Abstract
Background
The trans-eyebrow supraorbital keyhole approach, a minimal transcranial approach, has been widely used in different types of surgery for sellar and parasellar lesions. In this study, we investigated the outcome of this approach in the surgical treatment of suprasellar and third ventricular craniopharyngioma.
Methods
Twenty-seven patients with suprasellar and third ventricular craniopharyngioma underwent surgery via a supraorbital approach between June 2007 and June 2018. The medical data and follow-up results were retrospectively analyzed.
Results
All tumors were located in the suprasellar region and the third ventricle. The mean tumor size was 29.1 mm. The mean follow-up period was 49.6 months. Gross total resection (GTR) was achieved in 23 patients (85.2%). Of 17 patients with preoperative visual impairment, 12 patients (70.6%) showed improvement. Following surgery, 11 patients exhibited new-onset anterior hypopituitarism, ten developed diabetes insipidus, and two became overweight. One residual tumor relapsed 1 year after surgery. No perioperative death, cerebrospinal fluid (CSF) rhinorrhea, or meningitis occurred. All patients exhibited satisfactory cosmetic results. At the last follow-up, the Extended Glasgow Outcome Scale Score was 8 in 25 patients (92.6%).
Conclusion
The supraorbital trans-eyebrow keyhole approach is characterized by minimal invasion and a satisfactory cosmetic outcome. According to our experience, craniopharyngiomas located in the suprasellar region and the third ventricle can be safely resected via a trans-eyebrow supraorbital keyhole approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Craniopharyngiomas are histologically benign tumors and account for approximately 2–5% of primary intracranial tumors [1]. Resection is the mainstay of treatment for craniopharyngiomas [2,3,4,5,6]. However, due to their complicated location and proximity to vital neurovascular structures, resection of craniopharyngiomas still remains a substantial challenge for surgeons [7,8,9,10]. Multiple surgical approaches, such as the traditional transcranial approach [11,12,13,14,15,16,17] and the endoscopic endonasal approach (EEA) [18,19,20], are currently used in the surgical treatment of craniopharyngioma. Each type of approach has its own advantages and disadvantages, and controversies regarding the optimal surgical approach still exist.
The supraorbital keyhole approach is a minimally invasive transcranial approach that can offer excellent exposure of the anatomical structures of the anterior skull base, middle cranial fossa, and sellar region [21, 22]. In recent years, a supraorbital trans-eyebrow keyhole approach has been used in the surgical treatment of craniopharyngiomas; however, these studies either only included a small group of patients or lacked detailed surgical and follow-up data [23,24,25,26,27,28]. Since June 2007, we have employed the trans-eyebrow supraorbital keyhole approach for surgical resection of craniopharyngioma. The results achieved were retrospectively examined in this study.
Materials and methods
This study was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). A series of 27 patients with craniopharyngioma underwent surgical resection via a trans-eyebrow supraorbital keyhole approach at our hospital between June 2007 and June 2018. The medical records and follow-up results were retrospectively analyzed.
Prior to surgery, all patients underwent head computed tomography (CT) and enhancing magnetic resonance imaging (MRI) examination. The size of the tumor was determined by the maximal tumor diameter based on MR images. Tumors were classified according to the scheme of Samii et al. [29]. The degree of tumor removal was evaluated based on intraoperative observation and postoperative MRI images. Gross total resection (GTR) was considered when both the intraoperative impression and postoperative MRI indicated no residual tumor.
The ophthalmologic examination consisted of testing the visual acuity and visual field. The visual outcome was graded as improved, stable, or deteriorated.
The preoperative endocrine evaluation included measurement of thyroid stimulating hormone (TSH), free triiodothyronine (FT3), free thyroxine (FT4), prolactin, follicle stimulating hormone, luteinizing hormone, morning cortisol, adrenocorticotropic hormone, growth hormone, and insulin-like growth factor. A diagnosis of diabetes insipidus was determined according to urine volume (> 3 L/24 h) and urine osmolality (< 300 mOsm/kg). Body mass index (BMI) was measured. Weight categories were classified as normal (BMI < 25), overweight (BMI 25–30), and obesity (BMI > 30).
