Abstract
Diffuse intrinsic pontine glioma (DIPG) is an incurable disease with a median overall survival of 10 months. Immune modulating antibodies have recently emerged as a highly promising treatment modality in multiple cancer types. We present results from the first study to evaluate the immune modulating antibody MDV9300 (pidilizumab) in pediatric patients with DIPG. All patients aged 3 years and older, diagnosed with DIPG between February 2014 and June 2015 in Israel, were offered to participate in the study. Enrolled patients were started on biweekly 6 mg/kg MDV9300 after radiation completion. Treatment was continued until disease progression on imaging. Patients were followed biweekly for the occurrence of neurological deficit toxicities and side effects. Secondary endpoints were event free survival and overall survival. Of 13 children diagnosed with DIPG during the study period, nine were enrolled in the study. The patients underwent radiotherapy and none had chemotherapy. A total of 83 cycles of MDV9300 (range 2–16) were applied. The main side effects were neutropenia (CTCAE grade 1–3), mild to moderate fatigue, and acute elevation of blood pressure. Four patients died within 1 year of the diagnosis, another three died within 2 years and two children are still alive nearly 30 months from diagnosis, with stable disease. The median event free survival is 9.3 months (range 6.8–24) and the median overall survival is 15.6 months (range 6.9–28). Preliminary results demonstrate that MDV9300 treatment is safe and may be effective in the treatment of children with DIPG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse intrinsic pontine glioma (DIPG) is one of the most lethal pediatric malignant tumors. The only proven effective therapy for this disease is radiation. Unfortunately, this modality is palliative and almost all patients succumb to their disease within 10–12 months from diagnosis. Multiple chemotherapy regimens, tyrosine kinase inhibitors, and radiation techniques have shown no impact on overall or event free survival [1,2,3,4]. As molecular genetic data on DIPG await translation to clinical practice, new treatment modalities are urgently needed [5].
Several studies showed involvement of the immune system in high grade glial tumors. Yang et al. compared immune infiltrate between patients with WHO I and WHO IV gliomas. Glioblastomas exhibited significantly higher perivascular (CD8) T cell infiltration than pilocytic astrocytomas (62 vs. 29%, p = 0.0005). Perivascular (49%) and intratumoral (89%; p = 0.004) CD56-positive cells were more commonly associated with glioblastoma [1]. Elevated CD3+ and CD8+ tumor-infiltrating immune cells were shown to correlate with prolonged survival in glioblastoma patients [6]. Despite the presence of immune cells within the tumor, high grade gliomas evade tumor detection and immune killing through multiple mechanisms. These mechanisms include PD1 activation through PDL1 [5], induction of B7-H1 receptor (PDL1 receptor) expression on tumor associated macrophages [7], inhibition of expression of CD40 ligand and FAS ligand on tumor infiltrating T cells, and downregulation of CD 40, 80, and 86 on antigen presenting cells [6].
Studies evaluating the efficacy of immune modulating therapies in animal models of high grade glioma, have shown promising results. Fifteen of 38 (40%) mice treated with anti-PD1 antibodies and radiation [8] or indoleamine-2,3-dioxygenase inhibition (IDO) combined with anti-CTLA4 and anti-PD1/PDL1 antibodies [9] were long term survivors.
MDV9300 (pidilizumab) is an immune modulating humanised IgG1 monoclonal antibody. Pidilizumab was originally thought to bind to the programmed cell death 1 (PD-1) molecule, but this is currently uncertain. Yet, this monoclonal antibody seems to enhance endogenous antitumor immune responses [10]. In adults, pidilizumab showed clinical activity in hematologic malignancies. Tumor response was associated with elevation of the rates of CD4 tumor infiltrating T cells [10, 11] and CD4/CD8 central memory cells. To date, no study regarding the safety and efficacy of MDV9300 treatment in children was reported in the English literature.
We present our preliminary results regarding toxicity and efficacy of MDV9300 (pidilizumab) in pediatric patients with DIPG.
Methods
This national prospective study involved all pediatric neuro-oncology centers in Israel. The study (clinicaltrials.gov ID NCT01952769) was approved by the Israel Ministry of Health and by the IRB committees of each participating center; and was performed in accordance with the ethical standards as outlined in the 1964 Declaration of Helsinki and its later amendments.
Children aged 3 years and older, diagnosed with DIPG between February 2014 and June 2015, were offered to participate in the study. DIPG diagnosis was based on all the following: (a) symptoms starting less than 6 weeks prior to diagnosis. (b) Symptoms that include one or more of the following: cranial nerve deficit, cerebellar dysfunction, or long tract dysfunction, and (c) MRI revealing a lesion infiltrating > 50% of the pons [12], The MRI findings were confirmed by two senior radiologists. Patients were eligible to enrol regardless of clinical status or response to radiation.
