS of the patients after GKRS was significantly better than that before GKRS. This indicates that our method can improve the quality of life of patients. Similarly, this favorable result may be a benefit of this precise treatment.
Some limitations of this study should be addressed here. First, this is not a case–control study, which may have been more convincing; we had to compare our results with prior studies in many aspects. However, patients with rHGG have various clinical conditions and past medical histories, and it is almost impossible to control so many variables to be at an identical level. Second, due to the small number of patients, we did not analyze the survival results and prognostic factors for grade III tumors and GBM separately. Further studies using our method should explore these aspects.
In conclusion, we used absolute CBV maps, which were derived from quantitative DSC-PWI, to guide the re-irradiation of rHGG using a gamma knife radiotherapy planning system. This method uses the principle of precise treatment. Our results demonstrated that quantitative DSC-PWI-guided GKRS is feasible for the treatment of rHGG and that these outcomes remain to be validated. Despite this, we think that with strict selection, some patients could benefit from treatment with this method.
References
Goodenberger ML, Jenkins RB (2012) Genetics of adult glioma. Cancer Genet 205:613–621
Hou LC, Veeravagu A, Hsu AR, Tse VC (2006) Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus 20:E5
Krex D, Klink B, Hartmann C et al (2007) Long-term survival with glioblastoma multiforme. Brain 130:2596–2606
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Silbergeld DL, Chicoine MR (1997) Isolation and characterization of human malignant glioma cells from histologically normal brain. J Neurosurg 86:525–531
Kumar AJ, Leeds NE, Fuller GN et al (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384
Elaimy AL, Mackay AR, Lamoreaux WT et al (2013) Clinical outcomes of gamma knife radiosurgery in the salvage treatment of patients with recurrent high-grade glioma. World Neurosurg 80:872–878
Stockham AL, Tievsky AL, Koyfman SA et al (2012) Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J Neurooncol 109:149–158
Hsieh PC, Chandler JP, Bhangoo S et al (2005) Adjuvant gamma knife stereotactic radiosurgery at the time of tumor progression potentially improves survival for patients with glioblastoma multiforme. Neurosurgery 57:684–692
Tatter SB (2002) Recurrent malignant glioma in adults. Curr Treat Options Oncol 3:509–524
Souhami L, Seiferheld W, Brachman D et al (2004) Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys 60:853–860
Verma N, Cowperthwaite MC, Burnett MG, Markey MK (2013) Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro-Oncology 15:515–534
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Dhermain F (2014) Radiotherapy of high-grade gliomas: current standards and new concepts, innovations in imaging and radiotherapy, and new therapeutic approaches. Chin J Cancer 33:16–24
Shin W, Horowitz S, Ragin A et al (2007) Quantitative cerebral perfusion using dynamic susceptibility contrast MRI: evaluation of reproducibility and age- and gender-dependence with fully automatic image postprocessing algorithm. Magn Reson Med 58:1232–1241
Carroll TJ, Horowitz S, Shin W et al (2008) Quantification of cerebral perfusion using the “bookend technique”: an evaluation in CNS tumors. Magn Reson Imaging 26:1352–1359
Kim YR, Rebro KJ, Schmainda KM (2002) Water exchange and inflow affect the accuracy of T1-GRE blood volume measurements: implications for the evaluation of tumor angiogenesis. Magn Reson Med 47:1110–1120
Larson EW, Peterson HE, Lamoreaux WT et al (2014) Clinical outcomes following salvage gamma knife radiosurgery for recurrent glioblastoma. World J Clin Oncol 5:142–148
Gutman DA, Cooper LA, Hwang SN et al (2013) MR imaging predictors of molecular profile and survival: multi-institutional study of the TCGA glioblastoma data set. Radiology 267:560–569
Smits M, van den Bent MJ (2017) Imaging correlates of adult glioma genotypes. Radiology 284:316–331
Miwa K, Matsuo M, Ogawa S et al (2014) Re-irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol 9:181
Fokas E, Wacker U, Gross MW et al (2009) Hypofractionated stereotactic reirradiation of recurrent glioblastomas: a beneficial treatment option after high-dose radiotherapy? Strahlenther Onkol 185:235–240
Acknowledgements
We would like to thank Nature Research Editing Service for English language editing.
Funding
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (81641176), the Natural Science Foundation of Shandong Province (ZR2014HM069 and ZR20141TM002), the Science and Technology Planning Project of Shandong Province (2014A010105009, 2013CB834702, 2014GSF118046, and 2016GSF201092), Major scientific and technological innovation project of Shandong Province (2017CXGC1209) and The Taishan Scholars Program (No. tsqn20161070).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declare no relevant relationship with any funding agencies or commercial institutes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, B., Zhao, P., Zhang, Y. et al. Quantitative dynamic susceptibility contrast perfusion-weighted imaging-guided customized gamma knife re-irradiation of recurrent high-grade gliomas. J Neurooncol 139, 185–193 (2018). https://doi.org/10.1007/s11060-018-2859-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2859-8