Abstract
Malignant gliomas (MG) are very aggressive tumors. In an effort to improve the outcome, the patients receive multi-modal therapies such as surgery, radiation and chemotherapy (temozolomide followed in many cases by bevacizumab). The survivors are affected by multiple learning and memory deficits. Greater deterioration over time in hippocampal specific cognitive tasks was shown in patients receiving bevacizumab in addition to radiation and temozolomide for a longer period of time (RTOG 0825). The rate of hippocampal atrophy in patients treated with radiation and temozolomide followed by bevacizumab is not yet determined, and is the goal of the present study. We used the serial MRIs obtained as parts of standard clinical care in patients with MG. Measurements were done using the Medical Image Processing, Analysis and Visualization (MIPAV) software. The hippocampus in the contralateral hemisphere was manually traced and measured, to avoid morphological structure changes induced by the tumor, radiation fields or surgical markers. We determined a longitudinal progression of hippocampal atrophy—with the maximum volume loss (33.26 %) for the patients that were on treatment for 5 years. There was no detectable hippocampal atrophy during the chemo-radiation followed by adjuvant temozolomide. A significant decrease in the absolute hippocampus volume was noted after 6 months of continuous bevacizumab treatment (p < 0.05). The hippocampal volume loss progressed over the next 3 years, and was higher than the one previously reported in Alzheimer disease patients. The hippocampal volume loss is minimal during the 1 month after diagnosis, when the patients receive chemo-radiation and adjuvant temozolomide. However, prolonged treatment including bevacizumab is associated with a significant rate of hippocampal volume loss.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Treatment advances lead to prolongation of survival for patients with high-grade astrocytic gliomas (anaplastic astrocytoma, AA and glioblastoma, GBM) and have made increasingly important to study long-term treatment related effects, including cognitive deficits. Treatments for malignant gliomas include radiation and temozolomide chemotherapy [1]. However, radiotherapy can induce cognitive decline [2] and in extreme cases can cause dementia [3]. The use of temozolomide with radiotherapy enhances neurotoxicity and contributes to further cognitive deficits [4]. Bevacizumab is commonly used in patients with recurrent MG that have failed temozolomide [5]. Bevacizumab is a monoclonal antibody against vascular endothelial growth factor A (VEGF-A) [6]. VEGF-A stimulates tumor angiogenesis, but it also promotes neurogenesis in the brain, particularly in the hippocampus, effecting fundamental processes needed for learning and memory [7]. Therefore, bevacizumab might contribute to cognitive impairment, especially in patients receiving this agent for prolonged periods of time [8]. Previous research (Bag et al.) suggested that high-grade glioma patients using bevacizumab experience a significant increase in ventricle volume over time as well as a significant decrease in whole brain volume and grey matter volume [9]. However, the authors were unable to segment grey matter, white matter, or hippocampus due to the poor contrast resolution of their images [9].
Cognitive impairments in brain tumor patients resemble clinically those seen in other neurodegenerative conditions such as the mild cognitive impairment (MCI) and Alzheimer dementia (AD). The process of hippocampal atrophy (both expressed as an absolute loss of hippocampal volume, as well as the percentage of volume loss each year) is more severe in the MCI and AD patients compared to the normal population [10, 11]. The question if similar findings can be identified in malignant glioma patients treated for long periods of time is the focus of our present article.
Methods
Patient selection and study design
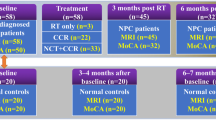
To be eligible for inclusion in our retrospective, Institutional Review Board (IRB) approved study, the patients needed to meet the following criteria: newly-diagnosed, supratentorial malignant glioma (GBM or AA), treated at the University of California, Irvine Medical Center’s (UCIMC) Comprehensive Brain Tumor Clinic, on active treatment for at least 18 months, and on bevacizumab for at least 6 months of their clinical course, with sagittal, fine cuts 3D contrast enhanced MPRAGE T1 weighted images available at the key analysis points. The 13 patients identified had surgical resection to remove tumor after the initial diagnosis and 6 weeks of radiotherapy with concomitant temozolomide. In absence of tumor progression, the patients received post-radiation adjuvant temozolomide, and were started on bevacizumab at the time of first tumor progression. Clinical variables for each patient are outlined in Table 1. Brain MRI’s were obtained before and after surgery, 2 weeks after completion of radiotherapy, and every 4–8 weeks during the chemotherapy treatment, as clinically indicated.
