Abstract
Introduction
Immune checkpoint inhibition through PD-1 and CTLA-4 blockade has shown efficacy in some adult malignancies and generated interest in pediatrics, including central nervous system (CNS) tumors. We describe our experience with immune checkpoint inhibition in recurrent/refractory pediatric CNS tumors.
Methods
We performed a retrospective chart review of pediatric patients with recurrent or refractory CNS tumors treated with ipilimumab, nivolumab and/or pembrolizumab at Dana-Farber/Boston Children’s Hospital between 2018 and 2019.
Results
Eleven patients were identified. Diagnoses included diffuse intrinsic pontine glioma (DIPG) (n = 2), high-grade glioma (HGG) (n = 5), ependymoma (n = 1), craniopharyngioma (n = 1), high-grade neuroepithelial tumor (n = 1) and non-germinomatous germ cell tumor (NGGCT) (n = 1). Eight patients had recurrent disease, while three had refractory disease. Nine patients received combination therapy (ipilimumab/nivolumab); two patients received either nivolumab or pembrolizumab. Median time from diagnosis-to-treatment was 8 months (range 0.8–156). All patients received prior radiation therapy (RT), with median time from RT-to-immunotherapy was 3.8 years. One patient received concurrent then adjuvant immunotherapy with RT. Median duration of treatment was 6.1 months (range 1–25). Therapy was discontinued in nine patients: seven due to disease progression and two due to toxicity (colitis; transaminitis). Other pertinent toxicities included Type 1 diabetes mellitus, hypothyroidism and skin toxicity. Based on iRANO criteria, best responses included partial response (n = 3), stable disease (n = 7) and progressive disease (n = 1). Durable response was noted in two patients.
Conclusion
Immune checkpoint inhibition was relatively well tolerated in a cohort of pediatric patients spanning several CNS tumor diagnoses. Results from prospective clinical trials will be critical to inform clinical decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint inhibitors (ICIs) are a class of immunotherapy drugs that block co-inhibitory signaling pathways and promote T cell mediated immune response against tumor cells [1, 2]. Most commonly, these inhibitors act by either blocking the interaction between programed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) or through the inhibition of cytotoxic T lymphocyte antigen 4 (CTLA4) [1]. Blockade of the PD-1/PD-L1 pathway leads to the activation of T-cells in the tumor microenvironment and reversal of T-cell exhaustion, while CTLA4 inhibition prevents T-cell inhibition and promotes the activation and proliferation of effector T-cell. Ipilimumab activates the immune system by targeting CTLA4, whereas nivolumab and pembrolizumab target and block PD-1 [1,2,3,4]. These agents have shown significant efficacy in adult cancers, leading to regulatory approval of numerous drugs in this class for the treatment of melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma and others [1, 5].
Recurrent pediatric high-grade central nervous system (CNS) tumors remain a significant therapeutic challenge. They are associated with dismal outcome and a lack of standardized treatment approaches [6, 7]. Despite significant improvement in our understanding of tumor biology and cancer genomics, therapeutic options for children with high-risk tumors remain limited. ICI therapy is currently being evaluated in adults with CNS tumors through several clinical trials; initial results from these studies show minimal improvement in survival outcomes in the upfront and recurrent setting [8, 9]. Notably, in a small randomized study of adults with recurrent glioblastoma multiforme, neoadjuvant pembrolizumab given at the time of surgery followed by continued adjuvant pembrolizumab showed durable improvement in progression-free and overall survival when compared to adjuvant pembrolizumab alone. In this study, neoadjuvant pembrolizumab was associated with activation of tumor-infiltrating lymphocytes and upregulation of interferon-gamma, resulting in enhanced local and systemic antitumor immune response [10]. This may provide a unique approach in the treatment of high-grade CNS tumors, and is currently under evaluation in pediatrics (NCT04323046).