Follow-up evaluations were scheduled at 1, 3, and 6 months after discharge and annually thereafter. During each visit, the neurological, ophthalmological, and endocrine statuses were assessed, and an imaging assessment was conducted. Prior to the writing of this article, all patients had participated in a telephone questionnaire for Extended Glasgow Outcome Scale evaluation.
Surgical technique
Tumors were approached from the nondominant side. In other cases, the approach was from the side of the most compromised visual function or tumor lateral extension below the sphenoid ridge. The patient was placed supine with the head secured in a 3-pin Mayfield head holder, raised 15° and rotated 0°–15° to the contralateral side. Once the patient was positioned, the location of the frontal sinus was mapped. Ipsilateral ventriculostomy drainage was placed prior to the supraorbital craniotomy in cases of preoperative hydrocephalus.
A 4–5 cm incision was made along the eyebrow, laterally to the supraorbital notch (Fig. 1a). The subcutaneous tissue was dissected to expose the corrugators, frontal muscles, and temporalis fascia. The scalp was superiorly retracted with silk threads. The pericranium was incised as superiorly as possible to create an inferior-based U-shaped pericranial flap that reflected inferiorly. Laterally, a small portion of the temporalis muscle was dissected and retracted backward to expose the keyhole region. During the dissection, care was taken to avoid injury to the supraorbital nerve.
Supraorbital keyhole approach on the left side. a The location of the incision; b The bone window and dural incision; c Resection of tumor through the prechiasmatic window; d Resection of tumor through the opticocarotid window; e Resection of tumor through the carotid-oculomotor window; f Resection of tumor through the lamina terminalis
Using a high-speed drill with a footplate attachment, a burr hole was drilled a few millimeters above the frontosphenoidal suture, and a semicircular craniotomy of approximately 3 × 2 cm was performed above the orbital rim and lateral to the frontal sinus (Fig. 1b). If the frontal sinus was entered, the mucosa was removed, and the sinus was packed with streptomycin bone wax. Under microscopic visualization, a small dura and arachnoid incision was made to allow for cerebrospinal fluid (CSF) egress. The inner table of the craniotomy and the bony protuberance of the orbital roof were flattened. For some tumors exhibiting lateral growth below the sphenoid ridge, the lateral part of the sphenoid ridge was removed to increase the exposure of tumors laterally to the internal carotid artery.
The dura mater was opened in a “U-shaped” manner that reflected inferiorly over the orbital rim. The proximal sylvian fissure and opticocarotid and optic oculomotor cisterns were opened for further CSF egress. Following adequate brain relaxation, additional dissection of the arachnoid membrane at the base of the frontal lobe and within the proximal sylvian fissure was performed to further free the frontal lobe and allow it to fall away from the skull base. Then, both optic nerves, the optic chiasm, both internal carotid arteries, and the tumor were identified. Surgical debulking of the tumor was then accomplished using a standard microsurgical technique with or without ultrasonic aspiration. Once adequate tumor debulking and neuro-vascular relaxation were achieved, the tumor was removed in pieces through the prechiasmatic, opticocarotid, and carotid-oculomotor windows (Fig. 1c–e). The lamina terminalis was opened to remove tumors in most patients with ventricular tumors (Fig. 1f). For patients who were suspected to have residual tumors in the pituitary fossa, posteroinferior area of the ipsilateral optic nerve and chiasm, and ipsilateral third ventricular wall, endoscopy was used to confirm adequate tumor resection (Fig. 2b–d).
Endoscopy-assisted supraorbital keyhole approach. a Micro-surgical field after tumor resection, the suspected pituitary stalk was in prechiasmatic window; b Endoscopic exploration revealed the pituitary stalk in the rear and residual tumor in the anterior; c Endoscopy-assisted tumor resection; d Endoscopy revealed that the pituitary stalk was retained and no residual tumor was observed in the pituitary fossa and inferior side of the optic chiasm; e Satisfactory postoperative cosmetic outcome
Once the lesion was satisfactorily resected, a watertight dural closure with a dural graft was performed. The bone flap was replaced with a titanium plating system. The muscular and subcutaneous layers and the skin were meticulously closed to ensure a satisfactory cosmetic result.
