Abstract
Brain metastases are a serious relatively common complication of breast cancer. We evaluated prognostic factors for survival after diagnosis of brain metastases from breast cancer in a contemporary cohort of patients. Patients diagnosed with breast cancer brain metastases at our institution between 1999 and March 2016 were evaluated. Overall survival was defined as time from brain metastasis diagnosis to death or last follow-up. Patients were classified according to the Breast cancer-specific Graded Prognostic Assessment (BS-GPA), based on age, Karnofsky performance score and breast cancer phenotype. 181 patients were identified. Tumor phenotype distribution was as follows: triple negative (TN, 18.8%), hormone receptor (HR)−HER2+ (16.6%), HR+HER2+ (23.2%) and HR+HER2− (30.9%), not available (10.5%). Median overall survival from brain metastasis diagnosis was 7.7 mos (95% CI 5.4–10.0 mos). Although TN patients experienced the worse outcome, no significant difference was observed across tumor phenotypes (median 5.1, 7.7, 11.0 and 8.6 months in TN, HR−HER2+, HR+HER2+, HR+HER2−, p = 0.081). The BS-GPA index was significantly associated with overall survival (median 18.8, 8.8, 6.2 and 3.6 months, respectively, for BS-GPA categories 3.5–4, 2.5–3, 1.5–2 and 0–1, p = 0.014). Increased number of local treatments for brain metastasis (radiotherapy or neurosurgery) or the administration of systemic therapy after brain metastasis diagnosis were also significant predictors of better overall survival (p < 0.001) and, when evaluated in multivariate analysis with BS-GPA, both added independent prognostication beyond BS-GPA. Patient-related features, tumor phenotype and multimodal treatments all independently contribute to modulate prognosis of patients diagnosed with breast cancer brain metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the commonest causes of brain metastasis [1]: approximately 10–15% of patients diagnosed with metastatic breast cancer will eventually develop brain metastases during the course of their disease [2]. In addition, autopsy studies suggest that the real incidence of brain metastases may be even higher, with rates up to 30% [3]. Even if all breast cancer patients are at risk for brain metastases, this event occurs more frequently in Human epidermal growth factor receptor type 2 (HER2) positive and triple-negative (TN) subtypes, with approximately 30 and 50% of metastatic patients developing brain metastases, respectively [4–6].
Breast cancer brain metastases have been traditionally viewed as a late complication of progressive metastatic disease, with poor prognosis, reported median survival historically ranging between 2 and 16 months, and few effective treatment options [1, 7]. More recently, due to the increase of therapeutic possibilities, the capacity to adequately predict the outcome of patients with breast cancer brain metastases has grown increasingly important. Several local treatments, such as whole-brain radiotherapy, stereotactic radiosurgery or surgical resection, are now available. In addition, the pool of available systemic treatments has been expanded beyond chemotherapy and endocrine therapy, thanks to the approval of targeted treatments.
Several studies have reported profound differences in survival after a diagnosis of brain metastasis depending on many factors, including performance status, age, number of brain metastases, the presence and status of extracranial disease (controlled vs. uncontrolled), tumor histology, subtypes and receipt of local or systemic therapy [8–11]. Based on these observations, several prognostic scores have been developed, often using data from patients included in clinical trials, with the aim to adequately predict outcome and tailor patients’ care accordingly [8–11]. One of the most used prognostic scores, the Graded Prognostic Assessment (GPA), was originally developed from a cohort of 1960 patients accrued in Radiation Therapy Oncology Group protocols for patients with brain metastases [10]. In a further refinement, Sperduto et al. identified molecular tumor subtype, Karnofsky performance score (KPS) and age as independent prognostic factors in patients receiving radiation therapy for newly diagnosed breast cancer brain metastases and incorporated all of these factors in the breast specific-GPA score (BS-GPA) [11].
In this context, data concerning real life patient cohorts should be considered, since patients included in clinical trials constitute a selected population, not perfectly representing patients seen in daily clinic.
We here report a retrospective analysis of breast cancer patients treated for brain metastases at the Istituto Oncologico Veneto of Padua over 17 years. The primary aim of the present analysis is to evaluate prognostic factors affecting overall survival from the development of brain metastases in a real-life cohort of patients treated with multiple modalities.