The success of ICI therapy in a subset of adult cancers has generated substantial interest in its use in pediatric malignancies, including CNS tumors [2, 8, 11,12,13,14]. In a phase 1 dose escalation clinical trial in pediatric patients with advanced solid tumors, ipilimumab was shown to be safe in children, with a similar profile of immune-related adverse events (irAE) when compared to adult studies [11]. Colitis, rash, transaminitis, and endocrinopathies were the most common adverse events; they were reported in approximately 55% of patients, with 27% developing grade 3 or 4 toxicities [11]. Various ICIs are currently being investigated in children with recurrent, refractory or progressive CNS tumors. Several of these clinical trial include treatment with pembrolizumab (NCT02359565), nivolumab/ipilimumab (NCT03130959) and REGN2810 (NCT0390869).
In recent years, there is evidence of the increasing use of commercially available ICIs in the treatment of pediatric CNS tumors, with limited data on safety and efficacy [12]. Herein, we report our single institutional experience using ICIs in the treatment of children and adolescents with recurrent and/or refractory CNS tumors.
Methods
Research design
Patients were identified using an institutional database and included in this retrospective study if they were treated with ICI therapy for recurrent or refractory pediatric CNS tumors between January 2018 and December 2019. Patients were excluded if this treatment was given on a clinical trial. Data collected from the electronic medical record included patient demographics, histopathological features and diagnosis, prior therapy, immunotherapy treatment type, toxicities related to immune checkpoint therapy, duration of therapy and radiographic response. The Dana-Farber Cancer Institute IRB approved this retrospective review.
Molecular analysis and tumor mutational burden (TMB)
Results from OncoPanel, a validated targeted next-generation sequencing assay for the detection of somatic variants of cancer, was used to report TMB and molecular variations [15].
PD-1/PD-L1
PD-1 (740-4859, Ventana, predilute) and PD-L1 (SP142, Spring Bioscience, predilute) immunohistochemistry (IHC) testing was performed with adequate controls in a CLIA-certified histology laboratory on 4 microns formalin-fixed paraffin-embedded tissue sections. The sections were examined by a neuropathologist (SA) with the pattern and intensity of staining noted. Positive PD-1 expression on IHC was defined as more than 1% immunolabeling of lymphocytes associated with tumor. Positive PD-L1 expression on IHC was defined as more than 1% immunolabeling of tumor cells.
Radiographic analysis
Radiographic response evaluations were performed by a pediatric neuro-radiologist (JC) based on iRANO (Immunotherapy Response Assessment in Neuro-Oncology) criteria [16].
Toxicity
Grading for toxicity were based on the Common Terminology Criteria for Adverse Events v5.0.
Statistical analysis
Continuous variables were reported as mean, standard deviation, and/or median and ranges. Categorical variables were described by frequency and/or percentage.
Results
Eleven patients, five male and six female, with recurrent or refractory CNS tumors treated with ICI therapy were identified, with a median age of 13.9 years (range 4.1–20.7) at time of treatment initiation with ICI. In this patient cohort, diagnoses included diffuse intrinsic pontine glioma (DIPG; n = 2), high grade glioma (HGG; n = 5), ependymoma (n = 1), craniopharyngioma (n = 1), high-grade neuroepithelial tumor (HGNET; n = 1) and non-germinomatous germ cell tumor (NGGCT; n = 1). The patient with NGGCT had tumor markers and histopathology consistent with the diagnosis of a choriocarcinoma, both at diagnosis and at time of recurrence. All patients received up-front standard-of-care therapy according to their disease type, with chemotherapy administered to six patients prior to ICI therapy. Two patients were treated with ICI as second line treatment, six as third line therapy and the remaining three after receiving three or more prior treatment regimens.
Eight patients had recurrent disease (five localized; 3 disseminated recurrence), while three had refractory disease. All patients in this cohort were previously treated with radiation therapy, with seven patients previously treated with focal radiation therapy (RT), while four underwent craniospinal irradiation. The median time from completion of RT to start of ICI therapy was 3.8 years (range 0.1–14.5). Patient characteristics are listed in Tables 1 and 2.