Results
Patient population
A total of 27 patients (14 males and 13 females) were included in this study. One patient with recurrent craniopharyngioma underwent placement of an Ommaya reservoir and radiosurgery. The mean age at the time of operation was 40.0 years (range 3–66 years), and the mean follow-up period was 49.6 months (range 3–131 months).
The main preoperative complaints included visual acuity loss (14 patients, 51.9%), headache (seven patients, 25.9%), and polyuria (five patients, 18.5%). The mean tumor size was 29.1 mm (range 10–51 mm); 11 tumors (40.7%) were solid, eight tumors (29.6%) were cystic, and the remaining eight tumors (29.6%) were mixed. Additionally, seven patients (25.9%) presented with preoperative hydrocephalus. A review of the pathologic findings revealed 19 adamantinomatous (70.4%) and eight papillary (29.6%) tumors. Detailed preoperative data are summarized in Table 1.
Extent of tumor removal and recurrence
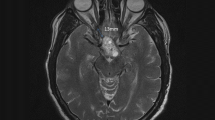
The tumors were resected with endoscopic assistance in 10 patients (37.0%). GTR was achieved in 23 patients (85.2%) (Fig. 3), and small residual tumors were found in 4 patients (14.8%). Intraoperatively, all residual tumors exhibited intimate adherence to the ventricular wall. At the 1-year follow-up evaluation, one residual tumor had relapsed. The pituitary stalks were preserved in 6 patients (25.9%) and sacrificed in 6 patients (22.2%). The results are summarized in Table 2.
Preoperative and their respective postoperative MR images confirm GTR of different grades of tumor. a, Case 1, a grade II tumor according to the classification system of Samii et al. [29]; b, Case 10, a grade III tumor, c, Case 22, a grade IV tumor; d, Case 9, a recurrent tumor of grade IV
Ophthalmological outcomes
Of the 17 patients (64.7%) with preoperative visual impairment, 12 patients (70.6%) improved, 3 patients remained stable, and 2 patients deteriorated (Table 2).
Endocrinologic outcomes
Before the operation, 7 patients (25.9%) had normal pituitary function, 16 (59.3%) exhibited partial hypopituitarism, 2 (7.4%) showed panhypopituitarism, 5 (18.5%) had diabetes insipidus, and one was overweight. After the operation, 11 (40.1%) patients developed new-onset anterior pituitary hypofunction, 10 (45.5%) developed permanent diabetes insipidus, two (cases 10 and 26) became overweight, two (cases 12 and 19) developed transient lethargy, and one (case 10) showed memory loss (Table 2).
Complete tumor resection was achieved in four patients who had completely normal postoperative endocrine function. Among them, three tumors were < 2 cm in size and were classified as grade 2 according to the classification scheme of Samii et al. [29]; the other tumor was larger than 2 cm and was classified as grade 4.
Hydrocephalus and perioperative complications
Seven patients had preoperative hydrocephalus (Table 1), and all patients experienced remission after the operation.
No perioperative death, CSF rhinorrhea, or meningitis occurred in this series. Postoperative pseudomeningocele formation was observed in two patients. One patient was cured via lumbar drainage for 5 days, and the other was cured with local compression over the incision. Violation of the frontal sinus occurred in one patient and was immediately repaired. Transient forehead numbness was reported by one patient. One patient exhibited a mild frontal lobe contusion with mental symptoms and was cured with conservative therapy. One patient had a postoperative sellar hematoma that did not require evacuation. The patient later developed hydrocephalus with bilateral visual deterioration and received a ventriculoperitoneal shunt. The hydrocephalus was resolved; however, no improvement of visual impairment was observed.
