Abstract
Purpose
Brain metastases (BM) occur in 15–35% of patients with metastatic breast cancer, conferring poor prognosis and impairing quality of life. Clinical scores have been developed to classify patients according to their prognosis. We aimed to check the utility of the Breast Graded Prognostic Assessment (B-GPA) and its modified version (mB-GPA) and compare them in routine clinical practice.
Methods
This is an ambispective study including all patients with breast cancer BM treated in a single cancer comprehensive center. We analyzed the overall survival (OS) from BM diagnosis until death. The Kaplan–Meier method and Cox proportional hazard regression model were used in the analyses. ROC curves were performed to compare both scores.
Results
We included 169 patients; median age was 50 years. HER2-positive and triple negative patients were 33.7% and 20.7%, respectively. At the last follow-up, 90% of the patients had died. Median OS was 12 months (95% confidence interval 8.0–16.0 months). OS was worse in patients with > 3 BM and in patients with triple negative subtype.
Conclusions
In our series, we confirm that B-GPA and mB-GPA scores correlated with prognosis. ROC curves showed that B-GPA and mB-GPA have similar prognostic capabilities, slightly in favor of mB-GPA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases (BM) are associated with functional deterioration and poor prognosis. Reports on the natural history of the disease found a median overall survival (OS) of 1 month if no treatment was given [1]. Before the development of targeted drugs and despite whole-brain radiotherapy (WBRT), median OS in the last century was less than 6 months [2]. However, BM in patients with breast cancer (BC) encompasses a wide range of prognostic groups of patients, all of whom require different approaches. Indeed, prognosis in some patients is fatal, while others might achieve more than 3 years of survival [3,4,5,6]. Among patients with advanced BC, up to 50% of those with HER2-positive disease and approximately 30–50% of patients with triple negative disease (TNBC) will develop BM. In contrast, about 10–15% of patients with hormone receptor (HR)-positive tumors will develop BM during the course of their disease [7, 8]. Median OS is about 24, 10 and 7 months for HER2+, HR+ and TNBC patients, respectively [6, 9].
The development of anti-HER2 drugs has substantially improved the survival outcomes of HER2-positive BC patients [10,11,12]. However, the penetration of some of these drugs across the blood–brain barrier is assumed to be limited, so many of these patients end up developing BM. Moreover, more accurate treatment strategies have emerged in recent years [13, 14]. This phenomenon, together with improved diagnostic imaging techniques, has led to the detection of an increase in BM incidence in all subtypes [15], prompting current efforts focusing on BM control.
Efforts must be made to improve survival while avoiding toxicities in patients with limited life expectancy. The addition of whole-brain radiation delays progression, but at the same time that improves overall survival, it also impairs cognitive function and, therefore, quality of life [16]. Because of this, in patients with a limited number of lesions, in a location amenable to brain surgery or stereotactic radiosurgery (SRS), those strategies are the preferred treatment options, since they minimize brain toxicity [17,18,19,20,21].
Several prognostic factors have been described in patients with BC and BM. The most important factors are performance status, tumor subtype, age and comorbidities. Other prognostic factors include the presence of active extracranial disease and, more recently, the number of BM [4, 22,23,24,25]. Prognostic predictor tools that include some of the clinical and histological factors described above have been developed to tailor treatment according to prognosis and efficacy of these treatments [25, 26]. The Breast Graded Prognostic Assessment (B-GPA) is a well-known score for assessing the prognosis of patients with BC and BM. The B-GPA proved to be more accurate than the traditional recursive partitioning analysis (RPA) score [27]. The B-GPA was later adapted by the same group to include other prognostic factors such as BC subtype, Karnofsky Performance Scale (KPS), and age at diagnosis of BM [4, 23, 25]. Despite its good correlation with survival, the B-GPA does not consider the number of BM. This issue is important as it determines suitability for local treatments such as SRS or surgery. In 2015, Subbiah et al. proposed a modified B-GPA score (mB-GPA) which includes the number of BM, proving the value of the new score in predicting the survival of these patients [5].
Some authors have attempted to validate these scores in different populations, but more evidence is needed to confirm their utility, and to determine which is the best score in this setting [3,4,5, 23,24,25,26, 28, 29]. The primary aim of our study was to validate the B-GPA and mB-GPA in routine clinical practice in a consecutive series of BC patients with BM treated at a single Spanish comprehensive cancer center. The secondary aim was to compare both scores to determine which is best for making clinical decisions.