Nine patients were treated with combination ICI therapy (ipilimumab/nivolumab). One patient received monotherapy with nivolumab and one patient received monotherapy with pembrolizumab. Dosing for the immune checkpoint inhibitors were as follows: ipilimumab, 1 mg/kg; nivolumab, 3 mg/kg; and pembrolizumab, 200 mg/dose; administered every 2–3 weeks (standard recommended dosing schedule). Median time from initial diagnosis to ICI treatment initiation was 8 months (range 0.8–156). Three patients were on stable doses of corticosteroids at the time of commencing ICI therapy. Median duration of treatment was 6.1 months (range 1–25), with therapy discontinued in nine patients. Seven patients discontinued therapy secondary to disease progression, while two patients suffered from significant irAE (colitis and/or transaminitis). Two patients remain on therapy at the time of data collection, after 18.5 and 25.2 months (NGGCT and secondary HGG), respectively.
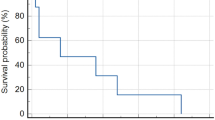
Based on iRANO criteria, best responses included partial (n = 3), stable (n = 7) and progressive disease (n = 1), All three patients who demonstrated partial response had recurrent/refractory high-grade tumors (Fig. 1). Durable response (defined here as > 12 months) was noted in three patients, one with HGG, one with anaplastic ependymoma and another with multiply recurrent progressive NGGCT (choriocarcinoma). Notably, one of these patients with durable response initially demonstrated tumor growth, which likely represented pseudo-progression, given that repeat imaging after initial growth demonstrated sustained stable disease for over 2 years (Fig. 1). Two additional patients were initially thought to have pseudo-progression. However, both these patients demonstrated continued tumor growth on repeat imaging, were subsequently classified as true progression, and therapy was discontinued. In patients in whom pseudo-progression was suspected, they were monitored closely and continued treatment with ICI. These patients did not require treatment with corticosteroids or other agents, and follow-up imaging to confirm pseudo-progression or progression were obtain after 2–3 months. Three patients (27%) eventually died of disease progression, while eight patients (72%) were alive at the time of data collection.
Ten patients (91%) developed toxicity of any grade while on therapy, six of these patients (54%) developed grade 3 or 4 toxicity (Table 3). Of those patients with grade 3/4 toxicity, two were treated with monotherapy (nivolumab or pembrolizumab), the rest with combination therapy. One DIPG patient remained on combination therapy for 2.6 months without any toxicities. Grade 3 toxicities included rash (n = 1), colitis (n = 1), mucositis (n = 1), type 1 diabetes (n = 1), fatigue (n = 1) and infection/wound dehiscence (n = 1). Two cases of grade 4 toxicity were noted, this included colitis with pembrolizumab and transaminitis/hyperbilirubinemia with ipilimumab/nivolumab, both of which were treated with corticosteroids. Treatment with corticosteroids was initiated for the patient who developed colitis and ICI therapy was held. After 5 weeks, symptoms resolved, steroids were weaned, and ICI therapy was resumed. At the time of data collection, this patent continued to receive therapy for three additional months without recurrence of colitis. For the other patient with grade 4 transaminitis and hyperbilirubinemia, ICI treatment was discontinued permanently for toxicity, with these irAEs subsequently treated successfully with a slow taper of corticosteroids over four months.
Tumor mutation burden was assessed in five patients at diagnosis, with a mean and median of 6.08 and 5.3 (range 3–10.6) coding somatic mutations per megabyte, respectively. In our patient cohort, one patient with a secondary HGG had underlying Gorlin syndrome, with no other known cancer predisposition syndrome known or found on testing. PD-1/PD-L1 expression by IHC was completed in six patients. All patients tested negative for PD-1 expression, while one patient (NGGCT) demonstrated positive PD-L1 expression.
Discussion
Despite the advancement in cancer genomics, the development of various targeted therapies and novel clinical trials, the outcomes for children and adolescent with recurrent/refractory CNS tumors have remained dismal with limited therapeutic successes. The approval of numerous ICIs in a number of adult malignancies and the development of pediatric immunotherapy clinical trials has consequently led to the widespread off-label use of ICI therapy, though with limited data on their safety and tolerability in children. As the use of these agents becomes more common, it is imperative to evaluate and document the efficacy of these drugs, understand the temporal and contextual situations that affect the immune microenvironment and more importantly, the side effects associated with the pediatric population.
