Abstract
The aim of the present study was to evaluate the safety and feasibility of hypofractionated stereotactic radiotherapy (SRT) with CyberKnife for growth hormone-secreting pituitary adenoma (GH-PA). Fifty-two patients with GH-PA were treated with hypofractionated SRT between September 2001 and October 2012. Eight patients had clinically silent GH-PA and 44 were symptomatic. Only 1 patient was inoperable. The other patients had recurrent or postoperative residual tumors on MRI. All patients had received pharmacotherapy prior to SRT with a somatostatin analog, dopamine agonist, and/or GH receptor antagonist. The marginal doses were 17.4–26.8 Gy for the 3-fraction schedule and 20.0–32.0 Gy for the 5-fraction schedule. Endocrinological remission was assessed by the Cortina consensus criteria 2010 (random GH <1 ng/ml or nadir GH after an oral glucose tolerance test <0.4 ng/ml and normalization of age- and sex-adjusted insulin-like growth factor-1). The median follow-up period was 60 months (range 27–137). The 5-year overall survival, local control, and disease-free survival rates were 100, 100, and 96 %, respectively. Nine patients (5 clinically silent and 4 symptomatic patients) satisfied the Cortina criteria without receiving further pharmacotherapy, whereas the remaining 43 patients did not. No post-SRT grade 2 or higher visual disorder occurred. Symptomatic post-SRT hypopituitarism was observed in 1 patient. CyberKnife hypofractionated SRT is safe and effective when judged by imaging findings for GH-PA. However, it may be difficult to satisfy the Cortina consensus criteria in most symptomatic patients with SRT alone. Further investigations of optimal treatments are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary adenoma (PA) is a benign tumor that constitutes approximately 15 % of all intracranial tumors [1, 2]. Functioning PA has been divided into growth hormone (GH)-secreting adenoma, prolactin-secreting adenoma, adrenocorticotropic hormone (ACTH)-secreting adenoma, thyroid-stimulating hormone-secreting adenoma, and gonadotropin (luteinizing hormone and follicle-stimulating hormone)-secreting adenoma. Approximately 25–30 % of patients with GH-secreting PA (GH-PA) and ACTH-secreting PA are free of symptoms despite hormone excess; these tumors are called clinically silent PA [3, 4]. Adenoma mostly develops during adulthood and rarely during childhood. GH-PA accounts for approximately 20 % of all PA cases. Acromegaly is a clinical syndrome that results from the excessive secretion of GH. Its clinical features have been attributed to high serum concentrations of pituitary-derived GH and liver-derived insulin-like growth factor-1 (IGF-1), which is GH-dependent [5]. Excess GH and IGF-1 have somatic and metabolic effects: acral and soft tissue overgrowth, skin thickening, hyperinsulinism, insulin resistance, and overt diabetes [6, 7]. They additionally elevate the risk of malignant tumors. If excess GH persists for a long time, elevations occur in the incidence of cardiovascular events, and failure to achieve a GH level within the biochemical criterion range has been reported to lead to a death rate that is two- to four-fold higher than that in healthy individuals and also decrease life expectancy by 10–15 years [8, 9].
Treatments for GH-PA aim to prevent the excessive secretion of growth hormones. A basic approach in the treatment of GH-PA is surgery (transcranial and transsphenoidal surgery) and drug therapy. Since decreases in GH and IGF-1 typically take years after radiotherapy (RT), GH-PA is not necessarily treated by immediate RT after resection, unlike non-functioning PA. However, RT is considered if residual or recurrent tumors invade the cavernous sinus or in cases in which repeated surgeries have resulted in fibrosis and inoperability. RT is also considered for poor responders to surgery and pharmacotherapy or cases requiring tumor control for the suppression of visual disorders.