Materials and methods
Study design and patients
This is an ambispective study of patients with BC and BM treated in a single comprehensive cancer center in Spain between January 2003 and April 2019. Inclusion criteria were: histologically proven invasive BC, age > 18 years at the time of BC diagnosis, BM confirmed by brain magnetic resonance imaging (MRI) or cerebral computed tomography scan. Patients with leptomeningeal disease were excluded unless they had concomitant BM. All subjects included prospectively gave their informed consent for inclusion in the study. The Ethics Committee also allowed the analysis of deceased patients. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of University Hospital of Bellvitge (PR189/20). Clinical data were collected retrospectively until 2014, and prospectively from 2014 to 2019.
Clinical and pathological data were collected for each patient, including patient demographics, KPS, number of BM, symptoms related to BM, BC subtype, histological grade, local and systemic treatments administered and response to each one. Estrogen receptor (ER), progesterone receptor (PR) and HER2 expression were determined by immunohistochemistry (IHC). Positivity for HR was defined as any receptor IHC spotting in at least 1% of tumor cells. HER2 status was defined as positive if IHC score 3 + or 2 + and amplification by fluorescent in situ hybridization (FISH). According to IHC and FISH, patients were classified into three subtypes: TN (HR−/HER2−), luminal-like (HR+/HER2−) and HER2+ (HR+ or −/HER2+).
Statistical analysis
B-GPA and mB-GPA scores [4, 5] were calculated for each patient (Table 1), classifying them into four groups. A higher score represents a better prognosis from 0 to 4 points.
Descriptive statistics were performed for demographic and clinical characteristics of the patients including sex, age and performance status at BM diagnoses, tumor subtype, stage at diagnosis, number of BM and BM treatment.
OS was defined as the time from BM diagnoses to death due to any cause. The Kaplan–Meier method was used to calculate OS and univariate analyses were conducted using the Cox proportional hazard regression model to calculate hazard ratios and 95% confidence intervals (CI).
Survival ROC curves were constructed to compare both scores to determine which correlated better with OS. Data analysis was performed using IBM SPSS version 18 and R software version 3.3.3.
Results
Patient characteristics
A total of 169 patients seen between January 2014 and April 2019 were included, all of whom were women. The median KPS was 80% [interquartile range (IQR) 70–90%]. Patient characteristics are listed in Table 2.
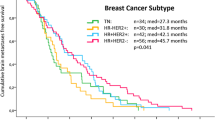
Most patients (54%) received multimodal treatment with surgery, SRS and/or WBRT. Patients who received multimodal treatment had a median KPS of 90% (IQR 90–100%), while patients who received only WBRT had a median KPS of 80% (IQR 70–90%). At a median follow-up of 12 months (0–162 months), 90% of the patients had died. Median OS was 12 months (95% CI 8.0–16.0). Median OS accordingly to tumor subtype was 26 months, 11 months and 8 months in HER2-positive, luminal and TNBC patients, respectively (p = 0.01 in the univariate analysis). OS was 22 months in patients with one BM, 11 months in those with 2–3 BM, and 8 months in patients with > 3 BM (p = 0.046). No other clinical and biological factors analyzed correlated with OS. There were no significant differences in patients diagnosed before 2010 or later.
B-GPA and mB-GPA score validation and comparison
Both B-GPA and mB-GPA scores were significant predictors of OS with a p value < 0.005 (Fig. 1). Table 3 shows median OS with confidence intervals for each group for both scores. Median OS was 6, 6, 17 and 24 months in patients with B-GPA scores ranging from 0–1.0, 1.5–2.0, 2.5–3.0 and 3.5–4.0, respectively, while median OS was 4, 7, 26 and 30 months in patients with mB-GPA ranging from 0–1.0, 1.5–2.0, 2.5–3.0 and 3.5–4.0 respectively.
The Kaplan–Meier curves show that the OS can be ranked using either B-GPA (a) and mB-GPA (b). The p value was < 0.005 for both scores. Patients with higher punctuation had longer survival in the two models. A higher punctuation than two is correlated with more than 1 year and a half of median overall survival in B-GPA score and more than 2 years in mB-GPA score
The survival ROC curves for B-GPA and mB-GPA were very similar, showing a slightly higher AUC for the mB-GPA curve (B-GPA AUC: 0.269 vs. mB-GPA AUC: 0.286).