MRI methods and image analysis
MRI imaging was conducted concurrently with clinical appointments every 4–8 weeks or earlier if a patient displayed evidence of progressive disease. 1.5 and 3.0 T MRIs were used to generate sagittal 3D contrast enhanced MPRAGE T1 weighted images. Of the 289 MRIs collected from eligible patients, 243 were suitable for volumetric measurement. Measurements were done using the Medical Image Processing, Analysis and Visualization (MIPAV) software by a research associate (BL) blinded to the patient treatment history, and then validated by another research associate (SS), who was independent from the first. The manual segmenting was also verified by the PI (DB). The manual tracing of hippocampal boundaries were done consecutively from the rostral to the caudal side of brain for each image. The hippocampus in the contralateral hemisphere was traced and measured, to avoid morphological structure changes induced by a tumor, shunt, radiation fields or surgical markers. MIPAV software calculates the absolute volume automatically, determined by the number of voxels in each delineated image, and a value is given in mm3.
Statistical analysis
Patient parameters and clinical variables (Table 1 ) were analyzed using descriptive statistics. Primary outcome measures for this study included monthly and annual rates of hippocampal atrophy (%), as well as the longitudinal progression of the mean total atrophy. Linear regression was used to evaluate significant (p < 0.05) differences in hippocampal volume overtime. There were two measure outcomes, similar with the outcomes reported previously in the MCI and AD studies [10, 11] (see also Table 2). The first outcome assessed hippocampal atrophy as a percent of total volume loss as compared with the hippocampal volume at the time of diagnosis. The second outcome calculated the rate of hippocampal atrophy using the difference in absolute volume (mm3) at each time interval. Pearson’s correlation examined the homogeneity between rates of volumetric change and patient descriptives (age, tumor grade, progression time, chemotherapy duration). Paired sample t tests determined whether the mean rates of atrophy were significant between different time periods. Multiple regression models assessed the effect of covariates (age, gender, tumor location, tumor grade, progression time, length of time on temozolomide, temozolomide cycles, bevacizumab duration, disease duration and survival status) on absolute and percent of hippocampal atrophy. Statistical analysis was performed using the newest version of SPSS 23.
Results
A total of 13 patients, six males and seven females were identified with a mean age of 54 + 14.183 (median = 55). Of the ten GBM and three AA tumors in study, seven were on the right hemisphere and six were on the left. All patients received radiotherapy with concomitant temozolomide, but two patients did not have post-radiation adjuvant temozolomide due to unequivocal tumor progression and were started directly on bevacizumab. For the 11 patients that received adjuvant temozolomide, the average time on adjuvant temozolomide was 8.71 months. At the first tumor progression all the patients received bevacizumab. The total duration of bevacizumab treatment for a single patient in our study ranged from 8 months to 55 months, with the overall average of 32.22 (median = 33.45) months. Median follow up time from diagnosis was 3.26 years, with 9 of the 13 patients still alive at the conclusion of the study. Median time from diagnosis to start of bevacizumab treatment was 7.86 months (34.14 weeks). The average number of MRIs measured per patient was 19.69 + 5.67 (median = 20).
We determined that the volume of hippocampus declined in all our patients over the duration of their treatment (see Fig. 1, representative patient). Figure 2a shows the longitudinal progression of hippocampal atrophy, with a maximal level of volume loss of 33.26 % being reached at almost 5 years after the initial diagnosis. Figure 2b shows identical data as a function of absolute volume loss.