Late performance outcomes
All patients exhibited satisfactory postoperative cosmetic results (Fig. 2e). Three patients remained overweight; however, their weight stabilized 6 months after the operation. Fourteen patients required cortisol with or without thyroxine hormonal replacement therapy, and 15 patients required oral desmopressin. At the last follow-up, the Extended Glasgow Outcome Scale Score was 8 in 25 patients (92.6%) and 7 and 6 in one patient each.
Discussion
For surgical management of craniopharyngiomas, the traditional transcranial approaches have the disadvantages of a large incision, unnecessary excessive brain exposure, and remarkable brain retraction [25], while an EEA has the drawbacks of a long working distance, poor vascular control, and the risk of CSF leakage or meningitis [30].
The supraorbital keyhole approach is an anterolateral minimally invasive transcranial approach. Anatomical studies have shown that the supraorbital keyhole approach offers a surgical field similar to traditional transcranial approaches with minimal brain retraction and limited craniotomy [31, 32]. The anatomical features (e.g., suprasellar craniopharyngiomas rarely have an arterial supply from the posterior circulation, and the expansile growth of tumors widens the neurovascular windows) facilitate resection of these tumors via an anterolateral transcranial approach. Reosch et al. [24] reported the largest group (39 patients) of craniopharyngiomas operated on via a supraorbital approach. The rates of GTR and visual improvement of their group were 74.3% and 66.6%, respectively. In another group of 22 craniopharyngiomas, Gazzeri et al. [25] reported a 90.9% GTR rate. Tawk et al. [28] recently reported that GTR was achieved in four patients (66.6%), with a visual improvement rate of 100%.
The GTR of craniopharyngioma is generally believed to be associated with tumor characteristics, the surgeon’s experience, and the surgical strategy. According to several large cohort studies, the GTR rate of craniopharyngioma is approximately 45–90% [11, 13, 20, 33,34,35]. With the goal of total resection, 23 patients (85.2%) achieved GTR in our study. During resection of a craniopharyngioma via the supraorbital approach, blind spots in the surgical field may preclude the accomplishment of GTR. Significant superior or inferior extension of the tumor may be difficult to address with the flat trajectory along the anterior cranial base offered by the supraorbital approach. In our study, no tumor exhibited apparent extension to an enlarged sella or lateral ventricle; the tumors were relatively small, and all were grade 2–4 according to Samii et al. classification scheme [29]. Tumors were resected through multiple neurovascular windows, such as the subchiasmatic, opticocarotid, lamina terminalis, and carotid-oculomotor windows, either in combination or alone. Intraoperative use of endoscopy allowed exposure of blind tumor spots for timely resection of residual tumors. Intraoperatively, close tumor-hypothalamus adhesion or blurred boundaries were identified in four patients with residual tumors; tiny tumor tissues were left to avoid further hypothalamus injury.
Craniopharyngioma is characterized by a propensity for postoperative recurrence, which is associated with the extent of resection and duration of follow-up. Using microsurgical transcranial approaches, Yasargil et al. [11] reported a 90% GTR rate and a 7% recurrence rate. Park et al. [20] reported a 46% GTR rate and a 15.5% overall tumor recurrence rate over a mean follow-up period of 35 months in their EEA group. In our study, during an average follow-up duration of 49.6 months, one residual tumor relapsed, yielding a recurrence rate of 3.7%.
After transcranial surgery, the reported rate of visual decline is approximately 10% [13, 35, 38]. After transsphenoidal surgery, the rate of visual improvement is reported to be 76.4–97% [29, 30]. In our study, 12 patients (70.6%) exhibited postoperative visual improvement, and 2 (7.4%) showed further visual decline. Of the two patients who exhibited postoperative visual impairment, patient No. 12 had bilateral decline, which was suspected to be associated with postoperative complications of hematomas in the sellar region and secondary hydrocephalus. The other patient presented with a decline in unilateral visual acuity, which might have resulted from intraoperative manipulation of the optic apparatus and its supplying perforators. During the operation, care must be taken to protect the optic apparatus and vascular structures. Particularly, it is important to fully relax the neurovascular structures by CSF release and sufficient tumor debulking before resection is performed through the neurovascular windows. Resection of large tumors in pieces through multiple neurovascular windows instead of a single window may minimize blind resection and manipulation of neurovascular structures.