We report our institutional experience with the off-label use of FDA-approved ICIs in children with recurrent/refractory CNS tumors. This represents one of the few case series reported in the pediatric population and adds to the emerging literature about the use of checkpoint inhibition in children and adolescent young adults with CNS tumors [12]. In this study, we report the overall tolerability of these agents, as well as the toxicities and clinical responses seen among a heterogenous population of CNS tumor patients. As best response, the majority of patients demonstrated either partial response or stable disease, with one patient having progressive disease. Impressively, durable response and continued treatment with immune checkpoint inhibition for over 18 months was demonstrated in two patients with aggressive malignant tumors, one of whom remains on therapy for over 2 years. Both of these patients were previously treated with standard chemotherapy and radiation therapy and were noted to have progressive disease prior to commencing ICI therapy. Sustained response and treatment duration of more than 2 years in the patient with secondary progressive HGG is impressive, particularly given the poor historical pooled OS of 5.6 months (6.9 months with immunotherapy) in patients with recurrent HGG, as reported by Klein et al. [7]. In addition, the durable response seen with the patient with progressive NGGCT is notable, given the dismal prognosis of patients with NGGCT who progress following RT and high-dose myeloablative chemotherapy with autologous stem cell rescue [17]. Despite the small number of successes, our experience highlights the potential of immunotherapy in a subset of pediatric CNS tumors. This is consistent with the experiences seen in numerous adult cancers, where some patients seems to respond better to ICI therapy than others [11, 12, 18,19,20,21].
In adult studies, monotherapy ICI has been associated with grade 3/4 toxicities in approximately 10–30% of patients. PD-1 inhibitors generally demonstrate better tolerability in comparison to CTLA4 inhibitors, when used as single agent [20, 22,23,24,25]. Combination therapy with ipilimumab and nivolumab was reported to cause significant grade 3/4 adverse events in up to 40–60% of adult cases, as reported in the CheckMate studies [19, 20, 22, 25, 26]. In pediatrics, several monotherapy ICI early phase clinical trials have been published. In a phase 1/2 clinical trial in pediatric patients with recurrent/refractory solid tumors or lymphoma, single agent nivolumab was associated with grade 3/4 adverse events in 36% of patients, with 12% demonstrating grade 3/4 irAE. Monotherapy ipilimumab was shown to be associated with grade 3/4 irAE in 27% of patients in a pediatric phase 1 study for children with advanced solid tumors [11, 27]. In our patient cohort, ICI therapy was generally well tolerated, with transaminitis being the most common toxicity, followed by headache, fatigue and skin toxicity (Table 3). Among the nine patients who received combination therapy with nivolumab and ipilimumab, four experienced grade 3 or 4 toxicities, accounting for approximately 44% of this group. Only two patients experienced significant grade 4 toxicity for which treatment was discontinued, accounting for less than 20% of the entire cohort. The frequency of overall irAEs and significant grade 3/4 toxicity in our pediatric patient cohort is similar to those reported in other pediatric and adult studies, suggesting relative safety and tolerability of ICI in this pediatric cohort. Of note, our cohort included two patients with DIPG, both of whom received therapy for a short duration before discontinuation of ICI secondary to disease progression. Despite minimal toxicity seen in this two patients, special consideration with ICI therapy should be taken when treating patients with CNS tumor who have large disease burden and limited space. This was exemplified in the Pediatric Brain Tumor Consortium study with pembrolizumab, where patients with DIPG had rapid neurological deterioration and shorter median PFS than expected, leading to early closure of the study to patients with recurrent DIPG [14].