Conventional RT was previously used to treat these cases; several investigators reported their results, which is a relatively safe option for tumors close to organs at risk (OAR) such as the brainstem and optic apparatus [10, 11]. However, owing to the convenience and precision of treatment, as well as the reduced risk of hypopituitarism, the use of stereotactic irradiation is increasing. The outcomes of Gamma Knife stereotactic radiosurgery (SRS) have been reported [12–14], with relatively favorable outcomes being achieved. However, several issues have been identified in previous studies. First, the biochemical criteria for the normalization of GH used in earlier and recent studies markedly differ. The Cortina consensus criteria have been adopted since 2010, and the satisfaction of a stricter criterion has begun to be recommended [15]. The strict criteria are as follows; random GH <1 ng/ml or nadir GH after an oral glucose tolerance test <0.4 ng/ml and the normalization of age- and sex-adjusted IGF-1. Second, while the targeting accuracy and dose fall-off of the Gamma Knife treatment are excellent, a single fraction treatment may not be appropriate for tumors that are large or adjacent to optic pathways because the dose limitation for these structures is considered to be 8–10 Gy when given in a single session [16, 17]. The sparing of normal tissues, especially late-responding tissues presumably with a low α/β ratio (≤3 Gy), such as the optic pathways and brain stem, may be more efficiently treated by lower daily doses with fractionated radiation than with SRS [18, 19]. In order to achieve increased local control while maintaining low optic pathway toxicity, we started protocol-based hypofractionated SRT with the CyberKnife system for GH-PA in 2001. We used a hypofractionated schedule, which was similar to that for the other types of PA. In the present study, we analyzed the safety and efficacy of hypofractionated SRT with CyberKnife for GH-PA at multiple institutions.
Methods
Study design, patient eligibility, and characteristics
This was a prospective study based on protocols designed by the Clinical Study Committees of the Japanese Red Cross Medical Center, Yokohama CyberKnife Center, and Okayama Kyokuto Hospital and was approved by the Institutional Review Boards. The eligibility criteria were as follows: (1) histologically confirmed GH-PA, (2) failure to satisfy the Cortina consensus criteria following surgery and drug therapy for more than 6 months or inoperability, (3) no prior radiotherapy or chemotherapy for cranial disease, and (4) written informed consent. Fifty-two patients with GH-PA were treated with hypofractionated SRT using CyberKnife between September 2001 to October 2012. As pharmacotherapy, a somatostatin analogue was administered as an initial choice. If hormone levels did not satisfy the Cortina consensus with surgery and the somatostatin analogue, a dopamine agonist and/or growth hormone-receptor antagonist was added. Since older Cortina consensus criteria were adopted previously, some patients were not administered a somatostatin analogue as the first choice. Patient and tumor characteristics are summarized in Table 1. Most of the tumors were adjacent to the OARs.
Treatment protocols
SRT was delivered in either 3 or 5 fractions, and the 5-fraction schedule was used for young patients (<30 years old) and those with tumors that were large (≥10 cc), taking the possibility of late toxicities into consideration. Radiation doses were prescribed at the margin [95 % volume border of the planning target volume (PTV)]. The planned dose was, as a rule, either 21 Gy in 3 fractions or 25 Gy in 5 fractions; when the doses delivered to the OAR (optic nerve, chiasm, and brain stem) exceeded these levels, the marginal doses were reduced. Thus, the maximum doses allowed for the OAR were 21 Gy in 3 fractions or 25 Gy in 5 fractions. When the OAR doses were kept below these levels, dose escalations were performed as much as possible. All irradiation was given once a day, 3–5 days a week.
CyberKnife system and treatment planning
The CyberKnife system (Accuray, Sunnyvale, CA, USA) is equipped with a 6-MV photon-beam accelerator, robotic arm that can be moved in 6 dimensions of freedom, and target locating system (TLS). These features have already been described in detail in our previous studies [19–21]. It has very high precision, and the geometrical accuracy of CyberKnife was previously shown to be less than 0.5 mm [22].