Endocrinologic disturbances associated with radical resection of craniopharyngiomas are very common. The reported incidences of panhypopituitarism and diabetes insipidus are 24–66% and 43–79% [11, 13, 36, 37], respectively. In this study, 11 patients (40.1%) exhibited new-onset anterior hypopituitarism, and 10 patients (45.4%) showed new-onset permanent diabetes insipidus. Eight patients who retained all or part of the pituitary stalk postoperatively exhibited transient diabetes insipidus. In contrast, the seven patients in whom the pituitary stalk was sacrificed all developed postoperative permanent diabetes insipidus. Our results suggest that a residual pituitary stalk may reduce the severity of postoperative diabetes insipidus.
Except for mild approach-related complications, no major complications, including perioperative death, CSF rhinorrhea, and meningitis, occurred in our case series. This result may have occurred because no giant tumors were included in our cohort, only limited brain retraction and exposure were needed in this approach, and the hypothalamus was preserved when the tumor involvement was closed.
Controversies regarding optimal surgical approach selection for sellar tumors still exist [38,39,40,41]. Most of the tumors included in our study could be safely addressed via the EEA or a traditional transcranial approach. Compared to the EEA, most neurosurgeons are more familiar with the microsurgery of the supraorbital approach, which yields superior surgical control and a low incidence of CSF leakage. However, this approach also has some drawbacks, including inevitable manipulation of the neurovascular structures and the presence of blind resection. The issue of blind surgical spots can be overcome by intraoperative endoscopy, but this technique might aggravate the problem of a crowded operative corridor due to the keyhole approach. Compared to a traditional transcranial approach, the supraorbital keyhole approach has the advantages of reduced trauma and improved cosmetic outcomes. Nevertheless, this approach also has the following apparent limitations: (i) a narrow bone window that increases the difficulty of operation and (ii) high experience requirements for surgeons. Hence, before using the trans-eyebrow supraorbital keyhole approach, it is necessary to consider the tumor characteristics, the surgeon’s familiarity with this approach, and thorough preoperative planning.
The present study bears all the disadvantages of a respective study design. A larger group of patients with longer duration of follow-up and a similar group of patients operated on via the EEA or traditional transcranial approach are required for further studies.
Conclusion
The supraorbital trans-eyebrow keyhole approach provides excellent exposure of the sellar and parasellar anatomical structures with minimal invasion and satisfactory cosmetic outcomes. Intraoperative application of endoscopy can further augment the exposure of relevant anatomical structures and lesions. Favorable results were achieved in our series of suprasellar and third ventricular craniopharyngiomas. Based on the tumor characteristics and surgeon’s experience, this approach can be employed as an alternative minimally invasive approach for radical resection of suprasellar and third ventricular craniopharyngiomas.