Hippocampal atrophy. a The longitudinal progression of hippocampal volume after start of bevacizumab presented as percent of hippocampal atrophy from baseline volume at diagnosis. b The longitudinal progression of hippocampal volume after start of bevacizumab presented as the mean volume (mm3) of hippocampus at specific time point. c The longitudinal progression of hippocampal volume after start of adjuvant temozolomide presented as percent of hippocampal atrophy from baseline volume at diagnosis. d The longitudinal progression of hippocampal volume after start of adjuvant temozolomide presented as the mean volume (mm3) of hippocampus at specific time point. The error bars represent standard error mean (SEM) for each time point. The numbers are (n) patients included at that time point. The stars show a significant mean difference from start of a specific chemotherapy (bevacizumab or temozolomide). The triangles show a significant mean difference compared to initial diagnosis. e Baseline (100 %) is start of bevacizumab. The stars show a significant mean difference from start of bevacizumab. The diamonds show a significant mean difference compared to the previous year. (*p < 0.05, **p < 0.01, ***p < 0.001; ♦p < 0.05, ♦♦p < 0.01, ♦♦♦p < 0.001)
Hippocampal atrophy rates were also calculated separately for each step in the treatment (radiation and concomitant temozolomide, adjuvant temozolomide and bevacizumab). We did not find a significant loss of hippocampal volume from the time of diagnosis until the end of radiation and the completion of the first 5 months of adjuvant temozolomide. Figure 2c shows the longitudinal progression of hippocampal atrophy, with a modest level of volume loss of 7.07 % being reached at the time of tumor progression. Figure 2b shows identical data as a function of absolute volume loss. A significance (p < 0.05) in percent volume was detected only at one single time point early in the treatment course (after 6 months of temozolomide treatment), but was not confirmed by the absolute volume analysis.
A significant decrease in the absolute hippocampus volume was noted after 6 months of continuous bevacizumab treatment (p < 0.05). A similar trend was noted also for the rate of hippocampal volume atrophy, with the statistically significant difference being detected 18 months after the start of bevacizumab (p < 0.01). The volume loss continued for as long as the patients received bevacizumab (Fig. 2a, b). In Fig. 2e we compared hippocampal volume changes at the start of bevacizumab and after 1–3 years of bevacizumab treatment. For the patients that were able to receive 3 years of continuous bevacizumab treatment (n = 6), the hippocampal volume continued to decline every year.
Independent samples t test were then used to determine whether particular group mean differences were significant. The number of temozolomide cycles or the length of time on temozolomide did not predict either the yearly hippocampal atrophy rate (p = 0.067), or the monthly hippocampal atrophy rate (p = 0.065). The GBM patients had higher monthly and annual rates of total and bevacizumab atrophy as compared with the AA patients (p < 0.05). No detectable significance for gender, bevacizumab duration, or disease duration was found.
Discussion
Chemotherapeutic drugs such as temozolomide and bevacizumab have led to improved progression free survival and overall survival in patients with MG. However, many patients exhibit patterns of cognitive impairment involving hippocampal related learning and memory paradigms (such as the Controlled Oral Word Association Test and the Trail Making Test [12]) and experience lower quality of life (QOL)—which potentially associates with prolonged bevacizumab use (such as the GBM patients who received bevacizumab from the initial diagnosis in the RTOG 0825 study) [13]. At the same time, no changes were seen in the hippocampal volumes for the GBM patients receiving standard radiation and temozolomide treatment [14]. Nevertheless, the Prust et al. study followed the patients for only a short period of time (35 weeks after the initial diagnosis—less than 9 months) [14], while our study followed our patients for up to 6 years—which supports the hypothesis that hippocampal atrophy is a delayed effect of malignant glioma treatment. Though we cannot separate the effects of radiation and the temozolomide for the effects of bevacizumab our pilot study suggests that the hippocampal atrophy might be accelerated by long-term bevacizumab use—as our patients reached a statistically significant level of hippocampal atrophy as expressed absolute volume loss only after 6 months of bevacizumab treatment. We also did not find any measurable hippocampal atrophy when we compared the values obtained at the time of diagnosis with the post-radiation MRIs and the MRIs obtained after 6 months of temozolomide treatment (approximately also 35 weeks after the initial diagnosis), similar with Prust’s study [14].