Consistent with its mechanism of action, many adverse events associated with ICI treatment are related to the robust activation of the immune system, leading to uninhibited immune responses and autoimmunity. Despite the severity of irAE which frequently requires intensive care, these events have been positively associated with anti-tumor response and improved outcomes. This correlation was first reported in melanoma, where cutaneous adverse events, specifically rash and vitiligo, along with any reported grade 3 or higher irAEs were associated with statistically significant overall survival benefit [28]. In addition, this finding was also shown in NSCLC, where irAEs were positively associated with survival outcome with median PFS and OS of 9.2 months and undefined, respectively, for patients with irAEs, in comparison to median PFS and OS of 4.8 months and 11.1 months, respectively, in patients without irAEs [29]. More recently, a meta-analysis evaluating correlation between irAEs of ICI and clinical benefit of Nivolumab treatment across various malignancies in 48 clinical trials showed a positive correlation in overall response rate with skin, gastrointestinal and endocrine irAEs in melanoma, NSCLC, urothelial carcinoma, anal cancer and head and neck squamous cell carcinoma [30]. Similarly, a positive correlation in overall response rate and skin and gastrointestinal irAEs was also seen in melanoma, renal cell carcinoma, colorectal carcinoma and malignant pleural mesothelioma patients when treated with combination ipilimumab and nivolumab [30]. Interestingly in our cohort of patients, among patients who were treated with ICI therapy for 12 months or longer, 50% (2/4) sustained immune-related toxicities. Perhaps more importantly, both these patients who developed irAE sustained durable response to ICI therapy, with mucositis and type 1 diabetes in the NGGCT patient, and transaminitis and hypothyroidism in the patient with secondary HGG. Despite the small numbers, our findings are supportive of the positive correlation between irAEs and treatment response, and this warrant further investigation in pediatric CNS tumors.
The presence of immune infiltrate in the tumor microenvironment has been shown to be correlated with response to ICI therapy, namely higher quantification of T-lymphocyte was predictive of better response to ICI therapy in various malignancies [31,32,33]. It is unclear if this concept is similarly predictive in pediatrics. In fact, the prognostic significance of specific biomarkers such as PD-1/PD-L1 expression, tumor mutational burden and immune infiltrate in pediatrics is unknown. Nonetheless, the correlation of immune infiltrate and response to ICI therapy is interesting, as CNS germinomas have traditionally been characterized by a robust lymphocytic infiltrate on histopathology [34,35,36,37,38]. While this is thought to be less prevalent in CNS NGGCT, there is evidence in extracranial NGGCT of extensive infiltration of CD8 + T-cells within an immunosuppressive tumor microenvironment characterized by the presence of T-regulatory cells and PD-L1 expressing tumor cells [39]. In review of the histopathology for our patient with NGGCT, his tumor was noted to be diffusely positive for PD-L1 expression. Therefore, his sustained response and survival from an aggressive, multiply recurrent disease with dismal prognosis has therapeutic implications, as it highlights the potential use of ICI therapy in pediatric CNS tumors with known immune infiltrate and/or expression of PD-1/PD-L1. In addition, it supports the consideration of a clinical trial evaluating the use of immune checkpoint inhibition in patients with progressive/recurrent CNS germ cell tumors.
In this study, we attempted to retrospectively evaluate the level of PD-1/PD-L1 expression as well as tumor mutation burden for each patient and correlate those findings with clinical outcomes. While the prognostic significance of PD-1/PD-L1 expression is controversial in adults, there is evidence that TMB is associated with improved outcome with immune checkpoint inhibition [11, 12, 40,41,42,43,44,45]. This may be in part due to the association between mutational load and neoantigen formation, where a higher mutational load is associated with an increase in neoantigen formation and more robust activation of T-cells [3, 46, 47]. Unfortunately, in our patient cohort, TMB for the majority of cases was not tested. In our cohort, only one patient demonstrated PD-L1 positivity out of the six patients who were tested, and this patient had a durable response to treatment. Given the small number of patients, it is not feasible to draw any substantive conclusions with regards to the relationship between PD-1/PD-L1 expression and response to ICI therapy in children with CNS tumors.
This study has several limitations, related to it being a single institutional retrospective review with a small sample size. As a retrospective review, we recognize that this paper is subject to patient selection and informational bias which may have affected the observed results. Additionally, we report the result from heterogenous group of patients with varied histology in this small cohort, which limits our ability to interpret the efficacy of immune checkpoint inhibition in any specific tumor type. We acknowledge that our analysis would likely be more robust if the level of tumor mutational burden, PD-1/PD-L1 expression and additional IHC testing for immune cell infiltration were available for all patients, however, we were limited by the availability of tumor tissue.
Conclusion
With the expanding use of ICI therapy in pediatric malignancies, this report describes the safety and tolerability of these drugs in a cohort of children with recurrent or refractory CNS tumors. Our experience showed that these agents are fairly well tolerated and this case series supports the continued formal exploration of ICI therapy in pediatric CNS tumors. Results from larger prospective trials currently underway will be critical in further understanding the role of these drugs in pediatric CNS tumors.