References
Hoffman HJ (1994) Surgical management of craniopharyngioma. Pediatr Neurosurg 21(Suppl 1):44–49
Constine LS, Woolf PD, Cann D et al (1993) Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med 328(2):87–94
Mulhern RK, Hancock J, Fairclough D, Kun L (1992) Neuropsychological status of children treated for brain tumors: a critical review and integrative analysis. Med Pediatr Oncol 20(3):181–191
Kang JK, Song JU (1988) Results of the management of craniopharyngioma in children. An endocrinological approach to the treatment. Childs Nerv Syst 4(3):135–138
Rajan B, Ashley S, Gorman C et al (1993) Craniopharyngioma—a long-term results following limited surgery and radiotherapy. Radiother Oncol 26(1):1–10
Shiminski-Maher T, Rosenberg M (1990) Late effects associated with treatment of craniopharyngiomas in childhood. J Neurosci Nurs 22(4):220–226
Chakrabarti I, Amar AP, Couldwell W, Weiss MH (2005) Long-term neurological, visual, and endocrine outcomes following transnasal resection of craniopharyngioma. J Neurosurg 102:650–657
Qi S, Lu Y, Pan J, Zhang X, Long H, Fan J (2011) Anatomic relations of the arachnoidea around the pituitary stalk: relevance for surgical removal of craniopharyngiomas. Acta Neurochir (Wien) 153:785–796
Steno J, Malacek M, Bizik I (2004) Tumor-third ventricular relationships in supradiaphragmatic craniopharyngiomas: correlation of morphological, magnetic resonance imaging, and operative findings. Neurosurgery 54:1051–1058 discussion 58–60
Wang KC, Hong SH, Kim SK, Cho BK (2005) Origin of craniopharyngiomas: implication on the growth pattern. Childs Nerv Syst 21:628–634
Yaşargil MG, Curcic M, Kis M, Siegenthaler G, Teddy PJ, Roth P (1990) Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg 73(1):3–11
Inoue HK, Fujimaki H, Kohga H, Ono N, Hirato M, Ohye C (1997) Basal interhemispheric supra- and/or infrachiasmal approaches via superomedial orbitotomy for hypothalamic lesions: preservation of hypothalamo-pituitary functions in combination treatment with radiosurgery. Childs Nerv Syst 13(5):250–256
Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M (1999) Surgical treatment of craniopharyngiomas: experience with 168 patients. J Neurosurg 90(2):237–250,1999
Erşahin Y, Yurtseven T, Ozgiray E, Mutluer S (2005) Craniopharyngiomas in children: Turkey experience. Childs Nerv Syst 21(8–9):766–772
Zhang YQ, Ma ZY,WuZB, Luo SQ, Wang ZC (2008) Radical resection of 202 pediatric craniopharyngiomas with special reference to the surgical approaches and hypothalamic protection. Pediatr Neurosurg 44(6):435–443
Samii M, Bini W (1991) Surgical treatment of craniopharyngiomas. Zentralbl Neurochir 52:17–23
Du C, Feng CY, Yuan XR, Liu Q, Peng ZF, Jiang XJ, Li XJ, Xiao GL, Xiong T (2016) Microsurgical management of craniopharyngiomas via a unilateral subfrontal approach: a retrospective study of 177 continuous cases. World Neurosurg 90:454–468
Kassam AB, Gardner PA, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM (2008) Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg 108(4):715–728
Dho YS, Kim YH, Se YB, Han DH, Kim JH, Park CK, Wang KC, Kim DY (2017) Endoscopic endonasal approach for craniopharyngioma: the importance of the relationship between pituitary stalk and tumor. J Neurosurg 29:1–9
Park HR, Kshettry VR, Farrel CJ, Lee JM, Kim YH, Won TB, Han DH, Do H, Nyguist G, Rosen M, Kim DG, Evans JJ, Paek SH (2017) Clinical outcome after extended endoscopic endonasal resection of craniopharyngiomas: two-institution experience. World Neurosurg 103:465–474
Wilson DA, Duong H, Teo C, Kelly DF (2014) The supraorbital endoscopic approach for tumors. World Neurosurg 82(65):S72–S80
Jallo GI, Bognár L (2006) Eyebrow surgery: the supraciliary craniotomy: technical note. Neurosurgery 59(Suppl 1):ONSE157–ONSE158
Mclaughlin N, Ditzel Filho LF, Shalaie K, Solari D, Kassam AB, Kelly DF (2011) The supraorbital approach for recurrent or residual suprasellar tumors. Minim Invasive Neurosurg 54:155–161
Reisch R, Perneczky A (2005) Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery 57(Suppl 4):242–255