Following diagnosis, patients were referred for standard tumor bed irradiation, and symptomatic patients were started on steroids until stabilization. Enrolment in the study was allowed at least two weeks following radiation completion. The maximal daily dexamethasone dose allowed by the study was 2 mg/m2.
Trial design and statistics
Each treatment cycle consisted of 2 weeks, and started with intravenous administration of 6 mg/kg of MDV9300. Drug dose was based on prior data from adult studies, which showed a good toxicity profile of MDV9300 when given at the therapeutic dose [13, 14]. Accrual of patients in groups of three was planned. Accrual of a subsequent group of three was allowed if no dose-limiting toxicities (DLTs) were observed within 14 days of treatment. Dose reduction of 25% was planned if a DLT would occur in 1 of 3 patients. All patients were followed for the occurrence of toxicities by means of biweekly evaluations that included routine physical examinations, Karnofsky Performance Status evaluation, blood count, and assessments of electrolytes and liver function. ECG, TSH, T3, and T4 levels were assessed every 12 weeks. Adverse events were graded using the National Cancer Institute (Rockville, MD, USA) Common Terminology Criteria for AE Version 4.3 (NCICTC-AE 4.3). Secondary endpoints were event free survival (EFS) and overall survival (OS), calculated from the date of diagnosis. Kaplan–Meier methodology was used to estimate time-to-event outcomes.
Results
Thirteen children, aged 3–18 years, were diagnosed with DIPG in Israel during the study period. Of them, 9 were enrolled in the present study. Four children were not enrolled due to parents’ preference. Patient characteristics are presented in Table 1. The median age at diagnosis was 6.5 years (range 3–19 years). The Karnofsky score at diagnosis ranged from 40 to 100. At the end of the radiation therapy, one patient improved his Karnofsky score to 70 (patient #7), the others had the same score before and after the radiotherapy. One patient (#3) underwent a biopsy of a tumor with an exophytic component. Pathology was consistent with glioblastoma multiforme. PD-1 analysis was not done on the specimen since the biopsy was taken before the patient was enrolled in the study. All patients were treated with standard tumor bed irradiation of 54 Gray. The median time between diagnosis and trial start day was 4 months (range 2–8 months). The time frame from the end of the radiotherapy to the initiation of treatment was dependent on the referral time and on the time to obtain parental consent Patients’ current status and follow up data are summarized in Table 1.
A total of 83 cycles of MDV9300 (range 2–16) were applied. Mild to moderate fatigue was reported by the patients following 7 (8.5%) of 83 cycles. Ten cycles (12%) were followed by neutropenia (CTCAA grade 1–3) and 2 were followed by mild lymphopenia. No patient was diagnosed with neutropenia of less than 500 cells/mm3 and no patient had a positive blood culture or a hospital admission due to a febrile illness. One grade 3 adverse event was noted, namely, a single event of blood pressure elevation to a maximum of 180/120 during MDV9300 infusion. Drug infusion was stopped and the patient was treated with one dose of nifedipine with blood pressure normalization. The patient underwent eight further treatment cycles with no significant side effects nor hypertension. Side effects and their rates are listed in Table 2.
Overall survival at 6 and 12 months was 100 and 55% respectively. Four patients survived less than 1 year (7–12 months); for 3 of them the Karnofsky score was less than 60 upon diagnosis. Three other patients survived 14–19 months, and two patients are alive 18 and 30 months after diagnosis. The median event free survival is 9.3 months (range 6.8–24) and the median overall survival is 15.6 months (range 6.9–28 months).
Case illustration # 1
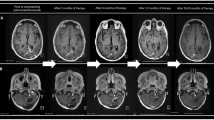
A 10-year-old boy (patient number 7 on Table 1) presented with left hemiparesis and MRI consistent with tumor infiltration of the pons (Fig. 1). Following radiation therapy, he developed quadriplegia and his general status deteriorated to a Karnofsky score of 40. MRI revealed progression or pseudoprogression of the pontine lesion. The first dose of MDV9300 was given 6 months after diagnosis. Imaging studies at 3, 6, and 12 months following the first dose (9, 12, and 18 months after diagnosis) revealed progressive tumor shrinkage and the patient gradually improved clinically. One year later the Karnofsky score rose to 80. He had mild hemiparesis and the MRI showed a smaller lesion. One year after completion of treatment (28 months following diagnosis), the child is stable both clinically and radiologically.