References
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264. https://doi.org/10.1038/nrc3239
Johnson DB, Sullivan RJ, Menzies AM (2017) Immune checkpoint inhibitors in challenging populations. Cancer 123:1904–1911. https://doi.org/10.1002/cncr.30642
Teng F, Meng X, Kong L, Yu J (2018) Progress and challenges of predictive biomarkers of anti PD-1/PD-L1 immunotherapy: a systematic review. Cancer Lett 414:166–173. https://doi.org/10.1016/j.canlet.2017.11.014
Wang SS, Bandopadhayay P, Jenkins MR (2019) Towards immunotherapy for pediatric brain tumors. Trends Immunol 40:748–761. https://doi.org/10.1016/j.it.2019.05.009
Graziani G, Tentori L, Navarra P (2012) Ipilimumab: a novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol Res 65:9–22. https://doi.org/10.1016/j.phrs.2011.09.002
Sabel M, Fleischhack G, Tippelt S, Gustafsson G, Doz F, Kortmann R, Massimino M, Navajas A, von Hoff K, Rutkowski S, Warmuth-Metz M, Clifford SC, Pietsch T, Pizer B, Lannering B, Group S-EBT (2016) Relapse patterns and outcome after relapse in standard risk medulloblastoma: a report from the HIT-SIOP-PNET4 study. J Neurooncol 129:515–524. https://doi.org/10.1007/s11060-016-2202-1
Kline C, Felton E, Allen IE, Tahir P, Mueller S (2018) Survival outcomes in pediatric recurrent high-grade glioma: results of a 20-year systematic review and meta-analysis. J Neurooncol 137:103–110. https://doi.org/10.1007/s11060-017-2701-8
Filley AC, Henriquez M, Dey M (2017) Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget 8:91779–91794. https://doi.org/10.18632/oncotarget.21586
Bristol-Myers Squibb provides update on phase 3 Opdivo (nivolumab) checkmate -548 trial in patients with newly diagnosed MGMT-methylated glioblastoma multiforme. Bristol-Myers Squibb Company, Princeton, NJ. https://bit.ly/2ktBxYb. Accessed 5 Sept 2019
Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, Wang AC, Ellingson BM, Rytlewski JA, Sanders CM, Kawaguchi ES, Du L, Li G, Yong WH, Gaffey SC, Cohen AL, Mellinghoff IK, Lee EQ, Reardon DA, O’Brien BJ, Butowski NA, Nghiemphu PL, Clarke JL, Arrillaga-Romany IC, Colman H, Kaley TJ, de Groot JF, Liau LM, Wen PY, Prins RM (2019) Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 25:477–486. https://doi.org/10.1038/s41591-018-0337-7
Merchant MS, Wright M, Baird K, Wexler LH, Rodriguez-Galindo C, Bernstein D, Delbrook C, Lodish M, Bishop R, Wolchok JD, Streicher H, Mackall CL (2016) Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin Cancer Res 22:1364–1370. https://doi.org/10.1158/1078-0432.CCR-15-0491
Gorsi HS, Malicki DM, Barsan V, Tumblin M, Yeh-Nayre L, Milburn M, Elster JD, Crawford JR (2019) Nivolumab in the treatment of recurrent or refractory pediatric brain tumors: a single institutional experience. J Pediatr Hematol Oncol 41:e235–e241. https://doi.org/10.1097/MPH.0000000000001339
Blumenthal DT, Yalon M, Vainer GW, Lossos A, Yust S, Tzach L, Cagnano E, Limon D, Bokstein F (2016) Pembrolizumab: first experience with recurrent primary central nervous system (CNS) tumors. J Neurooncol 129:453–460. https://doi.org/10.1007/s11060-016-2190-1
Hwang E, Onar A, Young-Poussaint T, Mitchell D, Kilburn L, Margol A, Gilheeny S, Lin T, Dunkel I, Fouladi M (2018) IMMU-09, Outcome of patients with recurrent diffuse intrinsic pontine glioma (DIPG) treated with pembrolizumab (ANTI-PD-1): a pediatric brain tumor consortium study (PBTC045). Neuro-Oncology 20(Suppl 2):i100. https://doi.org/10.1093/neuonc/noy059.325
Garcia EP, Minkovsky A, Jia Y, Ducar MD, Shivdasani P, Gong X, Ligon AH, Sholl LM, Kuo FC, MacConaill LE, Lindeman NI, Dong F (2017) Validation of OncoPanel: a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med 141:751–758. https://doi.org/10.5858/arpa.2016-0527-OA
Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, Ellingson BM, Hashimoto N, Pollack IF, Brandes AA, Franceschi E, Herold-Mende C, Nayak L, Panigrahy A, Pope WB, Prins R, Sampson JH, Wen PY, Reardon DA (2015) Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 16:e534–e542. https://doi.org/10.1016/S1470-2045(15)00088-1