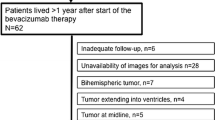
Our data are limited by the absence of control groups—patients that received only radiation and temozolomide, and did not require any other treatments for the next 1–3 years. We have tried to identify control MG patients with similar pathology that have survived similarly long periods of time (four or more years) without tumor progression and without receiving bevacizumab—but we were able to identify only two such patients in our large practice. In order to mitigate the effects of radiation, we measured the hippocampal volumes in the opposite side of brain then the tumor, and we made sure that the contralateral hippocampus was not affected by surgery and not included in the radiation fields.
In patients with MCI and AD, the rate of hippocampal atrophy correlates with disease progression and with the severity of cognitive loss [10, 11] (see Table 2). The annual percent change (APC) of hippocampal atrophy in normal (1.4–1.73), MCI (1.8–3.3), and AD (3.43–3.98) also correlates with the disease severity [10, 11]. As the annualized hippocampal volume loss measured in our study is higher than the one reported in Alzheimer disease patients, it is possible that treatment-induced hippocampal atrophy might directly explain the very high rate of memory deficits seen in long-term GBM survivors [15]. Data from clinical studies has identified severe treatment-induced dementia in a high number of long-term GBM survivors and cancer patients with brain metastasis [15, 16]. The evidence of such cognitive impairment has encouraged the use of particular AD drugs in brain cancer patients, to combat damaging neurological deficits resulting from the treatment of primary and metastatic brain tumors [17].
Bevacizumab is a VEGF inhibitor with anti angiogenic properties. Animal models have shown that VEGF expression is required for hippocampal neurogenesis involved in learning and memory [18]. It has been suggested that VEGF contributes to neuroprotection and neuronal repair in the central nervous system via its role in neurogenesis, long-term potentiation and cerebral blood flow following focal brain ischemia [19]. A recent study showed that prolonged treatment with bevacizumab is potentially associated with brain atrophy in malignant glioma patients [20]. The same study proposes that restricting VEGF may decrease the amount of neuronal repair, neurogenesis, and learning [20]. We propose that bevacizumab could contribute specifically to hippocampal atrophy by impairing hippocampal neurogenesis and healing of normal brain from surgical trauma, radiation and chemotherapy.
Previous papers report limitations due to resection, hemispheric tumor burden, and length of study, which may have impacted their ability to detect significant changes in the hippocampus over time [14]. It is suggested that the investigation into radiation and chemotherapy separately, in addition to novel targeted therapies over a longer period of time with stringent surgical parameters and a larger sample size, may be sufficient enough to determine further brain changes [14]. Our study analyzed sagittal cross sections of the hippocampus, imaged by magnetic resonance, over a span of several years beginning at diagnosis. The study detected concerning atrophy rates in glioma patients (8.903 %/year) over the course of their entire treatment—twice the reported rate of AD populations, three times the rate of MCI, and nearly four times the rate of normal aging (see Table 2). The retrospective nature of this study did not allow for concurrent investigation of cognitive impairment in our patients.
In conclusion, this study suggests that hippocampal atrophy is a relatively late phenomenon in the treatment of malignant glioma patients. The overall survival of malignant glioma patients is on the rise, and reached over 2 years in recent studies [21]—which potentially exposes the patients to prolonged use of chemotherapy drugs, including bevacizumab. We are currently planning a prospective study to examine the association between the rate of hippocampal atrophy, cognitive impairments and decreased QOL in malignant glioma patients on active chemotherapy for long periods of time.