The method of irradiation was also described in our previous studies [19, 20]. Briefly, radiation treatment was planned using a CT-based 3-dimensional treatment planning system (Ontarget [Accuray, Sunnyvale, CA, USA], until December 2008, or Multiplan [Accuray], from January 2009). Patients were lightly restrained with a custom-made thermoplastic face mask (WFR/Aquaplast Corp., Avondale, PA, USA), and 1.5-mm-thick CT images were taken after the administration of iopamidol. Magnetic resonance imaging (MRI) was performed with a 1.5-T system. T1-weighted contrast-enhanced images were obtained after the injection of meglumine gadopentetate (slice thickness: 2.0 mm, and slice interval: 0.5 mm). MRI images were then fused with CT images using Ontarget or Multiplan.
Lesions visible on CT and/or MRI were taken as the gross tumor volume. By taking the direction of tumor invasion into consideration, the clinical target volume (CTV) was adjusted based on information from the pre-operative images, discussion with the surgeon, and CT and/or MRI taken for planning. The PTV was equal to the CTV. Conformal treatment plans were designed for all cases using an inverse planning algorithm that involved setting dose constraints to minimize the irradiation delivered to critical structures such as optic pathways and the brain stem. The doses were calculated on the basis of the ray tracing algorithm.
Follow-up evaluation and statistical analysis
Patients were followed periodically after SRT. Regular follow-up studies included brain MRI, visual perception tests, and examinations of hormonal levels, applying the Cortina consensus criteria to the evaluation of biochemical hormone data. If hormone levels satisfied the Cortina consensus criteria, the doses of pharmacotherapy were reduced or interrupted. The overall survival, local control, and disease-free survival rates were calculated using the Kaplan–Meier method. Radiographic recurrences outside the PTV were not included in the calculation of local control rates. Differences between pre-treatment and last follow-up random GH and IGF-1 levels were examined by the paired t test. Values of P < 0.05 were considered to be significant. Statistical analyses were carried out with StatView Version 5 (SAS Institute Inc., Cary, NC, USA) and SPSS 11.0 J (SPSS Japan Inc., Tokyo, Japan). Toxicities were evaluated with the Common Terminology Criteria for Adverse Events version 4.0.
Results
Treatment characteristics
The 3-fraction and 5-fraction SRT schedules were delivered to 41 and 11 patients, respectively. In 18 patients, the total dose was reduced (median, 20.0 Gy; range, 17.4–24.5 Gy), because the doses delivered to the optic pathways or brain stem exceeded the maximum doses. In 6 patients, the dose was escalated (23.0 Gy; 22.0–32.0 Gy). A summary of the treatments is also shown in Table 1. The conformity and homogeneity indices were calculated according to the Radiation Therapy Oncology Group 1993 criteria [23] and the former was also calculated according to Paddick conformity index [24].
Survival and local control
All patients were observed for a minimum of 2 years. The median duration of follow-up was 60 months (range 27–137) for all patients. The 5-year overall survival rate was 100 % (95 % confidence interval [CI] 100–100 %). The 5-year local control and progression-free survival rates were 100 % (CI 100–100 %) and 96 % (CI 90–100 %), respectively (Fig. 1). At 5 years or more after the treatment, 3 patients developed local recurrence that was detectable by diagnostic imaging. Recurrence outside the irradiated field was detected in 2 cases (one in the contralateral cavernous sinus and the other in the skull base). All of these recurrent cases were symptomatic cases, and none of the 8 clinically silent cases developed recurrence. Figure 2 shows changes in random GH and IGF-1 levels from pre-irradiation measurements to measurements at the time of the last follow-up after irradiation. Figure 3 shows changes in serum hormone levels in symptomatic and clinically silent cases. Nine patients (5 clinically silent and 4 symptomatic patients) satisfied the Cortina consensus criteria without receiving further pharmacotherapy. Three clinically silent patients did not satisfy the Cortina consensus, but the pharmacotherapy was stopped because their random GH became <1.5 ng/ml and age- and sex-adjusted IGF-1 was normalized. On the other hand, the remaining 40 symptomatic patients failed to satisfy the criteria despite the continuous administration of pharmacotherapy. One of the clinically silent cases was treated with a dopamine agonist; however, medication has now been discontinued in all clinically silent cases. Although hypofractionated SRT significantly reduced hormone levels, complete endocrinological remission was only achieved in 17 % of all cases and 9 % of symptomatic cases when rated with the Cortina consensus criteria. There were no significant differences in radiographic control and hormonal control due to the fractionation schedule and radiation dose.