Materials and methods
Patients
Medical records of breast cancer patients with brain metastases referred to the Medical Oncology Division or Radiotherapy Division of the Istituto Oncologico Veneto between 1st December 1999 and 31st March 2016 were retrospectively reviewed. Inclusion criteria were: histologically proven invasive breast carcinoma, age >18 years at the time of breast cancer diagnosis, intradural brain metastasis radiologically confirmed using contrast-enhanced cerebral computed tomography scan and/or magnetic resonance imaging of the brain. Patients with breast cancer bone metastasis extending into the cranium were not included in the absence of intradural brain metastasis. Patients with diagnosis of leptomeningeal carcinomatosis concomitant to brain metastasis diagnosis and patients with diagnosis of leptomeningeal carcinomatosis alone were excluded (18 and 14 patients, respectively; Supplementary Material Image 1). Development of leptomeningeal disease subsequent to brain metastasis diagnosis was allowed.
Clinical and biological parameters for each patient, including patients demographics, primary tumor characteristics (including histological subtype, grade, stage at diagnosis, hormone receptor (HR)/HER2 status and Ki67), dates of diagnosis of primary breast cancer and subsequent distant and brain metastases and treatment received for breast cancer were included in a dedicated database.
Estrogen receptor (ER) and progesterone receptor (PgR) expression was determined by immunohistochemistry; positivity was defined as immunohistochemistry staining in at least 1% of tumor cells. HER2 status was defined as positive in case of immunohistochemistry score 3+ and/or amplification by fluorescent in situ hybridization.
Brain metastasis-specific data was also collected, including number of brain lesions, presence or absence of leptomeningeal involvement, status of extra cranial disease and KPS at time of diagnosis of breast cancer brain metastases. Local and systemic treatments administered from diagnosis of central nervous system metastases up to time of last follow-up were also recorded. The cut-off date for follow up is May 20, 2016. This study was approved by our institution ethics committee.
Statistical analysis
Patients were classified according to immunohistochemistry data (primary tumor) in the following breast cancer subtypes: ‘‘luminal type” (HR-positive/HER2-negative); ‘‘luminal-HER2+ type” (HR-positive/HER2-positive); ‘‘HER2+ type” (HR-negative/HER2-positive) and ‘‘triple negative TN type” (HR-negative/HER2-negative).
BS-GPA score was calculated, based on age, KPS and breast cancer subtype according to previously defined criteria [11].
Time to brain metastasis was defined as the time interval from diagnosis of primary breast cancer to diagnosis of brain metastasis. Overall survival was defined as the time from brain metastasis diagnosis to death from any cause or last follow-up. Patients alive without event were censored at cut-off date of this analysis (May 20th, 2016).
Descriptive statistics were performed for the patient demographics and clinical characteristics. For continuous variables median and range values were computed.
The Pearson’s Chi-2 test was used to study the association between categorical clinicopathological variables.
Median time to brain metastasis and overall survival were estimated using the Kaplan–Meier method and reported with their 95% confidence intervals (95% CIs). Kaplan–Meier curves were used to present time to brain metastasis and overall survival for patients in each group. Univariate and multivariate Cox regression modelling for proportional hazards was used to calculate HR and 95% CI. All reported p values are two-sided, and significance level was set at 5% (p < 0.05).
Analyses were performed using IBM SPSS (version 22).
Results
Patients characteristics at the time of primary breast cancer diagnosis
A total of 181 patients were included in this analysis. Patient and tumor characteristics at the time of primary breast cancer diagnosis are detailed in Table 1.
The median age at breast cancer diagnosis was 51 years (range 24–80 years). Most patients presented tumors with invasive ductal histology (n 158, 87.3%) and grade 3 (n 122, 67.4%). The majority of patients (n 103, 56.9%) had positive axillary lymph nodes at diagnosis. In addition, 29.3% (n 53) of patients had stage III disease and 19.9% (n 36) had metastases at time of diagnosis.
HR and HER2 status was known in 99 and 89% of patients, respectively. Among patients with known status, 63.5% (n 115) were HR positive, 39.8% (n 72) were HER2 positive.
Overall, 34 patients (18.8%) were categorized as triple negative, 30 (16.6%) as HR−HER2+, 42 (23.2%) as HR+HER2+ (luminal-HER2+type) and 56 (30.9%) as HR + HER2− (luminal type).