Discussion
Our study showed that both B-GPA and mB-GPA scores are useful tools for predicting OS in BC patients with BM, identifying a subgroup of patients with better survival outcomes. However, mB-GPA seemed to be a better prognostic tool, since it showed a slightly higher AUC and it seemed to better discriminate than B-GPA among patients with worse prognoses (OS of 4 vs. 7 months using mB-GPA and 6 vs. 6 months using B-GPA for patients with 0–1 vs. 1.5–2 points, respectively). Likewise, mB-GPA discriminates better the two top prognosis groups classified showing better survival and higher differences than B-GPA when giving the same punctuation (OS of 26 using mB-GPA and 17 months using B-GPA for patients with 2.5–3 points). The addition of the number of BM can help us to decision-making.
Our series has similar characteristics to those described in the literature for BC patients with BM [3,4,5, 29]. All patients were women with a median age of 50 years, mostly HER2+ and HR+, with a KPS greater than 70%. In the univariate analysis, our study confirms the predictive value of both the BC subtype and the number of BM, as shown in many other studies [3, 5, 29].
Regarding systemic treatment, advances in effective new targeted therapies in HER2+ and HR+ patients are leading to improvements in both PFS and OS [30,31,32,33,34,35,36], but they do not seem effective in preventing BM [37]. Knowledge is rapidly expanding, and novel and promising anti-HER2 targeted drugs, such as tucatinib and neratinib, have emerged to treat BM in HER2+ BC [13, 38,39,40].
An established prognostic index can assist in selecting the best approach for each patient, taking into account all of these characteristics and expected survival after BM diagnosis. We compared B-GPA with mB-GPA using survival ROC curves, and found that the mB-GPA index was slightly superior in predicting SBM. This result, in line with the results of the previous studies and validation cohorts [3,4,5, 29], supports the use of mB-GPA in routine clinical practice for the appropriate stratification of patients and therapeutic decision-making.
Extracranial disease status has been proposed as a prognostic factor which could help determine the most suitable treatment [29]. Nevertheless, clinical guidelines recommend beginning by treating the affected areas that involve a risk to life [41]. That means extracranial involvement determines the timing more than the kind of treatments, so it was not taken into account in most of the studies [4, 23, 26].
This study has several strengths and limitations. It has a large sample size and long-term follow-up among breast cancer patients treated in a single Spanish comprehensive cancer center. We are aware, however, that almost 50% of the patients were included retrospectively, so not all the information regarding those patients was available and we could have been missing. We decided to include all patients to minimize the selection bias. Moreover, all patients were treated at the same institution, in the multidisciplinary Neuro-Oncology Unit (NOU), where patients are evaluated by different specialists involved in the management of patients with primary and secondary brain tumors. The use of these prognostic indexes, in combination with the decisions of the NOU committee, is helping us to determine the best therapeutic strategies for our BC patients with BM.
Conclusions
Our study validates previous results regarding the GPA scores for discriminating risk groups with significant different survival outcomes in patients with breast cancer and brain metastases. In the univariate analysis, B-GPA and mB-GPA, number of lesions, and tumor subtype were the most important prognostic factors mB-GPA, in conjunction with evaluation by a multidisciplinary team, can help select the best approach and adjust the therapeutic effort in each patient.
Data availability
Database encompassing all patient data is saved in the Hospital’s hardware.
Code availability
IBM SPSS version 18 and R software version 3.3.3.
References
Zimm S, Wampler GL, Stablein D, et al. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48:384–94.
Fokstuen T, Wilking N, Rutqvist LE, et al. Radiation therapy in the management of brain metastases from breast cancer. Breast Cancer Res Treat. 2000;62:211–6.
Griguolo G, Jacot W, Kantelhardt E, et al. External validation of Modified Breast Graded Prognostic Assessment for breast cancer patients with brain metastases: a multicentric European experience. Breast. 2018;37:36–41.
Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–25.
Subbiah IM, Lei X, Weinberg JS, et al. Validation and development of a modified breast graded prognostic assessment as a tool for survival in patients with breast cancer and brain metastases. J Clin Oncol. 2015;33:2239–45.
Martin AM, Cagney DN, Catalano PJ, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 2017;3:1069–77.
Lin NU, Claus E, Sohl J, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113:2638–45.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72.
Lin NU, Amiri-Kordestani L, Palmieri D, et al. CNS metastases in breast cancer: old challenge, new frontiers. Clin Cancer Res. 2013;19:6404–18.
Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2—positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–52.
Kast K, Schoffer O, Link T, et al. Trastuzumab and survival of patients with metastatic breast cancer. Arch Gynecol Obstet. 2017;296:303–12.
Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol. 2019;19(11):1758835919833519.
Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2019;382(7):597–609.
Achrol AS, Rennert RC, Anders C, et al. Brain metastases. Nat Rev Dis Primers. 2019;5(1):5.