Changes in GH (a) and IGF-1 (b) levels from pre-irradiation measurements to measurements at the time of the last follow-up after irradiation (total population). Pre-irradiation serum GH and IGF-1 levels were 5.0 (2.74–10.03) (median, 95 % confidence interval) ng/ml and 455 (353–600) ng/ml, respectively. Serum GH and IGF-1 levels in the last follow-up were 1.89 (1.40–2.50) ng/ml and 242 (193–300) ng/ml, respectively. Significant differences were observed in serum GH and IGF-1 levels between pre- and post-irradiation (P < 0.0001). Changes in GH (c) and IGF-1 (d) levels from pre-irradiation measurements to measurements at the time of the last follow-up after irradiation (in symptomatic and silent cases). Pre-irradiation serum GH and IGF-1 levels for symptomatic and silent pituitary adenoma were 5.5 (3.00–10.65) (median, 95 % confidence interval) ng/ml, 2.50 (1.98–4.60) ng/ml, 500 (408–600) ng/ml, and 375 (342–413) ng/ml, respectively. Serum GH and IGF-1 levels in the last follow-up for symptomatic and silent pituitary adenoma were 2.00 (1.52–2.88) ng/ml, 0.31 (0.20–1.33) ng/ml, 250 (205–300) ng/ml, and 187 (156–240) ng/ml, respectively. Significant differences were observed in serum GH and IGF-1 levels for each group between pre- and post-irradiation (P < 0.01)
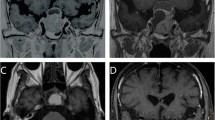
Example of hypofractionated SRT using CyberKnife for a patient with growth hormone-secreting pituitary adenoma. a Gadolinium-enhanced coronal MRI before SRT and the visual field test. b CyberKnife planning. c Gadolinium-enhanced coronal MRI and the visual field test 11 months after SRT. d Gadolinium-enhanced coronal MRI 120 months after SRT
Complications
No post-SRT Grade 2 or higher visual disorder was observed. Post-SRT hypopituitarism was observed in 1 patient who received no hormone replacement after surgery. No radiation-induced brain necrosis or paralysis of the oculomotor or abducens nerve was observed. No transient cyst enlargement was noted. There were no significant differences in late toxicities between the two fractionation schedules.
Discussion
Table 2 shows representative RT results for GH-PA [12–14, 25–28]. Various RT techniques, machines, and dose specifications were used at each institution. Representative studies of various SRS techniques indicated biochemical hormone control rates of 30–82 % with a median follow-up period of 5 years or longer [12–14, 25–28]. However, most evaluations in these studies were based on criteria such as GH <2.5 (ng/ml) rather than the Cortina consensus criteria. Many studies reported that 5–10 years or more are required before hormone levels decrease [10, 11]. In the present study, 60 % of all cases satisfied GH <2.5 and IGF-1< age- and sex-normalized levels, whereas only 17 % satisfied the Cortina consensus criteria. On the other hand, 55 % of symptomatic cases satisfied the previously used criteria of GH <2.5 ng/ml and IGF-1< age- and sex-normalized levels, whereas only 9 % satisfied the Cortina consensus criteria. Symptomatic cases had some difficulty satisfying the Cortina consensus criteria when treated with hypofractionated SRT alone. However, an increase in the drug dose and the addition of other drug types could be avoided to some extent by hypofractionated SRT. Although the relatively short follow-up period and small sample size were limitations in interpreting the results of this study, a higher radiation dose appeared to be needed in order to obtain better results. As a next step, we are planning to prescribe the dose at a 70 % isodose line and use 3-T MRI for planning to diagnose residual tumors precisely.