Case illustration #2
An 18-year-old boy (patient number 8 in Table 1) was diagnosed with Ollier’s syndrome, with secondary typical bone and cartilage lesions, and was diagnosed with pontine glioma based on imaging and new onset sixth nerve palsy. The patient underwent radiation with no response on post radiation MRI. He underwent radiotherapy, and 6 months later MDV9300 was given. Imaging studies done 6, 9, and 12 months after treatment initiation revealed significant ongoing tumor shrinkage (Fig. 2). The patient is clinically stable, with no neurological deficits other than ipsilateral sixth nerve palsy and partial neuronal hearing loss. The patient chose to continue therapy on a compassionate basis after completion of the planned 12 months of treatment and is now doing well, 22 months after diagnosis.
Discussion
DIPG is the most aggressive pediatric brain tumor. Most patients progress and die within 1 year and new treatments aimed at life prolongation are urgently needed. Immune modulating antibodies have shown promising results in multiple progressive malignancies, some of them unknown to be immune responsive [1, 4, 15, 16]. Most of such studies enrolled adult patients with primary extra CNS lesions. We present our preliminary results of the toxicity and efficacy of the antibody MDV9300 (pidilizumab) in pediatric patients with DIPG. To our best knowledge, this is the first study to investigate toxicity and efficacy of immune modulating antibodies in pediatric patients.
The toxicity profile of MDV9300 was favourable for the 9 children enrolled in this study,. Two patients experienced grade 3 adverse events. One of 83 treatment cycles was associated with grade 3 hypertension, which did not recur during further drug infusions. The only frequently reported adverse event was a grade 1–2 fatigue, recorded after 7 of 83 cycles, and asymptomatic grade 1–3 neutropenia after 10/83 cycles. One case of dyspnea was recorded. In a study of adults with multiple myeloma, toxicity rates related to MDV9300 treatment were similar [17]. Reported rates of anemia, thrombocytopenia, and neutropenia were 25%, 23%, and 34%, respectively. Other common grade 2–3 therapy-related adverse events were fatigue (50%), anorexia (17%), and hypophosphatemia (17%). None of the adverse effects were significant. Of importance, the exact mode of action of MDV9300 is currently under intensive investigation, and when further clarified, will contribute to the understanding of the expected toxicity profile.
Over the last 20 years, several clinical trials have evaluated various chemotherapy and radiotherapy regimens for patients with DIPG. In 2006, Hargrave et el [2]. summarized 28 chemoradiotherapy regimens and 4 studies evaluating targeted therapies; none of them showed an outcome significantly better than single agent radiation. Data for 973 patients who presented in that publication showed a median OS of 10.1 months (range 7–17); 1 year OS ranged from 25 to 53% and 2 year survival from 5 to 23%. Later studies summarizing the American and UK national experience with radiation and temozolomide showed OS of 9.6 and 9.5 months for cohorts of 63 and 43 patients, respectively [3, 4]. Unfortunately, no other agents have led to significant improvement in the extremely grim prognosis of this disease [5].
A multi-center retrospective study published by Jansen et al. [12] reviewed 316 patients with DIPG. The median survival was 10 months. The study divided patients into three risk groups based on age (> 3 years old), duration of symptoms prior to diagnosis, and the presence of ring enhancement within the tumor. Median survival was 13.7, 9.7, and 7 months in the low, intermediate, and high-risk groups, respectively. Based on these criteria, 8 of our patients had intermediate risk disease and 1 had high risk disease, with expected OS of 9.7 and 7 months, respectively. Data from our study show a median OS of 15.6 ± 3.1 months and a 1-year OS rate of 55%. Our cohort was too small to evaluate risk factors for early progression, but a Karnofsky score < 60, as presented in 3 of the 4 early progressing patients, and in none of the remaining patients, may predict aggressive disease.
Data regarding activity of immune modulating antibodies in the CNS originate from animal studies. Zeng et al. [8] published the outcome of mice that underwent irradiation in combination with anti-PD-1 antibodies. Median survival was 25 days in the control arm, 27 days in the anti-PD-1 antibody arm, 28 days in the radiation arm, and 53 days in the radiation plus anti-PD-1 therapy arm (P < 0.05 by log-rank Mantle-Cox). Long-term survival was observed only in the combined treatment arm, with a fraction (15–40%) of animals alive at day 180+ after treatment. Similar efficacy was demonstrated by other studies [18, 19]. Based on these data regarding the importance of combining multiple immune modulating treatments, the current study was planned to be continued with combined radiation, MDV9300 therapy, and low dose cyclophosphamide.
The main limitation of the current data is the small number of patients, which does not enable reaching final conclusions. Nonetheless, our preliminary data show that the immune modulating antibody MDV9300 may be well tolerated and possibly active in pediatric patients with DIPG. Further confirmation by other studies is required.