Gazzeri R, Nishiyama Y, Teo C (2014) Endoscopic supraorbital eyebrow approach for the surgical treatment of extraaxial and intraaxial tumors. Neurosurg Focus 37:1–8
Czirják S, Szeifert GT (2006) The role of the superciliary approach in the surgical management of intracranial neoplasms. Neurol Res 28(2):131–137
Prat R, Galeano I, Evangelista R, Pancucci G, Guarin J, Ayuso A, Misra M (2017) Trans-eyebrow supraorbital approach in large suprasellar craniopharyngioma surgery in adults: analysis of optic nerve length extent of tumor resection. Acta Neurochie 159:873–880
Tawk RG, Binning MJ, Thomas JM, Siddiqui AH, Grand W (2014) Transciliary supraorbital approach (Eyebrow Approach) for resection of retrochiasmatic craniopharyngiomas: an alternative approach, case series, and literature review. J Neurol Surg A 75:354–364
Samii M, Tatagbia M (1997) Surgical management of craniopharyngiomas: a review. Neurol Med Chir (Tokyo) 37:141–149
Zielinski G, Sajjad EA, Robak L, Koziarski A (2018) Subtemporal approach for gross total resection of retrochiasmatic craniopharyngiomas: our experience on 30 cases. World Neurosurg 109:E265–E273
Figueiredo EG, Deshmukh V, Nakaji P, Deshumkh P, Crusius MU, Crawford N, Spetzler RF, Preul MC. (2006) An anatomical evaluation of the mini-supraorbital approach and comparison with standard craniotomies. Neurosurgery 59(4 Suppl 2):ONS212–ONS220
Cheng CM, Noguchi A, Dogan A, Anderson GJ, Hsu FP, McMenomey SO, Delashaw JB Jr (2013) Quatitative verification of the keyhole concept: a comparison of area exposure in the parasellar region via supraorbital keyhole, frontotemporal pterional, and supraorbital approach. J Neurosurg 118:264–269
Weiner HL, Wisoff JR, Rosenberg ME, Kupersmith MJ, Cohen H, Zagzag D, Shiminski-Maher T, Flamm ES, Epstein FJ, Miller DC (1994) Craniopharyngiomas: a clinicopathological analysis of factors predictive of recurrence and functional outcome. Neurosurgery 35(6):1001–1011
Pan J, Qi S, Liu Y, Lu Y, Peng J, Zhang X, Xu YK, Huang G, Fan J (2016) Growth patterns of craniopharyngiomas: clinical analysis of 226 patients. J Neurosurg Pediatr 17:418–433
Fomichev D, Kalinin P, Kutin M, Sharipov O (2016) Extended transsphenoidal endoscopic endonasal surgery of suprasellar craniopharyngiomas. World Neurosurg 94:181–187
Van Effenterre R, Boch AL (2002) Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg 97:3–11
Shirane R, Su C, Kusaka Y, Jokura H, Yoshiomto T (2002) Surgical outcomes in 31 patients with craniopharyngiomas extending outside the suprasellar cistern: an evaluation of the frontobasal approach. J Neurosurg 96:704–712
Bowers CA, Altay T, Couldwell WT (2007) Surgical decision-making strategies in tuberculum sellae meningioma resection. Neurosurg Focus 30:E1
de Divitis E, Esposito F, Cappabianca P, Cavallo LM, de Divitis O (2008) Tuberculum sellae meningiomas: high route or low route? A series of 51 consecutive cases. Neurosurgery 62:556–563
Soni RS, Patel SK, Husain Q, Dahodwala MQ, Eloy JA, Liu JK (2014) From above or below: the controversy and historical evolution of tuberculum sellae meningioma resection from open to endoscopic skull base approaches. J Clin Neurosci 21:559–563
Liu JK, Sevak IA, Carmel PW, Eloy JA (2016) Microscopic versus endoscopic approaches for craniopharyngiomas: choosing the optimal surgical corridor for maximizing extent of resection and complication advoidance using a personalized, tailored approach. Neurosurg Focus 41:1–18
Funding
The funding was provide by The Science and Technology Project of Guangdong Province (NO:2015A020212016 and 2016A020214007), The Science and Technology Program of Guangzhou (NO: 201604020080), China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, M., Ye, Z., Ling, C. et al. Trans-eyebrow supraorbital keyhole approach in suprasellar and third ventricular craniopharyngioma surgery: the experience of 27 cases and a literature review. J Neurooncol 141, 363–371 (2019). https://doi.org/10.1007/s11060-018-03041-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03041-7