Time to brain metastasis
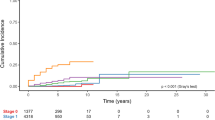
Median time to brain metastasis was 41.4 months (CI 95% 32.5–50.3 months). Breast cancer subtype significantly influenced time from breast cancer diagnosis to brain metastasis occurrence: median time to brain metastasis was 27.3, 31.8, 42.1 and 45.7 months in TN, HR−HER2+, HR+HER2+, HR+HER2−, respectively (p = 0.041) (Fig. 1).
Patients characteristics at the time of brain metastasis diagnosis
Patients characteristics at time of brain metastasis diagnosis are detailed in Table 2.
Median age was 56 years (range 31–84 years). Brain was the first site of recurrence in 17.7% (n 32) of patients, but only 18 of them had isolated brain involvement at time of brain metastasis diagnosis. Overall, 40 patients presented with a single brain metastasis, while 113 patients had more than 3 brain lesions. Extra-cranial disease was under control at time of brain metastasis progression in 68 patients (37.8%). 14 patients presented leptomeningeal involvement at some point during their clinical history.
Information on Performance Status at time of brain metastasis diagnosis was available for 169 out of 181 patients. Among these, 92 (50.8%) patients showed a deteriorated performance status evaluated as ≤70 according to the KPS scale, while 77 (42.5%) had a KPS >70.
BS-GPA score was calculated for 152 patients with available data (Table 2), only 11.5% of them were classified in the more favorable BS-GPA category (score 3.5–4).
Treatment modalities
Most patients received multimodality treatments (Table 2). Nevertheless, 30 patients (16.6%) did not receive neither local nor systemic treatment after the diagnosis of brain metastases. For one patient complete data regarding treatment was missing.
A total of 127 patients (70.2%) underwent local treatment for brain metastases. A minority of patients (n 21, 11.6%), were treated with neurosurgery. Most patients (n 124, 68.5%) received radiotherapy, in the form of either stereotactic radiotherapy or extensive radiotherapy fields such as whole brain radiation therapy, as primary treatment or after localized treatment. Most patients (n 104, 57.5%) received whole brain radiotherapy, while 16 (8.8%) patients received stereotactic radiotherapy and 13 (7.2%) patients received other kinds of radiotherapy, such as semi-localized boosts to the site of previous neurosurgery.
A total of 120 patients (66.3%) received at least a systemic treatment, namely chemotherapy, endocrine therapy or target therapy for 101 (55.8%), 36 (19.9%) and 50 (27.6%) patients, respectively. The median number of lines of systemic treatment received by patients after the diagnosis of brain metastases was one line per patient (range 0–9).
Prognostic factors for overall survival after brain metastasis diagnosis
At time of last follow-up, 159 patients (87.8%) had died. Median overall survival from brain metastasis diagnosis in the study cohort was 7.7 mos (95% CI 5.4–10.0 mos).
The impact of several known prognostic factors on overall survival from brain metastasis diagnosis was investigated using univariate Cox regression modeling (Table 3).
Overall survival after brain metastasis diagnosis was not significantly affected by breast cancer phenotype, although TN patients experienced the worse outcome. Median overall survival was 5.1 (95% CI 3.0–7.2), 7.7 (95% CI 4.1–11.2), 11.0 (95% CI 4.6–17.3) and 8.6 (95% CI 2.7–14.5) months in TN, HR−HER2+, HR+HER2+, HR+HER2− subgroups, respectively (p = 0.081).
Among clinical parameters evaluated at time of brain metastasis diagnosis, only KPS showed to significantly affect patients’ prognosis; patients with a KPS >70 had a significantly longer median overall survival than patients whose KPS was ≤70 (16.2 and 4.2 months respectively, p < 0.001).
Nevertheless, the BS-GPA score [11], which is calculated combining tumor phenotype with patient-related features (age and KPS), was significantly associated with overall survival after brain metastasis (p = 0.014) (Fig. 2; Table 3).
Treatment received also appeared to influence patient outcome. Increased number of local treatments received (radiotherapy or neurosurgery) or the administration of systemic treatment after brain metastasis diagnosis were significantly associated with better overall survival after brain metastasis (Table 3).