Moja L, Brambilla C, Compagnoni A, Pistotti V. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2006;2012(4):C006243.
Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388:2004–14.
Trovo M, Furlan C, Polesel J, et al. Radical radiation therapy for oligometastatic breast cancer: results of a prospective phase II trial. Radiother Oncol. 2018;126:177–80.
Muacevic A, Kreth FW, Tonn JC, Wowra B. Stereotactic radiosurgery for multiple brain metastases from breast carcinoma: feasibility and outcome of a local treatment concept. Cancer. 2004;100:1705–11.
Huang Z, Sun B, Shen G, et al. Brain metastasis reirradiation in patients with advanced breast cancer. J Radiat Res. 2017;58:142–8.
Yomo S, Hayashi M. The efficacy and limitations of stereotactic radiosurgery as a salvage treatment after failed whole brain radiotherapy for brain metastases. J Neurooncol. 2013;113:459–65.
Soon YY, Tham IWK, Lim KH, et al. Surgery or radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst Rev. 2014;2014(3):CD09454.
Niwinska A, Murawska M, Pogoda K. Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol. 2010;21:942–8.
Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–4.
Griguolo G, Dieci MV, Giarratano T, et al. Beyond breast specific—Graded Prognostic Assessment in patients with brain metastases from breast cancer: treatment impact on outcome. J Neurooncol. 2017;131:369–76.
Sperduto PW, Mesko S, Li J, et al. Beyond an updated graded Prognostic Assessment (Breast GPA): a prognostic index and trends in treatment and survival in breast cancer brain metastases from 1985 to today. Int J Radiat Oncol Biol Phys. 2020;107(2):334–43.
Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82:2111–7.
Laakmann E, Riecke K, Goy Y, et al. Comparison of nine prognostic scores in patients with brain metastases of breast cancer receiving radiotherapy of the brain. J Cancer Res Clin Oncol. 2016;142:325–32.
Tai CH, Wu CC, Hwang ME, et al. Single institution validation of a modified graded prognostic assessment of patients with breast cancer brain metastases. CNS Oncol. 2018;7:25–34.
Zhuang Q, Wong RX, Lian WX, et al. Validation of modified Breast Graded Prognostic Assessment for breast cancer patients with brain metastases: extra-cranial disease progression is an independent risk factor. Ann Palliat Med. 2019;8:390–400.
Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–36.
Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–46.
Hortobagyi GN. Ribociclib for the first-line treatment of advanced hormone receptor-positive breast cancer: a review of subgroup analyses from the MONALEESA-2 trial. Breast Cancer Res. 2018;20(1):123.
Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–36.
Gianni L, Dafni U, Gelber RD, et al. HERA 4-year follow-up. Lancet Oncol. 2011;12:236–44.
Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–205.
Prat A, Pineda E, Adamo B, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24:S26–35.
Von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–28.
Petrelli F, Ghidini M, Lonati V, et al. The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: a systematic review and pooled analysis. Eur J Cancer. 2017;84:141–8.
Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14:64–71.
Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610–9.
Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4) †. Immune-related Pathol Response Criteria. 2018;29:1634–57.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
AS, JL, MG, SR, MB and CF conceived and planned the hypothesis. MG, MS, RV and SP improved the overview of the study. AS, JL, SR, MB and CF planned the study protocol and collected the data VN, AS and CF analyzed the data and presented the results. CF took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MG declares consulting/advisory fees from Pfizer, Eisai, Genomic-Health, Agendia, Daiichi-Sankyo, Novartis, Roche Pharma and Kern and travel/accommodation grants from Daiichi-Sankyo, Kern, Novartis and Pfizer. SP declares consulting/advisory fees from Astra-Zeneca, Daiichi-Sankyo, Novartis, Polyphor and Roche and travel/accommodation grants from Novartis. CFZ declares facilities to congress attendance from Pfizer. RVV declares professional fees from Novartis, Pfizer and Roche. RVF declares professional fees from Novartis, Gilead, Eisai, Esteve and Takeda. The rest of the authors have no conflicts of interest to declare.
Ethical approval
The protocol and the informed consent were approved by the Ethics Committee of University Hospital of Bellvitge (PR189/20). (Appendix 1in ESM).
Consent to participate
Patients included prospectively signed the informed consent approved by ethics committee (Appendix 2in ESM).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fabregat-Franco, C., Stradella, A., Navarro, V. et al. Validation and comparison of Breast Graded Prognostic Assessment scores in patients with breast cancer and brain metastases. Clin Transl Oncol 23, 1761–1768 (2021). https://doi.org/10.1007/s12094-021-02577-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02577-x