The incidences of visual disorders and hypopituitarism were also similar to those reported previously. In the present study, approximately 20 % of patients had a large tumor (>10 cc) that would not normally be treated with Gamma Knife SRS. Moreover, most tumors were adjacent to the OARs such as optical pathways. Despite the presence of such difficult cases, our results for hypofractionated SRT were consistent with previous findings. Favorable outcomes may have been achieved by our use of hypofractionation [19, 20]. If our findings on 100 cases of non-functioning PA [19] and 40 cases of craniopharyngioma [20] are taken into account, the doses of 21 Gy/3 Fr and 25 Gy/5 Fr may be regarded as safe and rational for achieving a satisfactory long-term outcome in the optic pathway. However, comparison with GammaKnife data would be useful to evaluate the efficacy of hypofractionated SRT. In GammaKnife treatment for GH-PA, a single dose of 20–25 Gy was applied to the tumor [12–14, 25, 27], but considering the adverse effects on optic pathways, the dose to the OAR may better be reduced to 8–10 Gy [16, 17]. To estimate the corresponding single doses for our hypofractionated doses, the linear-quadratic (LQ) model is known to be unreliable, but the corresponding single dose for hypofractionated doses may be roughly estimated by adding 15–20 % to the single dose calculated by the LQ model [29, 30]; by applying this hypothesis, these hypofractionated doses appeared to correspond to approximately 14–17 Gy given in a single fraction (SRS). Most of the tumors in our cases were adjacent to the optic pathways, and these doses are apparently higher than the safe SRS doses of 8–10 Gy. Fractionation was considered desirable from the viewpoint of adverse events, and the results obtained in the present study are expected to serve as useful indicators of dose limits for hypofractionation targeting the optic pathway. There has not yet been any recommendation for the optic pathway dose when using hypofractionation.
Clinically silent and totally silent GH-PA is a concept reported previously [3, 4, 31, 32]. Wade et al. [3] showed that, when GH-PA was categorized into 4 types, approximately 1/3 of all cases belonged to the silent GH-PA group. A previous study also found GH-PA in 3–19 % of all surgically treated cases of non-functioning PA [33]. According to the findings of a more recent study, responses to this treatment vary depending on the histological features of GH-PA. Lee et al. [34] divided GH-PA into two histological subtypes (densely granulated and sparsely granulated somatotroph cell types) and found that the remission rate was markedly higher for the densely granulated somatotroph-cell adenoma group. Mori et al. [35] showed that, when GH-PA was analyzed immunohistochemically, approximately 45 % showed a monohormonal pattern and 55 % a plurihormonal pattern. Furthermore, one-quarter of the monohormonal GH adenomas had a dot-like pattern of cytokeratin immunoreactivity in most tumor cells (>80 %); they were significantly more common in female or younger patients and were slightly larger and more invasive than monohormonal GH adenomas with perinuclear cytokeratin. The results of the present study demonstrated that clinically silent GH-PA differed markedly from symptomatic GH-PA in terms of pathophysiology. These results suggest that a careful follow-up without additional treatment may suffice if total mass reductions are achievable. In the near future, we intend to explore how histological differences reflect responses to radiotherapy by analyzing outcomes in relation to pathological findings at the time of surgery.