References
Yang I, Han SJ, Sughrue ME, Tihan T, Parsa AT (2011) Immune cell infiltrate differences in pilocytic astrocytoma and glioblastoma: evidence of distinct immunological microenvironments that reflect tumor biology. J Neurosurg 115:505–511. doi:10.3171/2011.4.JNS101172
Hargrave D, Bartels U, Bouffet E (2006) Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7:241–248
Bailey S, Howman A, Wheatley K, Wherton D, Boota N, Pizer B, Fisher D, Kearns P, Picton S, Saran F et al (2013) Diffuse intrinsic pontine glioma treated with prolonged temozolomide and radiotherapy: results of a United Kingdom phase II trial (CNS 2007 04). Eur J Cancer 49:3856–3862. doi:10.1016/j.ejca.2013.08.006
Cohen KJ, Heideman RL, Zhou T, Holmes EJ, Lavey RS, Bouffet E, Pollack IF (2011) Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol 13:410–416. doi:10.1093/neuonc/noq205
Kaye EC, Baker JN, Broniscer A (2014) Management of diffuse intrinsic pontine glioma in children: current and future strategies for improving prognosis. CNS Oncol 3:421–431. doi:10.2217/cns.14.47
Kmiecik J, Poli A, Brons NH, Waha A, Eide GE, Enger PO, Zimmer J, Chekenya M (2013) Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol 264:71–83. doi:10.1016/j.jneuroim.2013.08.013
Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT (2013) Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res 19:3165–3175. doi:10.1158/1078-0432.CCR-12-3314
Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E et al (2013) Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys 86:343–349. doi:10.1016/j.ijrobp.2012.12.025
Castro MG, Baker GJ, Lowenstein PR (2014) Blocking immunosuppressive checkpoints for glioma therapy: the more the Merrier! Clin Cancer Res 20:5147–5149. doi:10.1158/1078-0432.CCR-14-0820
Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, Romaguera J, Hagemeister F, Fanale M, Samaniego F et al (2014) Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol 15:69–77. doi:10.1016/S1470-204
Moreno B, Parisi G, Robert L, Ribas A (2015) Anti-PD-1 therapy in melanoma. Seminoncology 42:466. doi:10.1053/j.seminoncol.2015.02.008
Jansen MH, Veldhuijzen van Zanten SE, Sanchez Aliaga E, Heymans MW, Warmuth-Metz M, Hargrave D, van der Hoeven EJ, Gidding CE, de Bont ES, Eshghi OS et al (2015) Survival prediction model of children with diffuse intrinsic pontine glioma based on clinical and radiological criteria. Neuro Oncol 17:160–166. doi:10.1093/neuonc/nou104
Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A (2008) Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 14:3044–3051. doi:10.1158/1078-0432.CCR-07-4079
Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, Kaminski MS, Holland HK, Winter JN, Mason JR et al (2013) Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B cell lymphoma: results of an international phase II trial. J Clin Oncol 31:4199–4206. doi:10.1200/JCO.2012.48.3685
Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A, Dieckmann K, Filipits M, Brandstetter A, Weller M et al (2015) Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol 17:1064–1075. doi:10.1093/neuonc/nou307
Dixit S (2014) Immunotherapy for high-grade glioma. Future Oncol 10:911–915. doi:10.2217/fon.14.20
Efebera Y, Rosko A, Hofmeister C, Benner J, Bakan C, Stamper K, Lamb T, Hollie D, Sell M, Avigan D et al (2015) First interim results of a phase I/II study of lenalidomide in combination with anti-PD-1 monoclonal antibody MDV9300 (CT-011) in patients with relapsed/refractory multiple myeloma. Blood 126:1838
Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, Massarelli E, Hong D, Naing A, Diab A et al (2014) Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res 2:831–838. doi:10.1158/2326-6066.CIR-14-0069
Seyedin SN, Schoenhals JE, Lee DA, Cortez MA, Wang X, Niknam S, Tang C, Hong DS, Naing A, Sharma P et al (2015) Strategies for combining immunotherapy with radiation for anticancer therapy. Immunotherapy 7:967–980. doi:10.2217/imt.15.65
Funding
Medication was provided by the company (CureTech Ltd. 42 Hayarkon St. Yavne 81227, Israel) no other funding was provided.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fried, I., Lossos, A., Ben Ami, T. et al. Preliminary results of immune modulating antibody MDV9300 (pidilizumab) treatment in children with diffuse intrinsic pontine glioma. J Neurooncol 136, 189–195 (2018). https://doi.org/10.1007/s11060-017-2643-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2643-1