Modak S, Gardner S, Dunkel IJ, Balmaceda C, Rosenblum MK, Miller DC, Halpern S, Finlay JL (2004) Thiotepa-based high-dose chemotherapy with autologous stem-cell rescue in patients with recurrent or progressive CNS germ cell tumors. J Clin Oncol 22:1934–1943. https://doi.org/10.1200/JCO.2004.11.053
Haslam A, Prasad V (2019) Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open 2:e192535. https://doi.org/10.1001/jamanetworkopen.2019.2535
Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS (2015) Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372:2006–2017. https://doi.org/10.1056/NEJMoa1414428
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A, Investigators K (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521–2532. https://doi.org/10.1056/NEJMoa1503093
Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J (2015) Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16:375–384. https://doi.org/10.1016/S1470-2045(15)70076-8
Spain L, Diem S, Larkin J (2016) Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44:51–60. https://doi.org/10.1016/j.ctrv.2016.02.001
Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, Lebbé C, Ferraresi V, Smylie M, Weber JS, Maio M, Konto C, Hoos A, de Pril V, Gurunath RK, de Schaetzen G, Suciu S, Testori A (2015) Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 16:522–530. https://doi.org/10.1016/S1470-2045(15)70122-1
Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, Larkin JMG, Lorigan P, Neyns B, Blank CU, Petrella TM, Hamid O, Su SC, Krepler C, Ibrahim N, Long GV (2019) Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 20:1239–1251. https://doi.org/10.1016/S1470-2045(19)30388-2
Larkin J, Hodi FS, Wolchok JD (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:1270–1271. https://doi.org/10.1056/NEJMc1509660
Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, Powles T, Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R, Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D, Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B, Investigators C (2018) Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277–1290. https://doi.org/10.1056/NEJMoa1712126
Davis KL, Fox E, Merchant MS, Reid JM, Kudgus RA, Liu X, Minard CG, Voss S, Berg SL, Weigel BJ, Mackall CL (2020) Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol 21:541–550. https://doi.org/10.1016/S1470-2045(20)30023-1
Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS (2016) Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 22:886–894. https://doi.org/10.1158/1078-0432.CCR-15-1136
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, Nakagawa K (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4:374–378. https://doi.org/10.1001/jamaoncol.2017.2925
Xing P, Zhang F, Wang G, Xu Y, Li C, Wang S, Guo Y, Cai S, Wang Y, Li J (2019) Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO + IPI: a systematic review and meta-analysis. J Immunother Cancer 7:341. https://doi.org/10.1186/s40425-019-0779-6
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964. https://doi.org/10.1126/science.1129139
Fridman WH, Pagès F, Sautès-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12:298–306. https://doi.org/10.1038/nrc3245
Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Börger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grützmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Léonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Mușină AM, Scripcariu DV, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, Galon J (2018) International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391:2128–2139. https://doi.org/10.1016/S0140-6736(18)30789-X
Liu B, Arakawa Y, Yokogawa R, Tokunaga S, Terada Y, Murata D, Matsui Y, Fujimoto KI, Fukui N, Tanji M, Mineharu Y, Minamiguchi S, Miyamoto S (2018) PD-1/PD-L1 expression in a series of intracranial germinoma and its association with Foxp3 + and CD8 + infiltrating lymphocytes. PLoS ONE 13:e0194594. https://doi.org/10.1371/journal.pone.0194594