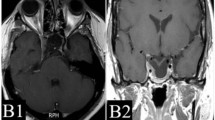
In the present study, there were many cases in which tumor shrinkage or complete response was observed after SRT when evaluated by diagnostic imaging; however, the effects observed on these images were not reflected in biochemical data. Figure 3 shows one such case. This was a 25-year-old woman with acromegaly who had visual field abnormalities after multiple surgeries and drug therapy, and was then judged to be inoperable. Before hypofractionated SRT, GH was 3.5 ng/ml and IGF-1 was 310 ng/ml. She underwent hypofractionated SRT using CyberKnife with 31.7 Gy in 5 fractions to her 12.4 cc tumor. Eleven months after hypofractionated SRT, the tumor achieved a partial response, and improvements were noted in the visual field. At 120 months, the tumor achieved a complete response when rated by diagnostic imaging. However, hormone levels failed to completely satisfy the Cortina consensus (GH 5.2 ng/ml, IGF-1 305 ng/ml). Thus, in the present study, there were many cases in which the control of a macroscopic tumor did not satisfy the Cortina consensus criteria. Even when tumor volumes are controllable by hypofractionated SRT, achieving the suppression of GH appears to be difficult with this therapy alone. Although the possibility that hormones are produced by another co-existing microscopic tumor cannot be ruled out, the satisfactory control of symptomatic GH-PA requires multidisciplinary treatments, involving a combination of surgery, drug therapy, and irradiation. Landolt et al. [36] reported that patients in whom drug therapies were stopped before SRS had improved hormonal control than those who continued drug therapies. Although that was a small retrospective nonrandomized study, separating the two groups may be better considered in the future study. The conventional belief that complete endocrinological remission is achievable with radiotherapy alone needs to be reevaluated.
In conclusion, CyberKnife hypofractionated SRT is safe for GH-PA and rated as effective on the basis of diagnostic imaging findings. However, when SRT is applied to patients with symptomatic GH-PA without being combined with any other therapy, it may be difficult to satisfy the Cortina consensus criteria in most cases. Further investigations of hypofractionated SRT with a longer follow-up are warranted in order to define its role in the treatment of GH-PA. Since GH-PA cases undergoing hypofractionated SRT are not frequently reported, our results will contribute to establishing a standard treatment for GH-PA.
References
Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, McCutcheon IE (2004) The prevalence of pituitary adenomas: a systematic review. Cancer 101:613–619
Sivakumar W, Chamoun R, Nguyen V, Couldwell WT. Incidental pituitary adenomas (2011) Neurosurg Focus 31:E18. doi:10.3171/2011.9.FOCUS11217
Wade AN, Baccon J, Grady MS, Judy KD, O’Rourke DM, Snyder PJ (2011) Clinically silent somatotroph adenomas are common. Eur J Endocrinol 165:39–44. doi:10.1530/EJE-11-0216
Mayson SE, Snyder PJ (2015) Silent pituitary adenomas. Endocrinol Metab Clin N Am 44:79–87. doi:10.1016/j.ecl.2014.11.001
Schneider HJ, Sievers C, Saller B, Wittchen HU, Stalla GK (2008) High prevalence of biochemical acromegaly in primary care patients with elevated IGF-1 levels. Clin Endocrinol (Oxf) 69:432–435. doi:10.1111/j.1365-2265.2008.03221.x
Bengtsson BA, Edén S, Ernest I, Odén A, Sjögren B (1988) Epidemiology and long-term survival in acromegaly. A study of 166 cases diagnosed between 1955 and 1984. Acta Med Scand 223:327–335