References
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Hilverda K, Bosma I, Heimans JJ et al (2010) Cognitive functioning in glioblastoma patients during radiotherapy and temozolomide treatment: initial findings. J Neurooncol 97:89–94
Asai A, Matsutani M, Kohno T et al (1989) Subacute brain atrophy after radiation therapy for malignant brain tumor. Cancer 63:1962–1974
Taphoorn MJ, Klein M (2004) Cognitive deficits in adult patients with brain tumours. Lancet Neurol 3:159–168
Vredenburgh JJ, Desjardins A, Herndon JE 2nd et al (2007) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13:1253–1259
Norden AD, Young GS, Setayesh K et al (2008) Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 70:779–787
Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA 99:11946–11950
Ng T, Cheung YT, Ng QS, Ho HK, Chan A (2014) Vascular endothelial growth factor inhibitors and cognitive impairment: evidence and controversies. Expert Opin Drug Saf 13:83–92
Bag AK, Kim H, Gao Y et al (2015) Prolonged treatment with bevacizumab is associated with brain atrophy: a pilot study in patients with high-grade gliomas. J Neurooncol 122:585–593
Chiang GC, Insel PS, Tosun D et al (2010) Hippocampal atrophy rates and CSF biomarkers in elderly APOE2 normal subjects. Neurology 75:1976–1981
Jack CR Jr, Petersen RC, Xu Y et al (2000) Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 55:484–489
Ross TP, Calhoun E, Cox T, Wenner C, Kono W, Pleasant M (2007) The reliability and validity of qualitative scores for the controlled oral word association test. Arch Clin Neuropsychol 22:475–488
Gilbert MR, Dignam JJ, Armstrong TS et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699–708
Prust MJ, Jafari-Khouzani K, Kalpathy-Cramer J et al (2015) Standard chemoradiation for glioblastoma results in progressive brain volume loss. Neurology 85:683–691
Schmidinger M, Linzmayer L, Becherer A et al (2003) Psychometric- and quality-of-life assessment in long-term glioblastoma survivors. J Neurooncol 63:55–61
Jack CR Jr, Petersen RC, Xu Y et al (1998) Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology 51:993–999
Jack CR Jr, Shiung MM, Gunter JL et al (2004) Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 62:591–600
Schuff N, Woerner N, Boreta L et al (2009) MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain 132:1067–1077
Ridha BH, Anderson VM, Barnes J et al (2008) Volumetric MRI and cognitive measures in Alzheimer disease: comparison of markers of progression. J Neurol 255:567–574
Bag AK, Kim H, Gao Y, et al (2015) Prolonged treatment with bevacizumab is associated with brain atrophy: a pilot study in patients with high-grade gliomas. J Neurooncol 122:585–593
Stupp R, Hegi ME, Gorlia T, et al (2014) Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 15:1100–1108
Funding
This study was supported by the National Institute for Neurological Diseases and Stroke Award number NS072234, by the UCI Cancer Center Award Number P30CA062203 from the National Cancer Institute and by research funds donated by various donors to UC Irvine Neurological Oncology Program.
Author contributions
Shantell C. Nolen: gathered data, wrote the manuscript, performed statistical analysis. Brian Lee: performed MRI measurements. Shruti Shantharam: performed MRI measurements, selected eligible patients for the study. Hon J. Yu: taught BL and SS how to performed MRI measurements, collected data, reviewed the manuscript. Lydia Su: participated in project design, critically edited the manuscript, provided imaging expertise. John Billimek: supervised and guided SCN through the statistical analysis of the data. Daniela A. Bota: formulate the project, obtained IRB approvals for gathering and analyzing the patient data, conducted the retrospective review of the patient’s medical records, supervised and critically edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Neither of the authors has any conflict of interests.
Disclosures
The authors (Shantell C. Nolen, Brian Lee, Shruti Shantharam, Hon J. Yu, Lydia Su, John Billimek and Daniela A. Bota) have no financial relationships relevant to this manuscript.
Additional information
Shantell C. Nolen and Brian Lee have equally contributed to this research and they carried out the statistical analysis.
Rights and permissions
About this article
Cite this article
Nolen, S.C., Lee, B., Shantharam, S. et al. The effects of sequential treatments on hippocampal volumes in malignant glioma patients. J Neurooncol 129, 433–441 (2016). https://doi.org/10.1007/s11060-016-2188-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2188-8