Wildeman ME, Shepard MJ, Oldfield EH, Lopes MBS (2018) Central nervous system germinomas express programmed death ligand 1. J Neuropathol Exp Neurol 77:312–316. https://doi.org/10.1093/jnen/nly008
Willis SN, Mallozzi SS, Rodig SJ, Cronk KM, McArdel SL, Caron T, Pinkus GS, Lovato L, Shampain KL, Anderson DE, Anderson RC, Bruce JN, O’Connor KC (2009) The microenvironment of germ cell tumors harbors a prominent antigen-driven humoral response. J Immunol 182:3310–3317. https://doi.org/10.4049/jimmunol.0803424
Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L (2016) Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol 27:1482–1492. https://doi.org/10.1093/annonc/mdw168
Chan AK, Shi ZF, Lo KW, Ng HK, Lau CC (2019) P14.47 Tissue immune markers for central nervous system germinoma. Neuro-Oncology 21:377–378. https://doi.org/10.1093/neuonc/noz126.282
Boldrini R, De Pasquale MD, Melaiu O, Chierici M, Jurman G, Benedetti MC, Salfi NC, Castellano A, Collini P, Furlanello C, Pistoia V, Cifaldi L, Terenziani M, Fruci D (2019) Tumor-infiltrating T cells and PD-L1 expression in childhood malignant extracranial germ-cell tumors. Oncoimmunology 8:e1542245. https://doi.org/10.1080/2162402X.2018.1542245
Steuer CE, Ramalingam SS (2018) Tumor mutation burden: leading immunotherapy to the era of precision medicine? J Clin Oncol 36:631–632. https://doi.org/10.1200/JCO.2017.76.8770
Galuppini F, Dal Pozzo CA, Deckert J, Loupakis F, Fassan M, Baffa R (2019) Tumor mutation burden: from comprehensive mutational screening to the clinic. Cancer Cell Int 19:209. https://doi.org/10.1186/s12935-019-0929-4
Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A, Rosso R, Zuber M, Muraro MG, Amicarella F, Cremonesi E, Heberer M, Iezzi G, Lugli A, Terracciano L, Sconocchia G, Oertli D, Spagnoli GC, Tornillo L (2013) Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 49:2233–2242. https://doi.org/10.1016/j.ejca.2013.02.015
Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL (2014) In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 20:2773–2782. https://doi.org/10.1158/1078-0432.CCR-13-2702
Mino-Kenudson M (2016) Programmed cell death ligand-1 (PD-L1) expression by immunohistochemistry: could it be predictive and/or prognostic in non-small cell lung cancer? Cancer Biol Med 13:157–170. https://doi.org/10.20892/j.issn.2095-3941.2016.0009
Hwang K, Koh EJ, Choi EJ, Kang TH, Han JH, Choe G, Park SH, Yearley JH, Annamalai L, Blumenschein W, Sathe M, McClanahan T, Jung H, Wang KC, Kim SK, Kim CY (2018) PD-1/PD-L1 and immune-related gene expression pattern in pediatric malignant brain tumors: clinical correlation with survival data in Korean population. J Neurooncol 139:281–291. https://doi.org/10.1007/s11060-018-2886-5
Gubin MM, Schreiber RD (2015) CANCER. The odds of immunotherapy success. Science 350:158–159. https://doi.org/10.1126/science.aad4140
Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, Durno C, Krueger J, Cabric V, Ramaswamy V, Zhukova N, Mason G, Farah R, Afzal S, Yalon M, Rechavi G, Magimairajan V, Walsh MF, Constantini S, Dvir R, Elhasid R, Reddy A, Osborn M, Sullivan M, Hansford J, Dodgshun A, Klauber-Demore N, Peterson L, Patel S, Lindhorst S, Atkinson J, Cohen Z, Laframboise R, Dirks P, Taylor M, Malkin D, Albrecht S, Dudley RW, Jabado N, Hawkins CE, Shlien A, Tabori U (2016) Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol 34:2206–2211. https://doi.org/10.1200/JCO.2016.66.6552
Funding
No external funding to report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interests or competing interests to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cacciotti, C., Choi, J., Alexandrescu, S. et al. Immune checkpoint inhibition for pediatric patients with recurrent/refractory CNS tumors: a single institution experience. J Neurooncol 149, 113–122 (2020). https://doi.org/10.1007/s11060-020-03578-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03578-6