Rosario PW (2011) Frequency of acromegaly in adults with diabetes or glucose intolerance and estimated prevalence in the general population. Pituitary 14:217–221. doi:10.1007/s11102-010-0281-0
Bihan H, Espinosa C, Valdes-Socin H, Salenave S, Young J, Levasseur S, Assayag P, Beckers A, Chanson P (2004) Long-term outcome of patients with acromegaly and congestive heart failure. J Clin Endocrinol Metab 89:5308–5313
Damjanovic SS, Neskovic AN, Petakov MS, Popovic V, Vujisic B, Petrovic M, Nikolic-Djurovic M, Simic M, Pekic S, Marinkovic J (2002) High output heart failure in patients with newly diagnosed acromegaly. Am J Med 112:610–616
Barrande G, Pittino-Lungo M, Coste J, Ponvert D, Bertagna X, Luton JP, Bertherat J (2000) Hormonal and metabolic effects of radiotherapy in acromegaly: long-term results in 128 patients followed in a single center. J Clin Endocrinol Metab 85:3779–3785
Jenkins PJ, Bates P, Carson MN, Stewart PM, Wass JA (2006) Conventional pituitary irradiation is effective in lowering serum growth hormone and insulin-like growth factor-I in patients with acromegaly. J Clin Endocrinol Metab 91:1239–1245
Attanasio R, Epaminonda P, Motti E, Giugni E, Ventrella L, Cozzi R, Farabola M, Loli P, Beck-Peccoz P, Arosio M (2003) Gamma-knife radiosurgery in acromegaly: a 4-year follow-up study. J Clin Endocrinol Metab 88:3105–3112
Wang MH, Liu P, Liu AL, Luo B, Sun SB (2003) Efficacy of gamma knife radiosurgery in treatment of growth hormone-secreting pituitary adenoma. Zhonghua Yi Xue Za Zhi 83:2045–2048
Ikeda H, Jokura H, Yoshimoto T (2001) Transsphenoidal surgery and adjuvant gamma knife treatment for growth hormone-secreting pituitary adenoma. J Neurosurg 95:285–291
Giustina A, Chanson P, Bronstein MD, Klibanski A, Lamberts S, Casanueva FF, Trainer P, Ghigo E, Ho K, Melmed S (2010) Acromegaly consensus group: A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab 95:3141–3148. doi:10.1210/jc.2009-2670
Leber KA, Berglöff J, Pendl G (1998) Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg 88:43–50
Stafford SL, Pollock BE, Leavitt JA, Foote RL, Brown PD, Link MJ, Gorman DA, Schomberg PJ (2003) A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 55:1177–1181
Hoban PW, Jones LC, Clark BG (1999) Modeling late effects in hypofractionated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 43:199–210
Iwata H, Sato K, Tatewaki K, Yokota N, Inoue M, Baba Y, Shibamoto Y (2011) Hypofractionated stereotactic radiotherapy with CyberKnife for nonfunctioning pituitary adenoma: high local control with low toxicity. Neuro Oncol 13:916–922. doi:10.1093/neuonc/nor055
Iwata H, Tatewaki K, Inoue M, Yokota N, Baba Y, Nomura R, Shibamoto Y, Sato K (2012) Single and hypofractionated stereotactic radiotherapy with CyberKnife for craniopharyngioma. J Neurooncol 106:571–577. doi:10.1007/s11060-011-0693-3
Inoue M, Shiomi H, Iwata H, Taguchi J, Okawa K, Kikuchi C, Inada K, Iwabuchi M, Murai T, Koike I, Tatewaki K, Ohta S, Inoue T (2015) Development of system using beam’s eye view images to measure respiratory motion tracking errors in image-guided robotic radiosurgery system. J Appl Clin Med Phys 16:5049. doi:10.1120/jacmp.v16i1.5049
Antypas C, Pantelis E (2008) Performance evaluation of a CyberKnife G4 image-guided robotic stereotactic radiosurgery system. Phys Med Biol 53:4697–4718. doi:10.1088/0031-9155/53/17/016
Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, Martin L (1993) Radiation therapy oncology group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys 27:1231–1239
Paddick I (2000) A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg 93:S219–S222