In consideration of the fact that the parameters combined in the BS-GPA index (age, KPS, tumor phenotype) may affect the physician’s therapeutic approach, we tested if there was an association between BS-GPA categories and the administration of local or systemic treatment. As expected, patients in the less favorable BS-GPA category (BS-GPA index ≤1) were less likely to receive systemic treatment after brain metastasis diagnosis compared to other BS-GPA categories (44% vs. 71%, p = 0.021). On the contrary, no significant association was observed between BS-GPA category and local treatment (80, 74, 71 and 61% of patients received at least 1 local treatment in BS-GPA categories 3.5–4, 2.5–3, 1.5–2, 0.5–1, respectively, p = 0.264).
Patients undergoing increased lines of local treatments where more likely to receive systemic therapy (43, 77 and 77% of patients treated respectively with 0, 1, and 2 or more local treatments also received systemic therapy, p < 0.001).
Therefore, to correct the prognostic role of treatments for patient-related features (resumed in the BS-GPA) avoiding potential bias, we performed two separate analyses: (a) Overall survival from brain metastasis diagnosis according to number of local treatments, corrected for BS-GPA category; (b) Overall survival from brain metastasis diagnosis according to systemic treatments corrected for BS-GPA category (patients with BS-GPA index ≤1 excluded). Both local and systemic treatment added independent prognostication beyond BS-GPA (Table 3).
Discussion
Consistently with prior studies [12–14], in our cohort of 181 patients diagnosed with breast cancer brain metastases, most patients had high-grade tumors (grade 3, 67.4%) with positive nodal status (56.9%) at time of breast cancer diagnosis.
The proportions of patients with HER2-positive (39.8%) and TN (18.8%) tumors in this cohort were higher than those commonly described in a general population of breast cancer patients not selected for brain metastasis [15, 16]. This is in agreement with the common notion that HER2-positive and TN status constitute risk factors for the development of breast cancer brain metastases [2, 5, 6, 17]. Consistently, breast cancer subtype also significantly influenced time from breast cancer diagnosis to brain metastasis occurrence, with shorter time to brain metastasis intervals in the TN and HER2+ subgroups. However, this result did not reflect in a difference in overall survival after brain metastasis diagnosis. Even if TN patients experienced the worse outcome, tumor phenotype alone was an insufficient predictor of overall survival after brain metastasis diagnosis in this cohort of patients, thus suggesting that other factors may contribute to the prognosis from the development of brain metastasis.
Median overall survival from brain metastasis diagnosis of patients in our study cohort was shorter (7.7 mos vs. 13.8 mos) than that reported by Sperduto et al. in their original publication [11], highlighting differences between a real-life unselected group of patients and selected cohorts of patients used to develop prognostic scores. In fact, patients were included in the original BS-GPA development cohort if receiving treatment for brain metastasis diagnosis, thus probably selecting a better prognosis cohort of patients. On the contrary, 30 patients (16.6%) in our cohort did not receive neither local nor systemic treatment after the diagnosis of brain metastasis, probably due to deteriorated general conditions and rapid unfavorable evolution. Consistently, a larger number of patients presented with a less favorable BS-GPA score (BS-GPA index ≤1) (9.9% vs. 6%) and a smaller number of patients presented with a more favorable BS-GPA score (BS-GPA index 3.5–4.0) (11.5% vs. 33%) than reported by Sperduto et al. [11]. This observation is confirmed by literature data from other real-life institutional databases. In fact, the largest published real-life mono-istitutional database, including 1552 patients treated at the MD Anderson Cancer Center, recently reported a median overall survival from brain metastasis diagnosis of 9.4 mos and a higher proportion of patients with less favourable BS-GPA scores (21.6% of patients having a BS-GPA index ≤1 and 12.1% of patients having a BS-GPA index 3.5–4.0) [12].
Nevertheless, despite these differences in patient population and treatment, the BS-GPA score maintained its prognostic value for post-brain metastasis overall survival even in our unselected cohort of patients (p = 0.014).
Even if patient-related factors, resumed in the BS-GPA index, at least partially influenced treatment (less favorable BS-GPA score patients being less likely to receive systemic treatment after brain metastasis diagnosis) both local and systemic treatment added independent prognostication beyond BS-GPA.
In fact, the number of local treatments received (radiotherapy or neurosurgery) and the administration of systemic treatment after brain metastasis diagnosis were significantly associated with better post-brain metastasis survival and this result was confirmed in multivariate analysis.
Although its mono-institutional, retrospective design and the long period of time covered (1999–2016) might limit the general applicability of these results, this observation should at least question our therapeutic attitude when facing a patient with breast cancer brain metastasis diagnosis.