Fukuoka S, Ito T, Takanashi M, Hojo A, Nakamura H (2001) Gamma knife radiosurgery for growth hormone-secreting pituitary adenomas invading the cavernous sinus. Stereotact Funct Neurosurg 76:213–217
Kobayashi T (2009) Long-term results of stereotactic gamma knife radiosurgery for pituitary adenomas. Specific strategies for different types of adenoma. Prog Neurol Surg 22:77–95. doi:10.1159/000163384
Lee CC, Vance ML, Xu Z, Yen CP, Schlesinger D, Dodson B, Sheehan J (2014) Stereotactic radiosurgery for acromegaly. J Clin Endocrinol Metab 99:1273–1281. doi:10.1210/jc.2013-3743
Yan JL, Chang CN, Chuang CC, Hsu PW, Lin JD, Wei KC, Lee ST, Tseng JK, Pai PC, Chen YL (2013) Long-term follow-up of patients with surgical intractable acromegaly after linear accelerator radiosurgery. J Formos Med Assoc 112:416–420. doi:10.1016/j.jfma.2012.01.020
Shibamoto Y, Otsuka S, Iwata H, Sugie C, Ogino H, Tomita N (2012) Radiobiological evaluation of the radiation dose as used in high-precision radiotherapy: effect of prolonged delivery time and applicability of the linear-quadratic model. J Radiat Res 53:1–9
Iwata H, Matsufuji N, Toshito T, Akagi T, Otsuka S, Shibamoto Y (2013) Compatibility of the repairable-conditionally repairable, multi-target and linear-quadratic models in converting hypofractionated radiation doses to single doses. J Radiat Res 54:367–373. doi:10.1093/jrr/rrs089
Naritaka H, Kameya T, Sato Y, Furuhata S, Otani M, Kawase T (1999) Morphological characterization and subtyping of silent somatotroph adenomas. Pituitary 1:233–241
Mohammed S, Syro L, Abad V, Salehi F, Horvath E, Scheithauer BW, Kovacs K, Cusimano M (2009) Silent somatotroph adenoma of the pituitary in an adolescent. Can J Neurol Sci 36:123–125
Cooper O (2015) Silent corticotroph adenomas. Pituitary 18:225–231. doi:10.1007/s11102-014-0624-3
Lee CC, Vance ML, Lopes MB, Xu Z, Chen CJ, Sheehan J (2015) Stereotactic radiosurgery for acromegaly: outcomes by adenoma subtype. Pituitary 18:326–334. doi:10.1007/s11102-014-0578-5
Mori R, Inoshita N, Takahashi-Fujigasaki J, Joki T, Nishioka H, Abe T, Fujii T, Yamada S (2013) Clinicopathological features of growth hormone-producing pituitary adenomas in 242 acromegaly patients: classification according to hormone production and cytokeratin distribution. ISRN Endocrinol 2013:723432. doi:10.1155/2013/723432
Landolt AM, Haller D, Lomax N, Scheib S, Schubiger O, Siegfried J, Wellis G (2000) Octreotide may act as a radioprotective agent in acromegaly. J Clin Endocrinol Metab 85:1287–1289
Acknowledgments
The authors are grateful to Mr. Kosaku Inada, Mr. Manabu Senda, Mr. Kohei Okawa, and Ms. Kumiko Ogawa for their valuable help in this research.
Financial disclosure
The authors declare that they are not involved in any relationships with companies or organizations that make products related to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest exist for any of the authors.
Ethical standards
The study was conducted in accordance with the Declaration of Helsinki and complies with the current laws of the countries in which it was performed. An independent ethics committee or institutional review board for each study site approved the study protocol. Informed consent was obtained from all individual patients included in the study.
Rights and permissions
About this article
Cite this article
Iwata, H., Sato, K., Nomura, R. et al. Long-term results of hypofractionated stereotactic radiotherapy with CyberKnife for growth hormone-secreting pituitary adenoma: evaluation by the Cortina consensus. J Neurooncol 128, 267–275 (2016). https://doi.org/10.1007/s11060-016-2105-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2105-1