When considering breast cancer related brain metastases, the past philosophy of fatalistic futileness of treatment has been progressively abandoned in the last decades, leaving place to the notion that breast cancer brain metastasis patients represent an extremely heterogeneous group of patients from both a clinical and biological point of view. This is reflected by the increasing number of publications concerning breast cancer brain metastasis prognostic indexes and their refinement [9–12]. However, it should be kept in mind that multimodality treatment itself contributes to patients’ prognosis; a notion that is particularly significant when considering recent advances in systemic therapies for some breast cancer subtypes and in radiation therapy techniques, which might be underestimated by prognostic indexes constructed using data from old, often selected, cohorts of patients.
Concluding, although brain metastasis remains a feared event in breast cancer patients, a body of evidence is now available supporting individualization of treatment for selected good-prognosis patients and several prognostic tools have been proposed to aid clinicians in these decisions.
Even if this unselected real-life cohort of breast cancer brain metastasis patients showed some significant differences in baseline characteristics when compared to the original BS-GPA development cohort, BS-GPA score confirmed its prognostic significance, corroborating its applicability to every-day clinic patients.
Moreover, in addition to patient-related features and tumor phenotype (summarized by BS-GPA), treatments, both local and systemic, independently contributed to modulate the prognosis of breast cancer brain metastasis patients. This highlights the relevance of adequately tailored multimodality treatment in patients with breast cancer brain metastases.
Funding
This study was supported in part by the Italian Ministry of Health, Project L02P16-2013.
References
Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS (2005) Breast cancer metastasis to the central nervous system. Am J Pathol 167:913–920
Lin NU, Bellon JR, Winer EP (2004) CNS metastases in breast cancer. J Clin Oncol 22:3608–3617. doi:10.1200/JCO.2004.01.175
Tsukada Y, Fouad A, Pickren JW, Lane WW (1983) Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer 52:2349–2354
Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, D’Amico R (2012) Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 4:CD006243. doi:10.1002/14651858.CD006243.pub2
Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, Bunnell C, Rue M, Gelman R, Winer E (2003) Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97:2972–2977. doi:10.1002/cncr.11436
Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP (2008) Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 113:2638–2645. doi:10.1002/cncr.23930
Engel J, Eckel R, Aydemir U, Aydemir S, Kerr J, Schlesinger-Raab A, Dirschedl P, Holzel D (2003) Determinants and prognoses of locoregional and distant progression in breast cancer. Int J Radiat Oncol Biol Phys 55:1186–1195
Niwinska A, Murawska M, Pogoda K (2010) Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol 21:942–948. doi:10.1093/annonc/mdp407
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745–751
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W (2008) A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 70:510–514
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J et al (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419–425. doi:10.1200/JCO.2011.38.0527
Subbiah IM, Lei X, Weinberg JS, Sulman EP, Chavez-MacGregor M, Tripathy D, Gupta R, Varma A, Chouhan J, Guevarra RP et al (2015) Validation and development of a modified breast graded prognostic assessment as a tool for survival in patients with breast cancer and brain metastases. J Clin Oncol 33:2239–2245. doi:10.1200/JCO.2014.58.8517
De Ieso PB, Schick U, Rosenfelder N, Mohammed K, Ross GM (2015) Breast cancer brain metastases: a 12 year review of treatment outcomes. Breast 24:426–433. doi:10.1016/j.breast.2015.03.007
Shen Q, Sahin AA, Hess KR, Suki D, Aldape KD, Sawaya R, Ibrahim NK (2015) Breast cancer with brain metastases: clinicopathologic features, survival, and paired biomarker analysis. Oncologist 20:466–473. doi:10.1634/theoncologist.2014-0107
Hess KR, Esteva FJ (2013) Effect of HER2 status on distant recurrence in early stage breast cancer. Breast Cancer Res Treat 137:449–455. doi:10.1007/s10549-012-2366-0
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, Cronin KA (2014) US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. doi:10.1093/jnci/dju055
Leyland-Jones B (2009) Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol 27:5278–5286. doi:10.1200/JCO.2008.19.8481
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Griguolo, G., Dieci, M.V., Giarratano, T. et al. Beyond breast specific—Graded Prognostic Assessment in patients with brain metastases from breast cancer: treatment impact on outcome. J Neurooncol 131, 369–376 (2017). https://doi.org/10.1007/s11060-016-2309-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2309-4